The alkali metals consist of the
chemical elements
lithium (Li),
sodium (Na),
potassium (K),
[The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names for the elements in some languages, such as German and Russian.] rubidium
Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher ...
(Rb),
caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that a ...
(Cs), and
francium
Francium is a chemical element with the symbol Fr and atomic number 87. It is extremely radioactive; its most stable isotope, francium-223 (originally called actinium K after the natural decay chain it appears in), has a half-life of only 22&nb ...
(Fr). Together with
hydrogen they constitute
group 1, which lies in the
s-block of the
periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
. All alkali metals have their outermost electron in an
s-orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any sp ...
: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of
group trends in properties in the periodic table, with elements exhibiting well-characterised
homologous
Homology may refer to:
Sciences
Biology
*Homology (biology), any characteristic of biological organisms that is derived from a common ancestor
*Sequence homology, biological homology between DNA, RNA, or protein sequences
* Homologous chrom ...
behaviour.
This family of elements is also known as the lithium family after its leading element.
The alkali metals are all shiny,
soft, highly
reactive metals at
standard temperature and pressure
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union o ...
and readily lose their
outermost electron to form
cations with
charge +1. They can all be cut easily with a knife due to their softness, exposing a shiny surface that tarnishes rapidly in air due to
oxidation by atmospheric moisture and
oxygen (and in the case of lithium,
nitrogen). Because of their high reactivity, they must be stored under oil to prevent reaction with air, and are found naturally only in
salts and never as the free elements. Caesium, the fifth alkali metal, is the most reactive of all the metals. All the alkali metals react with water, with the heavier alkali metals reacting more vigorously than the lighter ones.
All of the discovered alkali metals occur in nature as their compounds: in order of
abundance, sodium is the most abundant, followed by potassium, lithium, rubidium, caesium, and finally francium, which is very rare due to its extremely high
radioactivity; francium occurs only in minute
traces in nature as an intermediate step in some obscure side branches of the natural
decay chains. Experiments have been conducted to attempt the synthesis of
ununennium (Uue), which is likely to be the next member of the group; none were successful. However, ununennium may not be an alkali metal due to
relativistic effects, which are predicted to have a large influence on the chemical properties of
superheavy elements; even if it does turn out to be an alkali metal, it is predicted to have some differences in physical and chemical properties from its lighter homologues.
Most alkali metals have many different applications. One of the best-known applications of the pure elements is the use of rubidium and caesium in
atomic clocks, of which caesium atomic clocks form the basis of the
second
The second (symbol: s) is the unit of time in the International System of Units (SI), historically defined as of a day – this factor derived from the division of the day first into 24 hours, then to 60 minutes and finally to 60 seconds ...
. A common application of the compounds of sodium is the
sodium-vapour lamp, which emits light very efficiently.
Table salt, or sodium chloride, has been used since antiquity.
Lithium finds use as a psychiatric medication and as an
anode in
lithium batteries. Sodium, potassium and lithium are
essential element
In the context of nutrition, a mineral is a chemical element required as an essential nutrient by organisms to perform functions necessary for life. However, the four major structural elements in the human body by weight (oxygen, hydrogen, carbon ...
s, having major biological roles as
electrolytes, and although the other alkali metals are not essential, they also have various effects on the body, both beneficial and harmful.
__TOC__
History

Sodium compounds have been known since ancient times; salt (
sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
) has been an important commodity in human activities, as testified by the English word ''salary'', referring to ''salarium'', money paid to Roman soldiers for the purchase of salt. While
potash has been used since ancient times, it was not understood for most of its history to be a fundamentally different substance from sodium mineral salts.
Georg Ernst Stahl obtained experimental evidence which led him to suggest the fundamental difference of sodium and potassium salts in 1702,
and
Henri-Louis Duhamel du Monceau was able to prove this difference in 1736. The exact chemical composition of potassium and sodium compounds, and the status as chemical element of potassium and sodium, was not known then, and thus
Antoine Lavoisier did not include either alkali in his list of chemical elements in 1789.
Pure potassium was first isolated in 1807 in England by
Humphry Davy, who derived it from
caustic potash
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which explo ...
(KOH, potassium hydroxide) by the use of electrolysis of the molten salt with the newly invented
voltaic pile. Previous attempts at electrolysis of the aqueous salt were unsuccessful due to potassium's extreme reactivity.
Potassium was the first metal that was isolated by electrolysis.
Later that same year, Davy reported extraction of sodium from the similar substance
caustic soda (NaOH, lye) by a similar technique, demonstrating the elements, and thus the salts, to be different.
 Petalite
Petalite (
Li Al Si4 O10) was discovered in 1800 by the
Brazilian chemist
José Bonifácio de Andrada in a mine on the island of
Utö, Sweden.
However, it was not until 1817 that
Johan August Arfwedson, then working in the laboratory of the chemist
Jöns Jacob Berzelius
Baron Jöns Jacob Berzelius (; by himself and his contemporaries named only Jacob Berzelius, 20 August 1779 – 7 August 1848) was a Swedish chemist. Berzelius is considered, along with Robert Boyle, John Dalton, and Antoine Lavoisier, to be on ...
,
detected the presence of a new element while analysing petalite
ore.
This new element was noted by him to form compounds similar to those of sodium and potassium, though its
carbonate and
hydroxide were less
soluble in water and more
alkaline
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a base (chemistry), basic, ionic compound, ionic salt (chemistry), salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as ...
than the other alkali metals.
Berzelius gave the unknown material the name "''lithion''/''lithina''", from the
Greek word ''λιθoς'' (transliterated as ''lithos'', meaning "stone"), to reflect its discovery in a solid mineral, as opposed to potassium, which had been discovered in plant ashes, and sodium, which was known partly for its high abundance in animal blood. He named the metal inside the material "''lithium''".
Lithium, sodium, and potassium were part of the discovery of
periodicity, as they are among a series of triads of elements in the same
group that were noted by
Johann Wolfgang Döbereiner in 1850 as having similar properties.

Rubidium and caesium were the first elements to be discovered using the
spectroscope, invented in 1859 by
Robert Bunsen and
Gustav Kirchhoff.
The next year, they discovered caesium in the
mineral water from
Bad Dürkheim, Germany. Their discovery of rubidium came the following year in
Heidelberg, Germany, finding it in the mineral
lepidolite.
The names of rubidium and caesium come from the most prominent lines in their
emission spectra
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a atomic electron transition, transition from a high energy state to a lower energy st ...
: a bright red line for rubidium (from the
Latin word ''rubidus'', meaning dark red or bright red), and a sky-blue line for caesium (derived from the Latin word ''caesius'', meaning sky-blue).
Around 1865
John Newlands produced a series of papers where he listed the elements in order of increasing atomic weight and similar physical and chemical properties that recurred at intervals of eight; he likened such periodicity to the
octave
In music, an octave ( la, octavus: eighth) or perfect octave (sometimes called the diapason) is the interval between one musical pitch and another with double its frequency. The octave relationship is a natural phenomenon that has been refer ...
s of music, where notes an octave apart have similar musical functions. His version put all the alkali metals then known (lithium to caesium), as well as
copper,
silver, and
thallium (which show the +1 oxidation state characteristic of the alkali metals), together into a group. His table placed hydrogen with the
halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
s.

After 1869,
Dmitri Mendeleev
Dmitri Ivanovich Mendeleev (sometimes transliterated as Mendeleyev or Mendeleef) ( ; russian: links=no, Дмитрий Иванович Менделеев, tr. , ; 8 February Old_Style_and_New_Style_dates">O.S._27_January.html" ;"title="O ...
proposed his periodic table placing lithium at the top of a group with sodium, potassium, rubidium, caesium, and thallium. Two years later, Mendeleev revised his table, placing hydrogen in group 1 above lithium, and also moving thallium to the
boron group. In this 1871 version, copper, silver, and
gold were placed twice, once as part of
group IB, and once as part of a "group VIII" encompassing today's groups
8 to 11.
[In the 1869 version of Mendeleev's periodic table, copper and silver were placed in their own group, aligned with hydrogen and ]mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
, while gold was tentatively placed under uranium and the undiscovered eka-aluminium in the boron group. After the introduction of the 18-column table, the group IB elements were moved to their current position in the
d-block, while alkali metals were left in ''group IA''. Later the group's name was changed to ''group 1'' in 1988.
[ The trivial name "alkali metals" comes from the fact that the hydroxides of the group 1 elements are all strong ]alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
s when dissolved in water.[Adloff, Jean-Pierre; Kaufman, George B. (25 September 2005)]
Francium (Atomic Number 87), the Last Discovered Natural Element
. ''The Chemical Educator'' 10 (5). Retrieved 26 March 2007. Perey then attempted to determine the proportion of beta decay to alpha decay in actinium-227. Her first test put the alpha branching at 0.6%, a figure that she later revised to 1%.eka
Eka or EKA may refer to:
People
* Eka Budianta (born 1956), Indonesian poet
* Eka Darville (born 1989), Australian actor
* Eka Gigauri (born 1978), Georgian activist
* Eka Gurtskaia (born ), Georgian beauty pageant titleholder
* Eka Kurniawan ...
-francium) in the periodic table would be ununennium (Uue), element 119.[The ]asterisk
The asterisk ( ), from Late Latin , from Ancient Greek , ''asteriskos'', "little star", is a typographical symbol. It is so called because it resembles a conventional image of a heraldic star.
Computer scientists and mathematicians often voc ...
denotes an excited state
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to a ...
.
It is highly unlikelyperiod 8 element
An extended periodic table theorises about chemical elements beyond those currently known in the periodic table and proven. , the element with the highest atomic number known is oganesson (''Z'' = 118), which completes the seventh period (row) ...
on the extended periodic table, it may well be discovered in the near future through other reactions, and indeed an attempt to synthesise it is currently ongoing in Japan.
Occurrence
In the Solar System
 The
The Oddo–Harkins rule The Oddo–Harkins rule holds that an element with an even atomic number (such as carbon, element 6) is more abundant than both elements with the adjacently larger and smaller odd atomic numbers (such as boron, element 5, and nitrogen, element 7, ...
holds that elements with even atomic numbers are more common that those with odd atomic numbers, with the exception of hydrogen. This rule argues that elements with odd atomic numbers have one unpaired proton and are more likely to capture another, thus increasing their atomic number. In elements with even atomic numbers, protons are paired, with each member of the pair offsetting the spin of the other, enhancing stability.supernova
A supernova is a powerful and luminous explosion of a star. It has the plural form supernovae or supernovas, and is abbreviated SN or SNe. This transient astronomical event occurs during the last evolutionary stages of a massive star or when ...
e and not in stellar nucleosynthesis
Stellar nucleosynthesis is the creation (nucleosynthesis) of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a ...
. Lithium is also much less abundant than sodium and potassium as it is poorly synthesised in both Big Bang nucleosynthesis and in stars: the Big Bang could only produce trace quantities of lithium, beryllium and boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
due to the absence of a stable nucleus with 5 or 8 nucleons, and stellar nucleosynthesis could only pass this bottleneck by the triple-alpha process, fusing three helium nuclei to form carbon, and skipping over those three elements.
On Earth
 The Earth formed from the same cloud of matter that formed the Sun, but the planets acquired different compositions during the
The Earth formed from the same cloud of matter that formed the Sun, but the planets acquired different compositions during the formation and evolution of the solar system
The formation of the Solar System began about 4.6 billion years ago with the gravitational collapse of a small part of a giant molecular cloud. Most of the collapsing mass collected in the center, forming the Sun, while the rest flattened into a ...
. In turn, the natural history of the Earth caused parts of this planet to have differing concentrations of the elements. The mass of the Earth is approximately 5.98 kg. It is composed mostly of iron (32.1%), oxygen (30.1%), silicon (15.1%), magnesium (13.9%), sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
(2.9%), nickel (1.8%), calcium (1.5%), and aluminium (1.4%); with the remaining 1.2% consisting of trace amounts of other elements. Due to planetary differentiation, the core region is believed to be primarily composed of iron (88.8%), with smaller amounts of nickel (5.8%), sulfur (4.5%), and less than 1% trace elements.amphibole
Amphibole () is a group of inosilicate minerals, forming prism or needlelike crystals, composed of double chain tetrahedra, linked at the vertices and generally containing ions of iron and/or magnesium in their structures. Its IMA symbol is A ...
, cryolite, nitratine, and zeolite.Great Salt Lake
The Great Salt Lake is the largest saltwater lake in the Western Hemisphere and the eighth-largest terminal lake in the world. It lies in the northern part of the U.S. state of Utah and has a substantial impact upon the local climate, particula ...
and the Dead Sea
The Dead Sea ( he, יַם הַמֶּלַח, ''Yam hamMelaḥ''; ar, اَلْبَحْرُ الْمَيْتُ, ''Āl-Baḥrū l-Maytū''), also known by other names, is a salt lake bordered by Jordan to the east and Israel and the West Bank ...
.gallium
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group (aluminiu ...
and niobium
Niobium is a chemical element with chemical symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to pure titanium, and it has sim ...
. Commercially, the most important lithium mineral is spodumene, which occurs in large deposits worldwide.earth's crust
Earth's crust is Earth's thin outer shell of rock, referring to less than 1% of Earth's radius and volume. It is the top component of the lithosphere, a division of Earth's layers that includes the crust and the upper part of the mantle. The ...
at any time, due to its extremely short half-life of 22 minutes.
Properties
Physical and chemical
The physical and chemical properties of the alkali metals can be readily explained by their having an ns1 valence electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
, which results in weak metallic bonding. Hence, all the alkali metals are soft and have low densities,boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
s,vaporisation
Vaporization (or vaporisation) of an element or compound is a phase transition from the liquid phase to vapor. There are two types of vaporization: evaporation and boiling. Evaporation is a surface phenomenon, whereas boiling is a bulk phenomenon ...
, and dissociation.electrical conductivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allow ...
.strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is ex ...
, and barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
, as well as the divalent lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttr ...
s europium
Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lanth ...
and ytterbium, are pale yellow, though the colour is much less prominent than it is for caesium.halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
s to form the alkali metal halide
In chemistry, alkali metal halides, or alkali halides, are the family of inorganic compounds with the chemical formula MX, where M is an alkali metal and X is a halogen. These compounds are the often commercially significant sources of these m ...
s, which are white ionic crystalline compounds that are all soluble in water except lithium fluoride ( Li F).alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
ne hydroxides and thus should be handled with great care. The heavier alkali metals react more vigorously than the lighter ones; for example, when dropped into water, caesium produces a larger explosion than potassium if the same number of moles of each metal is used.periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s and phenols, gaseous ammonia, and alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s, the last demonstrating the phenomenal degree of their reactivity. Their great power as reducing agents makes them very useful in liberating other metals from their oxides or halides.stoichiometry
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equal ...
and low ionisation potentials. Alkalides are chemically similar to the electrides, which are salts with trapped electrons acting as anions.first coordination sphere
In coordination chemistry, the first coordination sphere refers to the array of molecules and ions (the ligands) directly attached to the central metal atom. The second coordination sphere consists of molecules and ions that attached in variou ...
, also known as the first, or primary, solvation shell. The bond between a water molecule and the metal ion is a dative covalent bond
In coordination chemistry, a coordinate covalent bond, also known as a dative bond, dipolar bond, or coordinate bond is a kind of two-center, two-electron covalent bond in which the two electrons derive from the same atom. The bonding of metal io ...
, with the oxygen atom donating both electrons to the bond. Each coordinated water molecule may be attached by hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
s to other water molecules. The latter are said to reside in the second coordination sphere. However, for the alkali metal cations, the second coordination sphere is not well-defined as the +1 charge on the cation is not high enough to polarise the water molecules in the primary solvation shell enough for them to form strong hydrogen bonds with those in the second coordination sphere, producing a more stable entity.
Lithium
The chemistry of lithium shows several differences from that of the rest of the group as the small Li+ cation polarises anions and gives its compounds a more covalent character.
Francium
Francium is also predicted to show some differences due to its high atomic weight, causing its electrons to travel at considerable fractions of the speed of light and thus making relativistic effects more prominent. In contrast to the trend of decreasing electronegativities and ionisation energies of the alkali metals, francium's electronegativity and ionisation energy are predicted to be higher than caesium's due to the relativistic stabilisation of the 7s electrons; also, its atomic radius is expected to be abnormally low. Thus, contrary to expectation, caesium is the most reactive of the alkali metals, not francium.
Nuclear
All the alkali metals have odd atomic numbers; hence, their isotopes must be either odd–odd (both proton and neutron number
The neutron number, symbol ''N'', is the number of neutrons in a nuclide.
Atomic number (proton number) plus neutron number equals mass number: . The difference between the neutron number and the atomic number is known as the neutron excess: . ...
are odd) or odd–even (proton number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
is odd, but neutron number is even). Odd–odd nuclei have even mass numbers, whereas odd–even nuclei have odd mass numbers. Odd–odd primordial nuclide
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the ...
s are rare because most odd–odd nuclei are highly unstable with respect to beta decay, because the decay products are even–even, and are therefore more strongly bound, due to nuclear pairing effects.Chernobyl accident
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the No. 4 reactor in the Chernobyl Nuclear Power Plant, near the city of Pripyat in the north of the Ukrainian SSR in the Soviet Union. It is one of only two nu ...
. Caesium-137 undergoes high-energy beta decay and eventually becomes stable barium-137. It is a strong emitter of gamma radiation. Caesium-137 has a very low rate of neutron capture and cannot be feasibly disposed of in this way, but must be allowed to decay.tracer
Tracer may refer to:
Science
* Flow tracer, any fluid property used to track fluid motion
* Fluorescent tracer, a substance such as 2-NBDG containing a fluorophore that is used for tracking purposes
* Histochemical tracer, a substance used for tr ...
in hydrologic studies, analogous to the use of tritium. Small amounts of caesium-134 and caesium-137 were released into the environment during nearly all nuclear weapon tests and some nuclear accidents, most notably the Goiânia accident and the Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the No. 4 reactor in the Chernobyl Nuclear Power Plant, near the city of Pripyat in the north of the Ukrainian SSR in the Soviet Union. It is one of only two nuc ...
. As of 2005, caesium-137 is the principal source of radiation in the zone of alienation around the Chernobyl nuclear power plant.
Periodic trends
The alkali metals are more similar to each other than the elements in any other group are to each other.
Atomic and ionic radii
 The atomic radii of the alkali metals increase going down the group.
The atomic radii of the alkali metals increase going down the group.
First ionisation energy
 The first ionisation energy of an element or molecule is the energy required to move the most loosely held electron from one mole of gaseous atoms of the element or molecules to form one mole of gaseous ions with electric charge +1. The factors affecting the first ionisation energy are the nuclear charge, the amount of shielding by the inner electrons and the distance from the most loosely held electron from the nucleus, which is always an outer electron in
The first ionisation energy of an element or molecule is the energy required to move the most loosely held electron from one mole of gaseous atoms of the element or molecules to form one mole of gaseous ions with electric charge +1. The factors affecting the first ionisation energy are the nuclear charge, the amount of shielding by the inner electrons and the distance from the most loosely held electron from the nucleus, which is always an outer electron in main group element
In chemistry and atomic physics, the main group is the group of elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, and fluorine as arrange ...
s. The first two factors change the effective nuclear charge the most loosely held electron feels. Since the outermost electron of alkali metals always feels the same effective nuclear charge (+1), the only factor which affects the first ionisation energy is the distance from the outermost electron to the nucleus. Since this distance increases down the group, the outermost electron feels less attraction from the nucleus and thus the first ionisation energy decreases.
Reactivity
The reactivities of the alkali metals increase going down the group. This is the result of a combination of two factors: the first ionisation energies and atomisation energies of the alkali metals. Because the first ionisation energy of the alkali metals decreases down the group, it is easier for the outermost electron to be removed from the atom and participate in chemical reactions, thus increasing reactivity down the group. The atomisation energy measures the strength of the metallic bond of an element, which falls down the group as the atoms increase in radius and thus the metallic bond must increase in length, making the delocalised electrons further away from the attraction of the nuclei of the heavier alkali metals. Adding the atomisation and first ionisation energies gives a quantity closely related to (but not equal to) the activation energy of the reaction of an alkali metal with another substance. This quantity decreases going down the group, and so does the activation energy; thus, chemical reactions can occur faster and the reactivity increases down the group.
Electronegativity
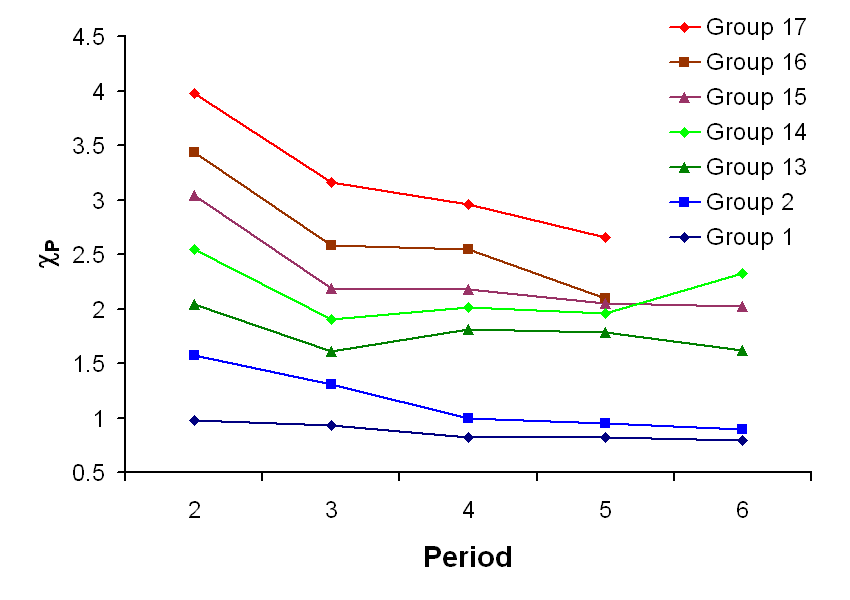 Electronegativity is a chemical property that describes the tendency of an atom or a functional group to attract electrons (or electron density) towards itself.
Electronegativity is a chemical property that describes the tendency of an atom or a functional group to attract electrons (or electron density) towards itself.sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
were covalent, the pair of shared electrons would be attracted to the chlorine because the effective nuclear charge on the outer electrons is +7 in chlorine but is only +1 in sodium. The electron pair is attracted so close to the chlorine atom that they are practically transferred to the chlorine atom (an ionic bond). However, if the sodium atom was replaced by a lithium atom, the electrons will not be attracted as close to the chlorine atom as before because the lithium atom is smaller, making the electron pair more strongly attracted to the closer effective nuclear charge from lithium. Hence, the larger alkali metal atoms (further down the group) will be less electronegative as the bonding pair is less strongly attracted towards them. As mentioned previously, francium is expected to be an exception.alkali metal hydroxide
The alkali hydroxides are a class of chemical compounds which are composed of an alkali metal cation and the hydroxide anion (OH−). The alkali hydroxides are:
*Lithium hydroxide (LiOH)
* Sodium hydroxide (NaOH)
*Potassium hydroxide (KOH)
*Rubi ...
that is not deliquescent.
Melting and boiling points
The melting point of a substance is the point where it changes state from solid to liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, a ...
while the boiling point of a substance (in liquid state) is the point where the vapour pressure of the liquid equals the environmental pressure surrounding the liquid and all the liquid changes state to gas. As a metal is heated to its melting point, the metallic bonds keeping the atoms in place weaken so that the atoms can move around, and the metallic bonds eventually break completely at the metal's boiling point.
Density
The alkali metals all have the same crystal structure ( body-centred cubic)room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
.
Compounds
The alkali metals form complete series of compounds with all usually encountered anions, which well illustrate group trends. These compounds can be described as involving the alkali metals losing electrons to acceptor species and forming monopositive ions.
Hydroxides
 All the alkali metals react vigorously or explosively with cold water, producing an
All the alkali metals react vigorously or explosively with cold water, producing an aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be re ...
of a strongly basic
BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Thomas E. Kurtz at Dartmouth College ...
alkali metal hydroxide and releasing hydrogen gas.oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
ic alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, whe ...
s. They easily react with carbon dioxide to form carbonates or bicarbonates, or with hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
to form sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds lar ...
s or bisulfides, and may be used to separate thiols from petroleum. They react with amphoteric oxides: for example, the oxides of aluminium, zinc, tin, and lead react with the alkali metal hydroxides to give aluminates, zincates, stannates, and plumbates. Silicon dioxide is acidic, and thus the alkali metal hydroxides can also attack silicate glass.
Intermetallic compounds
 The alkali metals form many intermetallic compounds with each other and the elements from groups 2 to 13 in the periodic table of varying stoichiometries,
The alkali metals form many intermetallic compounds with each other and the elements from groups 2 to 13 in the periodic table of varying stoichiometries,mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
, including Na5Hg8 and Na3Hg. Some of these have ionic characteristics: taking the alloys with gold, the most electronegative of metals, as an example, NaAu and KAu are metallic, but RbAu and CsAu are semiconductors.
Compounds with the group 13 elements
The intermetallic compounds of the alkali metals with the heavier group 13 elements ( aluminium, gallium
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group (aluminiu ...
, indium, and thallium), such as NaTl, are poor conductors or semiconductors, unlike the normal alloys with the preceding elements, implying that the alkali metal involved has lost an electron to the Zintl anions involved.[Sevov, S.C]
"Zintl Phases"
pp. 113–132 in ''Intermetallic Compounds, Principles and Practice: Progress'', Vol. 3. Westbrook, J.H.; Freisher, R.L.: Eds.; John Wiley & Sons. Ltd., Chichester, England Nevertheless, while the elements in group 14 and beyond tend to form discrete anionic clusters, group 13 elements tend to form polymeric ions with the alkali metal cations located between the giant ionic lattice. For example, NaTl consists of a polymeric anion (—Tl−—)n with a covalent diamond cubic structure with Na+ ions located between the anionic lattice. The larger alkali metals cannot fit similarly into an anionic lattice and tend to force the heavier group 13 elements to form anionic clusters.[S.M. Kauzlarich, Encyclopedia of Inorganic chemistry, 1994, John Wiley & Sons, ]
Boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
is a special case, being the only nonmetal in group 13. The alkali metal borides tend to be boron-rich, involving appreciable boron–boron bonding involving deltahedral structures,Wade's rules
In chemistry the polyhedral skeletal electron pair theory (PSEPT) provides electron counting rules useful for predicting the structures of cluster compound, clusters such as borane and carborane clusters. The electron counting rules were originall ...
to forming Zintl anions like the rest of group 13.
Compounds with the group 14 elements
Lithium and sodium react with carbon to form acetylides, Li2C2 and Na2C2, which can also be obtained by reaction of the metal with acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
. Potassium, rubidium, and caesium react with graphite; their atoms are intercalated between the hexagonal graphite layers, forming graphite intercalation compounds of formulae MC60 (dark grey, almost black), MC48 (dark grey, almost black), MC36 (blue), MC24 (steel blue), and MC8 (bronze) (M = K, Rb, or Cs). These compounds are over 200 times more electrically conductive than pure graphite, suggesting that the valence electron of the alkali metal is transferred to the graphite layers (e.g. ).fulleride
Fullerides are chemical compounds containing fullerene anions. Common fullerides are derivatives of the most common fullerenes, i.e. C60 and C70. The scope of the area is large because multiple charges are possible, i.e., 60sup>''n''− (''n ...
s M''n''C60; sodium, potassium, rubidium, and caesium can form fullerides where ''n'' = 2, 3, 4, or 6, and rubidium and caesium additionally can achieve ''n'' = 1.germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors s ...
, tin, and lead), ionic substances with cage-like structures are formed, such as the silicides M4 Si4 (M = K, Rb, or Cs), which contains M+ and tetrahedral ions.germanide A germanide is any binary compound of germanium and a more electropositive element. The composition of most germanides is analogous to that of the corresponding silicides and does not follow formal valence rules. The germanides of alkali and alkali ...
s, involving the germanide ion Ge4− and other cluster ( Zintl) ions such as , , , and Ge9)2sup>6−, is largely analogous to that of the corresponding silicides.stannide
A stannide can refer to an intermetallic compound containing tin combined with one or more other metals; an anion consisting solely of tin atoms or a compound containing such an anion, or, in the field of organometallic chemistry an ionic compound ...
s are mostly ionic, sometimes with the stannide ion ( Sn4−),
Nitrides and pnictides
 Lithium, the lightest of the alkali metals, is the only alkali metal which reacts with nitrogen at standard conditions, and its nitride is the only stable alkali metal nitride. Nitrogen is an
Lithium, the lightest of the alkali metals, is the only alkali metal which reacts with nitrogen at standard conditions, and its nitride is the only stable alkali metal nitride. Nitrogen is an unreactive
In chemistry, reactivity is the impulse for which a chemical substance undergoes a chemical reaction, either by itself or with other materials, with an overall release of energy.
''Reactivity'' refers to:
* the chemical reactions of a single sub ...
gas because breaking the strong triple bond in the dinitrogen molecule (N2) requires a lot of energy. The formation of an alkali metal nitride would consume the ionisation energy of the alkali metal (forming M+ ions), the energy required to break the triple bond in N2 and the formation of N3− ions, and all the energy released from the formation of an alkali metal nitride is from the lattice energy of the alkali metal nitride. The lattice energy is maximised with small, highly charged ions; the alkali metals do not form highly charged ions, only forming ions with a charge of +1, so only lithium, the smallest alkali metal, can release enough lattice energy to make the reaction with nitrogen exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
, forming lithium nitride. The reactions of the other alkali metals with nitrogen would not release enough lattice energy and would thus be endothermic, so they do not form nitrides at standard conditions.
'Elusive Binary Compound Prepared'
''Chemical & Engineering News'' 80 No. 20 (20 May 2002)azide
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant applic ...
salts involving the linear anion; due to the large size of the alkali metal cations, they are thermally stable enough to be able to melt before decomposing.[H.G. Von Schnering, W. Hönle ''Phosphides – Solid-state Chemistry'' Encyclopedia of Inorganic Chemistry Ed. R. Bruce King (1994) John Wiley & Sons ] While most metals form arsenides, only the alkali and alkaline earth metals form mostly ionic arsenides. The structure of Na3As is complex with unusually short Na–Na distances of 328–330 pm which are shorter than in sodium metal, and this indicates that even with these electropositive metals the bonding cannot be straightforwardly ionic.stibine
Stibine (IUPAC name: stibane) is a chemical compound with the formula SbH3. A pnictogen hydride, this colourless, highly toxic gas is the principal covalent hydride of antimony, and a heavy analogue of ammonia. The molecule is pyramidal with H–S ...
(SbH3). Indeed, they have some metallic properties, and the alkali metal antimonides of stoichiometry MSb involve antimony atoms bonded in a spiral Zintl structure.
Oxides and chalcogenides
All the alkali metals react vigorously with oxygen at standard conditions. They form various types of oxides, such as simple oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
s (containing the O2− ion), peroxides (containing the ion, where there is a single bond
In chemistry, a single bond is a chemical bond between two atoms involving two valence electrons. That is, the atoms share one pair of electrons where the bond forms. Therefore, a single bond is a type of covalent bond. When shared, each of th ...
between the two oxygen atoms), superoxides (containing the ion), and many others. Lithium burns in air to form lithium oxide, but sodium reacts with oxygen to form a mixture of sodium oxide and sodium peroxide. Potassium forms a mixture of potassium peroxide and potassium superoxide, while rubidium and caesium form the superoxide exclusively. Their reactivity increases going down the group: while lithium, sodium and potassium merely burn in air, rubidium and caesium are pyrophoric (spontaneously catch fire in air).submarine
A submarine (or sub) is a watercraft capable of independent operation underwater. It differs from a submersible, which has more limited underwater capability. The term is also sometimes used historically or colloquially to refer to remotely op ...
air purifiers; the presence of water vapour, naturally present in breath, makes the removal of carbon dioxide by potassium superoxide even more efficient.sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
, selenium, tellurium, and polonium), and all the alkali metal chalcogenides are known (with the exception of francium's). Reaction with an excess of the chalcogen can similarly result in lower chalcogenides, with chalcogen ions containing chains of the chalcogen atoms in question. For example, sodium can react with sulfur to form the sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds lar ...
( Na2S) and various polysulfides with the formula Na2S''x'' (''x'' from 2 to 6), containing the ions.
Halides, hydrides, and pseudohalides
The alkali metals are among the most electropositive elements on the periodic table and thus tend to bond ionically to the most electronegative elements on the periodic table, the halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
s (fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
, chlorine, bromine, iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
, and astatine), forming salts known as the alkali metal halides. The reaction is very vigorous and can sometimes result in explosions.sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
, otherwise known as common salt. All of the stable alkali metal halides have the formula MX where M is an alkali metal and X is a halogen. They are all white ionic crystalline solids that have high melting points.cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a ...
s. These are isostructural to the respective halides except for lithium cyanide
Lithium cyanide is an inorganic compound with the chemical formula LiCN. It is a toxic, white colored, hygroscopic, water-soluble salt that finds only niche uses.
Preparation
LiCN arises from the interaction of lithium hydroxide and hydrogen cyan ...
, indicating that the cyanide ions may rotate freely.
Coordination complexes
Alkali metal cations do not usually form coordination complexes with simple Lewis base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
s due to their low charge of just +1 and their relatively large size; thus the Li+ ion forms most complexes and the heavier alkali metal ions form less and less (though exceptions occur for weak complexes).aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be re ...
, the alkali metal ions exist as octahedral hexahydrate complexes ( (H2O)6)sup>+), with the exception of the lithium ion, which due to its small size forms tetrahedral tetrahydrate complexes ( i(H2O)4)sup>+); the alkali metals form these complexes because their ions are attracted by electrostatic forces of attraction to the polar water molecules. Because of this, anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achie ...
salts containing alkali metal cations are often used as desiccant
A desiccant is a hygroscopic substance that is used to induce or sustain a state of dryness (desiccation) in its vicinity; it is the opposite of a humectant. Commonly encountered pre-packaged desiccants are solids that absorb water. Desiccant ...
s.18-crown-6
18-Crown-6 is an organic compound with the formula 2H4O and the IUPAC name of 1,4,7,10,13,16-hexaoxacyclooctadecane. It is a white, hygroscopic crystalline solid with a low melting point. Like other crown ethers, 18-crown-6 functions as a li ...
for K+, and 21-crown-7 for Rb+) and cryptands due to electrostatic attraction.
Ammonia solutions
The alkali metals dissolve slowly in liquid ammonia, forming ammoniacal solutions of solvated metal cation M+ and solvated electron e−, which react to form hydrogen gas and the alkali metal amide (MNH2, where M represents an alkali metal): this was first noted by Humphry Davy in 1809 and rediscovered by W. Weyl in 1864. The process may be speeded up by a catalyst. Similar solutions are formed by the heavy divalent alkaline earth metals calcium, strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is ex ...
, barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
, as well as the divalent lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttr ...
s, europium
Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lanth ...
and ytterbium. The amide salt is quite insoluble and readily precipitates out of solution, leaving intensely coloured ammonia solutions of the alkali metals. In 1907, Charles Krause identified the colour as being due to the presence of solvated electrons, which contribute to the high electrical conductivity of these solutions. At low concentrations (below 3 M), the solution is dark blue and has ten times the conductivity of aqueous sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
; at higher concentrations (above 3 M), the solution is copper-coloured and has approximately the conductivity of liquid metals like mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
.diatomic
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear. Ot ...
alkali metal molecules (M2) and alkali metal anions (M−). These are unstable and eventually become the more thermodynamically stable alkali metal amide and hydrogen gas. Solvated electrons are powerful reducing agents and are often used in chemical synthesis.
Organometallic
Organolithium
 Being the smallest alkali metal, lithium forms the widest variety of and most stable organometallic compounds, which are bonded covalently. Organolithium compounds are electrically non-conducting volatile solids or liquids that melt at low temperatures, and tend to form
Being the smallest alkali metal, lithium forms the widest variety of and most stable organometallic compounds, which are bonded covalently. Organolithium compounds are electrically non-conducting volatile solids or liquids that melt at low temperatures, and tend to form oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
s with the structure (RLi)''x'' where R is the organic group. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3C ...
, organolithium compounds are extremely powerful bases and nucleophiles. For use as bases, butyllithiums are often used and are commercially available. An example of an organolithium compound is methyllithium ((CH3Li)''x''), which exists in tetrameric (''x'' = 4, tetrahedral) and hexameric (''x'' = 6, octahedral) forms.ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s by reaction with metal carbonyls. The reaction with nickel tetracarbonyl
Nickel carbonyl (IUPAC name: tetracarbonylnickel) is a nickel(0) organometallic compound with the formula Ni(CO)4. This colorless liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for producing very high-pur ...
, for example, proceeds through an unstable acyl nickel carbonyl complex which then undergoes electrophilic substitution
Electrophilic substitution reactions are chemical reactions in which an electrophile displaces a functional group in a compound, which is typically, but not always, aromatic. Aromatic substitution reactions are characteristic of aromatic compounds ...
to give the desired aldehyde (using H+ as the electrophile) or ketone (using an alkyl halide) product.carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
s (ArCO2H) and aryl ketones to tertiary carbinols (Ar'2C(Ar)OH). Finally, they may be used to synthesise other organometallic compounds through metal-halogen exchange.
Heavier alkali metals
Unlike the organolithium compounds, the organometallic compounds of the heavier alkali metals are predominantly ionic. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compound
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
s, which are commercially available and exhibit more convenient reactivity. The principal organosodium compound of commercial importance is sodium cyclopentadienide. Sodium tetraphenylborate can also be classified as an organosodium compound since in the solid state sodium is bound to the aryl groups. Organometallic compounds of the higher alkali metals are even more reactive than organosodium compounds and of limited utility. A notable reagent is Schlosser's base, a mixture of ''n''-butyllithium and potassium ''tert''-butoxide. This reagent reacts with propene to form the compound allylpotassium (KCH2CHCH2). ''cis''-2-Butene and ''trans''-2-butene equilibrate when in contact with alkali metals. Whereas isomerisation
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
is fast with lithium and sodium, it is slow with the heavier alkali metals. The heavier alkali metals also favour the steric hindrance, sterically congested conformation. Several crystal structures of organopotassium compounds have been reported, establishing that they, like the sodium compounds, are polymeric.
Representative reactions of alkali metals
''Reaction with oxygen''
Upon reacting with oxygen, alkali metals form oxides, peroxides, superoxides and suboxides. However, the first three are more common. The table below["Inorganic Chemistry" by Gary L. Miessler and Donald A. Tar, 6th edition, Pearson] shows the types of compounds formed in reaction with oxygen. The compound in brackets represents the minor product of combustion.
The alkali metal peroxides are ionic compounds that are unstable in water. The peroxide anion is weakly bound to the cation, and it is hydrolysed, forming stronger covalent bonds.
:Na2O2 + 2H2O → 2NaOH + H2O2
The other oxygen compounds are also unstable in water.
:2KO2 + 2H2O → 2KOH + H2O2 + O2
:Li2O + H2O → 2LiOH
''Reaction with sulfur''
With sulfur, they form sulfides and polysulfides.
:2Na + 1/8S8 → Na2S + 1/8S8 → Na2S2...Na2S7
Because alkali metal sulfides are essentially salts of a weak acid and a strong base, they form basic solutions.
:S2- + H2O → HS− + HO−
:HS− + H2O → H2S + HO−
''Reaction with nitrogen''
Lithium is the only metal that combines directly with nitrogen at room temperature.
:3Li + 1/3N2 → Li3N
Li3N can react with water to liberate ammonia.
:Li3N + 3H2O → 3LiOH + NH3
''Reaction with hydrogen''
With hydrogen, alkali metals form saline hydrides that hydrolyse in water.
:Na + H2 → NaH (at high temperatures)
:NaH + H2O → NaOH + H2
''Reaction with carbon''
Lithium is the only metal that reacts directly with carbon to give dilithium acetylide. Na and K can react with acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
to give acetylides.
:2Li + 2C → Li2C2
:Na + C2H2 → NaC2H + 1/2H2 (at 1500C)
:Na + NaC2H → Na2C2 (at 2200C)
''Reaction with water''
On reaction with water, they generate hydroxide ions and hydrogen gas. This reaction is vigorous and highly exothermic and the hydrogen resulted may ignite in air or even explode in the case of Rb and Cs.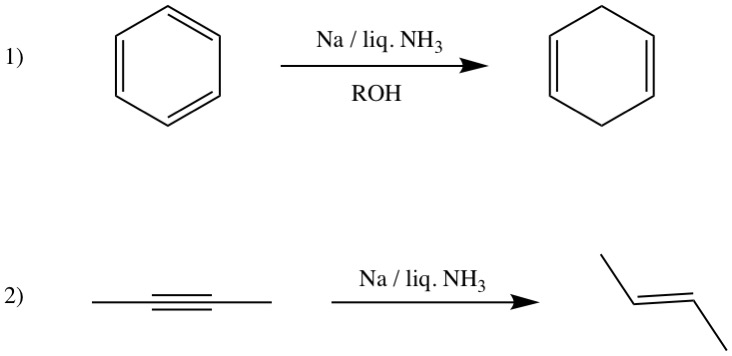 Reaction 1) is known as Birch reduction.
Other reductions
Reaction 1) is known as Birch reduction.
Other reductions
Extensions
 Although francium is the heaviest alkali metal that has been discovered, there has been some theoretical work predicting the physical and chemical characteristics of hypothetical heavier alkali metals. Being the first
Although francium is the heaviest alkali metal that has been discovered, there has been some theoretical work predicting the physical and chemical characteristics of hypothetical heavier alkali metals. Being the first period 8 element
An extended periodic table theorises about chemical elements beyond those currently known in the periodic table and proven. , the element with the highest atomic number known is oganesson (''Z'' = 118), which completes the seventh period (row) ...
, the undiscovered element ununennium (element 119) is predicted to be the next alkali metal after francium and behave much like their lighter Congener (chemistry), congeners; however, it is also predicted to differ from the lighter alkali metals in some properties. The stabilisation of ununennium's valence electron and thus the contraction of the 8s orbital cause its atomic radius to be lowered to 240 picometer, pm,
The stabilisation of ununennium's valence electron and thus the contraction of the 8s orbital cause its atomic radius to be lowered to 240 picometer, pm, Not as much work has been done predicting the properties of the alkali metals beyond ununennium. Although a simple extrapolation of the periodic table (by the aufbau principle) would put element 169, unhexennium, under ununennium, Dirac-Fock calculations predict that the next element after ununennium with alkali-metal-like properties may be element 165, unhexpentium, which is predicted to have the electron configuration [Og] 5g18 6f14 7d10 8s2 8p1/22 9s1.
Not as much work has been done predicting the properties of the alkali metals beyond ununennium. Although a simple extrapolation of the periodic table (by the aufbau principle) would put element 169, unhexennium, under ununennium, Dirac-Fock calculations predict that the next element after ununennium with alkali-metal-like properties may be element 165, unhexpentium, which is predicted to have the electron configuration [Og] 5g18 6f14 7d10 8s2 8p1/22 9s1.
Pseudo-alkali metals
Many other substances are similar to the alkali metals in their tendency to form monopositive cations. Analogously to the pseudohalogens, they have sometimes been called "pseudo-alkali metals". These substances include some elements and many more polyatomic ions; the polyatomic ions are especially similar to the alkali metals in their large size and weak polarising power.
Hydrogen
The element hydrogen, with one electron per neutral atom, is usually placed at the top of Group 1 of the periodic table for convenience, but hydrogen is not normally considered to be an alkali metal;[ and reacts easily with the ]halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
s,[ but the similarities mostly end there because of the small size of a bare proton H+ compared to the alkali metal cations.][ Its placement above lithium is primarily due to its ]electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
.fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
due to their similar chemical properties, though the resemblance is likewise not absolute.[Huheey, J.E.; Keiter, E.A. and Keiter, R.L. (1993) ''Inorganic Chemistry: Principles of Structure and Reactivity'', 4th edition, HarperCollins, New York, USA.][James, A.M. and Lord, M.P. (1992) ''Macmillan's Chemical and Physical Data'', Macmillan, London, UK.] As only one additional electron is required to fill in the outermost shell of the hydrogen atom, hydrogen often behaves like a halogen, forming the negative hydride ion, and is very occasionally considered to be a halogen on that basis. (The alkali metals can also form negative ions, known as alkalides, but these are little more than laboratory curiosities, being unstable.)rubidium
Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher ...
and caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that a ...
at 2000 K at the respective pressures when they undergo a nonmetal-to-metal transition.
The 1s1 electron configuration of hydrogen, while analogous to that of the alkali metals (ns1), is unique because there is no 1p subshell. Hence it can lose an electron to form the hydron (chemistry), hydron H+, or gain one to form the hydride ion H−.hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
s, which are an effect of charge-transfer, electrostatic, and electron correlative contributing phenomena.
Ammonium and derivatives
 The ammonium ion () has very similar properties to the heavier alkali metals, acting as an alkali metal intermediate between potassium and rubidium,
The ammonium ion () has very similar properties to the heavier alkali metals, acting as an alkali metal intermediate between potassium and rubidium,
Cobaltocene and derivatives
Cobaltocene, Co(C5H5)2, is a metallocene, the cobalt analogue of ferrocene. It is a dark purple solid. Cobaltocene has 19 valence electrons, one more than usually found in organotransition metal complexes, such as its very stable relative, ferrocene, in accordance with the 18-electron rule. This additional electron occupies an orbital that is antibonding with respect to the Co–C bonds. Consequently, many chemical reactions of Co(C5H5)2 are characterized by its tendency to lose this "extra" electron, yielding a very stable 18-electron cation known as cobaltocenium. Many cobaltocenium salts coprecipitate with caesium salts, and cobaltocenium hydroxide is a strong base that absorbs atmospheric carbon dioxide to form cobaltocenium carbonate.
Thallium
 Thallium is the heaviest stable element in group 13 of the periodic table. At the bottom of the periodic table, the inert pair effect is quite strong, because of the relativistic effects, relativistic stabilisation of the 6s orbital and the decreasing bond energy as the atoms increase in size so that the amount of energy released in forming two more bonds is not worth the high ionisation energies of the 6s electrons.
Thallium is the heaviest stable element in group 13 of the periodic table. At the bottom of the periodic table, the inert pair effect is quite strong, because of the relativistic effects, relativistic stabilisation of the 6s orbital and the decreasing bond energy as the atoms increase in size so that the amount of energy released in forming two more bonds is not worth the high ionisation energies of the 6s electrons.Dmitri Mendeleev
Dmitri Ivanovich Mendeleev (sometimes transliterated as Mendeleyev or Mendeleef) ( ; russian: links=no, Дмитрий Иванович Менделеев, tr. , ; 8 February Old_Style_and_New_Style_dates">O.S._27_January.html" ;"title="O ...
's 1869 periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
and Julius Lothar Meyer's 1868 periodic table.[.] While Tl+ is stabilised by the inert pair effect, this inert pair of 6s electrons is still able to participate chemically, so that these electrons are stereochemistry, stereochemically active in aqueous solution. Additionally, the thallium halides (except thallium(I) fluoride, TlF) are quite insoluble in water, and thallium(I) iodide, TlI has an unusual structure because of the presence of the stereochemically active inert pair in thallium.
Copper, silver, and gold
The group 11 element, group 11 metals (or coinage metals), copper, silver, and gold, are typically categorised as transition metals given they can form ions with incomplete d-shells. Physically, they have the relatively low melting points and high electronegativity values associated with post-transition metals. "The filled ''d'' subshell and free ''s'' electron of Cu, Ag, and Au contribute to their high electrical and thermal conductivity. Transition metals to the left of group 11 experience interactions between ''s'' electrons and the partially filled ''d'' subshell that lower electron mobility." Chemically, the group 11 metals behave like main-group metals in their +1 valence states, and are hence somewhat related to the alkali metals: this is one reason for their previously being labelled as "group IB", paralleling the alkali metals' "group IA". They are occasionally classified as post-transition metals. Their spectra are analogous to those of the alkali metals.mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
.
Production and isolation
The production of pure alkali metals is somewhat complicated due to their extreme reactivity with commonly used substances, such as water.azide
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant applic ...
, which yields the metal for sodium, potassium, rubidium, and caesium and the nitride for lithium.[
Lithium salts have to be extracted from the water of mineral springs, brine pools, and brine deposits. The metal is produced electrolytically from a mixture of fused lithium chloride and potassium chloride.]sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
by lowering the melting point of the substance to below 700 °C through the use of a Downs cell. For several years in the 1950s and 1960s, a by-product of the potassium production called Alkarb was a main source for rubidium. Alkarb contained 21% rubidium while the rest was potassium and a small fraction of caesium. Today the largest producers of caesium, for example the Tanco Mine in Manitoba, Canada, produce rubidium as by-product from pollucite.
For several years in the 1950s and 1960s, a by-product of the potassium production called Alkarb was a main source for rubidium. Alkarb contained 21% rubidium while the rest was potassium and a small fraction of caesium. Today the largest producers of caesium, for example the Tanco Mine in Manitoba, Canada, produce rubidium as by-product from pollucite.
Applications
 Lithium, sodium, and potassium have many applications, while rubidium and caesium are very useful in academic contexts but do not have many applications yet.
Lithium, sodium, and potassium have many applications, while rubidium and caesium are very useful in academic contexts but do not have many applications yet.
Biological role and precautions
Metals
Pure alkali metals are dangerously reactive with air and water and must be kept away from heat, fire, oxidising agents, acids, most organic compounds, halocarbons, plastics, and moisture. They also react with carbon dioxide and carbon tetrachloride, so that normal fire extinguishers are counterproductive when used on alkali metal fires.
Ions
 The bioinorganic chemistry of the alkali metal ions has been extensively reviewed.
Solid state crystal structures have been determined for many complexes of alkali metal ions in small peptides, nucleic acid constituents, carbohydrates and ionophore complexes.
Lithium naturally only occurs in traces in biological systems and has no known biological role, but does have effects on the body when ingested.
The bioinorganic chemistry of the alkali metal ions has been extensively reviewed.
Solid state crystal structures have been determined for many complexes of alkali metal ions in small peptides, nucleic acid constituents, carbohydrates and ionophore complexes.
Lithium naturally only occurs in traces in biological systems and has no known biological role, but does have effects on the body when ingested. Due to their similar atomic radii, rubidium and caesium in the body mimic potassium and are taken up similarly. Rubidium has no known biological role, but may help stimulate metabolism,
Due to their similar atomic radii, rubidium and caesium in the body mimic potassium and are taken up similarly. Rubidium has no known biological role, but may help stimulate metabolism,[ The median lethal dose (LD50) value for caesium chloride in mice is 2.3 g per kilogram, which is comparable to the LD50 values of potassium chloride and ]sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
. Caesium chloride has been promoted as an alternative cancer therapy, but has been linked to the deaths of over 50 patients, on whom it was used as part of a scientifically unvalidated cancer treatment.[Wood, Leonie. ]
Radioisotopes of caesium require special precautions: the improper handling of caesium-137 gamma ray sources can lead to release of this radioisotope and radiation injuries. Perhaps the best-known case is the Goiânia accident of 1987, in which an improperly-disposed-of radiation therapy system from an abandoned clinic in the city of Goiânia, Brazil, was scavenged from a junkyard, and the glowing caesium chloride, caesium salt sold to curious, uneducated buyers. This led to four deaths and serious injuries from radiation exposure. Together with caesium-134, iodine-131, and strontium-90, caesium-137 was among the isotopes distributed by the Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the No. 4 reactor in the Chernobyl Nuclear Power Plant, near the city of Pripyat in the north of the Ukrainian SSR in the Soviet Union. It is one of only two nuc ...
which constitute the greatest risk to health.
Notes
References
{{Authority control
Chemical compounds by element, A
Alkali metals,
Groups (periodic table)
Periodic table
Articles containing video clips
 Sodium compounds have been known since ancient times; salt (
Sodium compounds have been known since ancient times; salt ( Petalite ( Li Al Si4 O10) was discovered in 1800 by the Brazilian chemist José Bonifácio de Andrada in a mine on the island of Utö, Sweden. However, it was not until 1817 that Johan August Arfwedson, then working in the laboratory of the chemist
Petalite ( Li Al Si4 O10) was discovered in 1800 by the Brazilian chemist José Bonifácio de Andrada in a mine on the island of Utö, Sweden. However, it was not until 1817 that Johan August Arfwedson, then working in the laboratory of the chemist  Rubidium and caesium were the first elements to be discovered using the spectroscope, invented in 1859 by Robert Bunsen and Gustav Kirchhoff. The next year, they discovered caesium in the mineral water from Bad Dürkheim, Germany. Their discovery of rubidium came the following year in Heidelberg, Germany, finding it in the mineral lepidolite. The names of rubidium and caesium come from the most prominent lines in their
Rubidium and caesium were the first elements to be discovered using the spectroscope, invented in 1859 by Robert Bunsen and Gustav Kirchhoff. The next year, they discovered caesium in the mineral water from Bad Dürkheim, Germany. Their discovery of rubidium came the following year in Heidelberg, Germany, finding it in the mineral lepidolite. The names of rubidium and caesium come from the most prominent lines in their  After 1869,
After 1869,  The
The  The Earth formed from the same cloud of matter that formed the Sun, but the planets acquired different compositions during the
The Earth formed from the same cloud of matter that formed the Sun, but the planets acquired different compositions during the  Electronegativity is a chemical property that describes the tendency of an atom or a functional group to attract electrons (or electron density) towards itself. If the bond between sodium and chlorine in
Electronegativity is a chemical property that describes the tendency of an atom or a functional group to attract electrons (or electron density) towards itself. If the bond between sodium and chlorine in  All the alkali metals react vigorously or explosively with cold water, producing an
All the alkali metals react vigorously or explosively with cold water, producing an  The alkali metals form many intermetallic compounds with each other and the elements from groups 2 to 13 in the periodic table of varying stoichiometries, such as the sodium amalgams with
The alkali metals form many intermetallic compounds with each other and the elements from groups 2 to 13 in the periodic table of varying stoichiometries, such as the sodium amalgams with  Lithium, the lightest of the alkali metals, is the only alkali metal which reacts with nitrogen at standard conditions, and its nitride is the only stable alkali metal nitride. Nitrogen is an
Lithium, the lightest of the alkali metals, is the only alkali metal which reacts with nitrogen at standard conditions, and its nitride is the only stable alkali metal nitride. Nitrogen is an  Being the smallest alkali metal, lithium forms the widest variety of and most stable organometallic compounds, which are bonded covalently. Organolithium compounds are electrically non-conducting volatile solids or liquids that melt at low temperatures, and tend to form
Being the smallest alkali metal, lithium forms the widest variety of and most stable organometallic compounds, which are bonded covalently. Organolithium compounds are electrically non-conducting volatile solids or liquids that melt at low temperatures, and tend to form  Reaction 1) is known as Birch reduction.
Other reductions that can be carried by these solutions are:
:S8 + 2e− → S82-
:Fe(CO)5 + 2e− → Fe(CO)42- + CO
Reaction 1) is known as Birch reduction.
Other reductions that can be carried by these solutions are:
:S8 + 2e− → S82-
:Fe(CO)5 + 2e− → Fe(CO)42- + CO
 The ammonium ion () has very similar properties to the heavier alkali metals, acting as an alkali metal intermediate between potassium and rubidium, and is often considered a close relative. For example, most alkali metal salts are soluble in water, a property which ammonium salts share. Ammonium is expected to behave stably as a metal ( ions in a sea of delocalised electrons) at very high pressures (though less than the typical pressure where transitions from insulating to metallic behaviour occur around, 100 pascal (unit), GPa), and could possibly occur inside the Gas giant#Uranus and Neptune, ice giants Uranus and Neptune, which may have significant impacts on their interior magnetic fields. It has been estimated that the transition from a mixture of ammonia and dihydrogen molecules to metallic ammonium may occur at pressures just below 25 GPa. Under standard conditions, ammonium can form a metallic amalgam with mercury.
Other "pseudo-alkali metals" include the alkylammonium cations, in which some of the hydrogen atoms in the ammonium cation are replaced by alkyl or aryl groups. In particular, the quaternary ammonium cations () are very useful since they are permanently charged, and they are often used as an alternative to the expensive Cs+ to stabilise very large and very easily polarisable anions such as . Tetraalkylammonium hydroxides, like alkali metal hydroxides, are very strong bases that react with atmospheric carbon dioxide to form carbonates. Furthermore, the nitrogen atom may be replaced by a phosphorus, arsenic, or antimony atom (the heavier nonmetallic pnictogens), creating a phosphonium () or arsonium () cation that can itself be substituted similarly; while stibonium () itself is not known, some of its organic derivatives are characterised.
The ammonium ion () has very similar properties to the heavier alkali metals, acting as an alkali metal intermediate between potassium and rubidium, and is often considered a close relative. For example, most alkali metal salts are soluble in water, a property which ammonium salts share. Ammonium is expected to behave stably as a metal ( ions in a sea of delocalised electrons) at very high pressures (though less than the typical pressure where transitions from insulating to metallic behaviour occur around, 100 pascal (unit), GPa), and could possibly occur inside the Gas giant#Uranus and Neptune, ice giants Uranus and Neptune, which may have significant impacts on their interior magnetic fields. It has been estimated that the transition from a mixture of ammonia and dihydrogen molecules to metallic ammonium may occur at pressures just below 25 GPa. Under standard conditions, ammonium can form a metallic amalgam with mercury.
Other "pseudo-alkali metals" include the alkylammonium cations, in which some of the hydrogen atoms in the ammonium cation are replaced by alkyl or aryl groups. In particular, the quaternary ammonium cations () are very useful since they are permanently charged, and they are often used as an alternative to the expensive Cs+ to stabilise very large and very easily polarisable anions such as . Tetraalkylammonium hydroxides, like alkali metal hydroxides, are very strong bases that react with atmospheric carbon dioxide to form carbonates. Furthermore, the nitrogen atom may be replaced by a phosphorus, arsenic, or antimony atom (the heavier nonmetallic pnictogens), creating a phosphonium () or arsonium () cation that can itself be substituted similarly; while stibonium () itself is not known, some of its organic derivatives are characterised.
 Thallium is the heaviest stable element in group 13 of the periodic table. At the bottom of the periodic table, the inert pair effect is quite strong, because of the relativistic effects, relativistic stabilisation of the 6s orbital and the decreasing bond energy as the atoms increase in size so that the amount of energy released in forming two more bonds is not worth the high ionisation energies of the 6s electrons. It displays the +1 oxidation state that all the known alkali metals display, and thallium compounds with thallium in its +1 oxidation state closely resemble the corresponding potassium or silver compounds stoichiometrically due to the similar ionic radii of the Tl+ (164 picometer, pm), K+ (152 pm) and Ag+ (129 pm) ions. It was sometimes considered an alkali metal in continental Europe (but not in England) in the years immediately following its discovery, and was placed just after caesium as the sixth alkali metal in
Thallium is the heaviest stable element in group 13 of the periodic table. At the bottom of the periodic table, the inert pair effect is quite strong, because of the relativistic effects, relativistic stabilisation of the 6s orbital and the decreasing bond energy as the atoms increase in size so that the amount of energy released in forming two more bonds is not worth the high ionisation energies of the 6s electrons. It displays the +1 oxidation state that all the known alkali metals display, and thallium compounds with thallium in its +1 oxidation state closely resemble the corresponding potassium or silver compounds stoichiometrically due to the similar ionic radii of the Tl+ (164 picometer, pm), K+ (152 pm) and Ag+ (129 pm) ions. It was sometimes considered an alkali metal in continental Europe (but not in England) in the years immediately following its discovery, and was placed just after caesium as the sixth alkali metal in  For several years in the 1950s and 1960s, a by-product of the potassium production called Alkarb was a main source for rubidium. Alkarb contained 21% rubidium while the rest was potassium and a small fraction of caesium. Today the largest producers of caesium, for example the Tanco Mine in Manitoba, Canada, produce rubidium as by-product from pollucite. Today, a common method for separating rubidium from potassium and caesium is the fractional crystallization (chemistry), fractional crystallisation of a rubidium and caesium alum (Caesium, Cs, Rubidium, Rb) Al(Sulfate, SO4)2·12Water, H2O, which yields pure rubidium alum after approximately 30 recrystallisations. The limited applications and the lack of a mineral rich in rubidium limit the production of rubidium compounds to 2 to 4 tonnes per year. Caesium, however, is not produced from the above reaction. Instead, the mining of pollucite ore is the main method of obtaining pure caesium, extracted from the ore mainly by three methods: acid digestion, alkaline decomposition, and direct reduction. Both metals are produced as by-products of lithium production: after 1958, when interest in lithium's thermonuclear properties increased sharply, the production of rubidium and caesium also increased correspondingly. Pure rubidium and caesium metals are produced by reducing their chlorides with calcium metal at 750 °C and low pressure.
As a result of its extreme rarity in nature, most francium is synthesised in the nuclear reaction 197Gold, Au + 18 O → 210Francium, Fr + 5 neutron, n, yielding francium-209, francium-210, and francium-211. The greatest quantity of francium ever assembled to date is about 300,000 neutral atoms, which were synthesised using the nuclear reaction given above. When the only natural isotope francium-223 is specifically required, it is produced as the alpha daughter of actinium-227, itself produced synthetically from the neutron irradiation of natural radium-226, one of the daughters of natural uranium-238.
For several years in the 1950s and 1960s, a by-product of the potassium production called Alkarb was a main source for rubidium. Alkarb contained 21% rubidium while the rest was potassium and a small fraction of caesium. Today the largest producers of caesium, for example the Tanco Mine in Manitoba, Canada, produce rubidium as by-product from pollucite. Today, a common method for separating rubidium from potassium and caesium is the fractional crystallization (chemistry), fractional crystallisation of a rubidium and caesium alum (Caesium, Cs, Rubidium, Rb) Al(Sulfate, SO4)2·12Water, H2O, which yields pure rubidium alum after approximately 30 recrystallisations. The limited applications and the lack of a mineral rich in rubidium limit the production of rubidium compounds to 2 to 4 tonnes per year. Caesium, however, is not produced from the above reaction. Instead, the mining of pollucite ore is the main method of obtaining pure caesium, extracted from the ore mainly by three methods: acid digestion, alkaline decomposition, and direct reduction. Both metals are produced as by-products of lithium production: after 1958, when interest in lithium's thermonuclear properties increased sharply, the production of rubidium and caesium also increased correspondingly. Pure rubidium and caesium metals are produced by reducing their chlorides with calcium metal at 750 °C and low pressure.
As a result of its extreme rarity in nature, most francium is synthesised in the nuclear reaction 197Gold, Au + 18 O → 210Francium, Fr + 5 neutron, n, yielding francium-209, francium-210, and francium-211. The greatest quantity of francium ever assembled to date is about 300,000 neutral atoms, which were synthesised using the nuclear reaction given above. When the only natural isotope francium-223 is specifically required, it is produced as the alpha daughter of actinium-227, itself produced synthetically from the neutron irradiation of natural radium-226, one of the daughters of natural uranium-238.
 Lithium, sodium, and potassium have many applications, while rubidium and caesium are very useful in academic contexts but do not have many applications yet. Lithium is often used in lithium-ion battery, lithium-ion batteries, and lithium oxide can help process silica. Lithium stearate is a thickener and can be used to make lubricating greases; it is produced from lithium hydroxide, which is also used to absorb carbon dioxide in space capsules and submarines. Lithium chloride is used as a brazing alloy for aluminium parts. Metallic lithium is used in alloys with magnesium and aluminium to give very tough and light alloys.
Sodium compounds have many applications, the most well-known being sodium chloride as table salt. Sodium salts of fatty acids are used as soap. Pure sodium metal also has many applications, including use in sodium-vapor lamp, sodium-vapour lamps, which produce very efficient light compared to other types of lighting, and can help smooth the surface of other metals. Being a strong reducing agent, it is often used to reduce many other metals, such as titanium and zirconium, from their chlorides. Furthermore, it is very useful as a heat-exchange liquid in fast breeder nuclear reactors due to its low melting point, viscosity, and cross-section (physics), cross-section towards neutron absorption.
Potassium compounds are often used as fertilisers as potassium is an important element for plant nutrition. Potassium hydroxide is a very strong base, and is used to control the pH of various substances. Potassium nitrate and potassium permanganate are often used as powerful oxidising agents. Potassium superoxide is used in breathing masks, as it reacts with carbon dioxide to give potassium carbonate and oxygen gas. Pure potassium metal is not often used, but its alloys with sodium may substitute for pure sodium in fast breeder nuclear reactors.
Rubidium and caesium are often used in atomic clocks. Caesium atomic clocks are extraordinarily accurate; if a clock had been made at the time of the dinosaurs, it would be off by less than four seconds (after 80 million years). For that reason, caesium atoms are used as the definition of the second. Rubidium ions are often used in purple fireworks, and caesium is often used in drilling fluids in the petroleum industry.
Francium has no commercial applications, but because of francium's relatively simple atomic structure, among other things, it has been used in spectroscopy experiments, leading to more information regarding energy levels and the coupling constants between subatomic particles. Studies on the light emitted by laser-trapped francium-210 ions have provided accurate data on transitions between atomic energy levels, similar to those predicted by quantum mechanics, quantum theory.
Lithium, sodium, and potassium have many applications, while rubidium and caesium are very useful in academic contexts but do not have many applications yet. Lithium is often used in lithium-ion battery, lithium-ion batteries, and lithium oxide can help process silica. Lithium stearate is a thickener and can be used to make lubricating greases; it is produced from lithium hydroxide, which is also used to absorb carbon dioxide in space capsules and submarines. Lithium chloride is used as a brazing alloy for aluminium parts. Metallic lithium is used in alloys with magnesium and aluminium to give very tough and light alloys.
Sodium compounds have many applications, the most well-known being sodium chloride as table salt. Sodium salts of fatty acids are used as soap. Pure sodium metal also has many applications, including use in sodium-vapor lamp, sodium-vapour lamps, which produce very efficient light compared to other types of lighting, and can help smooth the surface of other metals. Being a strong reducing agent, it is often used to reduce many other metals, such as titanium and zirconium, from their chlorides. Furthermore, it is very useful as a heat-exchange liquid in fast breeder nuclear reactors due to its low melting point, viscosity, and cross-section (physics), cross-section towards neutron absorption.
Potassium compounds are often used as fertilisers as potassium is an important element for plant nutrition. Potassium hydroxide is a very strong base, and is used to control the pH of various substances. Potassium nitrate and potassium permanganate are often used as powerful oxidising agents. Potassium superoxide is used in breathing masks, as it reacts with carbon dioxide to give potassium carbonate and oxygen gas. Pure potassium metal is not often used, but its alloys with sodium may substitute for pure sodium in fast breeder nuclear reactors.
Rubidium and caesium are often used in atomic clocks. Caesium atomic clocks are extraordinarily accurate; if a clock had been made at the time of the dinosaurs, it would be off by less than four seconds (after 80 million years). For that reason, caesium atoms are used as the definition of the second. Rubidium ions are often used in purple fireworks, and caesium is often used in drilling fluids in the petroleum industry.
Francium has no commercial applications, but because of francium's relatively simple atomic structure, among other things, it has been used in spectroscopy experiments, leading to more information regarding energy levels and the coupling constants between subatomic particles. Studies on the light emitted by laser-trapped francium-210 ions have provided accurate data on transitions between atomic energy levels, similar to those predicted by quantum mechanics, quantum theory.
 The bioinorganic chemistry of the alkali metal ions has been extensively reviewed.
Solid state crystal structures have been determined for many complexes of alkali metal ions in small peptides, nucleic acid constituents, carbohydrates and ionophore complexes.
Lithium naturally only occurs in traces in biological systems and has no known biological role, but does have effects on the body when ingested. Lithium carbonate is used as a mood stabiliser in psychiatry to treat bipolar disorder (manic-depression) in daily doses of about 0.5 to 2 grams, although there are side-effects. Excessive ingestion of lithium causes drowsiness, slurred speech and vomiting, among other symptoms, and poisons the central nervous system, which is dangerous as the required dosage of lithium to treat bipolar disorder is only slightly lower than the toxic dosage. Its biochemistry, the way it is handled by the human body and studies using rats and goats suggest that it is an essential element, essential trace element, although the natural biological function of lithium in humans has yet to be identified.
Sodium and potassium occur in all known biological systems, generally functioning as electrolytes inside and outside cell (biology), cells. Sodium is an essential nutrient that regulates blood volume, blood pressure, osmotic equilibrium and pH; the minimum physiological requirement for sodium is 500 milligrams per day. Sodium chloride (also known as common salt) is the principal source of sodium in the diet, and is used as seasoning and preservative, such as for pickling and jerky (food), jerky; most of it comes from processed foods. The Dietary Reference Intake for sodium is 1.5 grams per day, but most people in the United States consume more than 2.3 grams per day, the minimum amount that promotes hypertension; this in turn causes 7.6 million premature deaths worldwide.
Potassium is the major cation (positive ion) inside cell (biology), animal cells, while sodium is the major cation outside animal cells. The concentration differences of these charged particles causes a difference in electric potential between the inside and outside of cells, known as the membrane potential. The balance between potassium and sodium is maintained by ion transporter proteins in the cell membrane. The cell membrane potential created by potassium and sodium ions allows the cell to generate an action potential—a "spike" of electrical discharge. The ability of cells to produce electrical discharge is critical for body functions such as neurotransmission, muscle contraction, and heart function. Disruption of this balance may thus be fatal: for example, ingestion of large amounts of potassium compounds can lead to hyperkalemia strongly influencing the cardiovascular system. Potassium chloride is used in the United States for lethal injection executions.
The bioinorganic chemistry of the alkali metal ions has been extensively reviewed.
Solid state crystal structures have been determined for many complexes of alkali metal ions in small peptides, nucleic acid constituents, carbohydrates and ionophore complexes.
Lithium naturally only occurs in traces in biological systems and has no known biological role, but does have effects on the body when ingested. Lithium carbonate is used as a mood stabiliser in psychiatry to treat bipolar disorder (manic-depression) in daily doses of about 0.5 to 2 grams, although there are side-effects. Excessive ingestion of lithium causes drowsiness, slurred speech and vomiting, among other symptoms, and poisons the central nervous system, which is dangerous as the required dosage of lithium to treat bipolar disorder is only slightly lower than the toxic dosage. Its biochemistry, the way it is handled by the human body and studies using rats and goats suggest that it is an essential element, essential trace element, although the natural biological function of lithium in humans has yet to be identified.
Sodium and potassium occur in all known biological systems, generally functioning as electrolytes inside and outside cell (biology), cells. Sodium is an essential nutrient that regulates blood volume, blood pressure, osmotic equilibrium and pH; the minimum physiological requirement for sodium is 500 milligrams per day. Sodium chloride (also known as common salt) is the principal source of sodium in the diet, and is used as seasoning and preservative, such as for pickling and jerky (food), jerky; most of it comes from processed foods. The Dietary Reference Intake for sodium is 1.5 grams per day, but most people in the United States consume more than 2.3 grams per day, the minimum amount that promotes hypertension; this in turn causes 7.6 million premature deaths worldwide.
Potassium is the major cation (positive ion) inside cell (biology), animal cells, while sodium is the major cation outside animal cells. The concentration differences of these charged particles causes a difference in electric potential between the inside and outside of cells, known as the membrane potential. The balance between potassium and sodium is maintained by ion transporter proteins in the cell membrane. The cell membrane potential created by potassium and sodium ions allows the cell to generate an action potential—a "spike" of electrical discharge. The ability of cells to produce electrical discharge is critical for body functions such as neurotransmission, muscle contraction, and heart function. Disruption of this balance may thus be fatal: for example, ingestion of large amounts of potassium compounds can lead to hyperkalemia strongly influencing the cardiovascular system. Potassium chloride is used in the United States for lethal injection executions.
 Due to their similar atomic radii, rubidium and caesium in the body mimic potassium and are taken up similarly. Rubidium has no known biological role, but may help stimulate metabolism, and, similarly to caesium, replace potassium in the body causing hypokalemia, potassium deficiency. Partial substitution is quite possible and rather non-toxic: a 70 kg person contains on average 0.36 g of rubidium, and an increase in this value by 50 to 100 times did not show negative effects in test persons. Rats can survive up to 50% substitution of potassium by rubidium. Rubidium (and to a much lesser extent caesium) can function as temporary cures for hypokalemia; while rubidium can adequately physiologically substitute potassium in some systems, caesium is never able to do so. There is only very limited evidence in the form of deficiency symptoms for rubidium being possibly essential in goats; even if this is true, the trace amounts usually present in food are more than enough.
Caesium compounds are rarely encountered by most people, but most caesium compounds are mildly toxic. Like rubidium, caesium tends to substitute potassium in the body, but is significantly larger and is therefore a poorer substitute. Excess caesium can lead to hypokalemia, arrythmia, and acute cardiac arrest, but such amounts would not ordinarily be encountered in natural sources. As such, caesium is not a major chemical environmental pollutant. The median lethal dose (LD50) value for caesium chloride in mice is 2.3 g per kilogram, which is comparable to the LD50 values of potassium chloride and
Due to their similar atomic radii, rubidium and caesium in the body mimic potassium and are taken up similarly. Rubidium has no known biological role, but may help stimulate metabolism, and, similarly to caesium, replace potassium in the body causing hypokalemia, potassium deficiency. Partial substitution is quite possible and rather non-toxic: a 70 kg person contains on average 0.36 g of rubidium, and an increase in this value by 50 to 100 times did not show negative effects in test persons. Rats can survive up to 50% substitution of potassium by rubidium. Rubidium (and to a much lesser extent caesium) can function as temporary cures for hypokalemia; while rubidium can adequately physiologically substitute potassium in some systems, caesium is never able to do so. There is only very limited evidence in the form of deficiency symptoms for rubidium being possibly essential in goats; even if this is true, the trace amounts usually present in food are more than enough.
Caesium compounds are rarely encountered by most people, but most caesium compounds are mildly toxic. Like rubidium, caesium tends to substitute potassium in the body, but is significantly larger and is therefore a poorer substitute. Excess caesium can lead to hypokalemia, arrythmia, and acute cardiac arrest, but such amounts would not ordinarily be encountered in natural sources. As such, caesium is not a major chemical environmental pollutant. The median lethal dose (LD50) value for caesium chloride in mice is 2.3 g per kilogram, which is comparable to the LD50 values of potassium chloride and