|
Carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2. The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, CaCO3, the chief constituent of limestone (as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions in water. Historically, it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process. Hydrates Sodium carbonate is obtained as three hydrates and as the anhydrous salt: * sodium carbonate decahydrate (natron), Na2CO3·10H2O, which readily effloresces to form the monohydrate. * sodium carbonate heptahydrate (not known in mineral form), Na2CO3·7H2O. * sodium carbonate monohydrate (thermonatrite), Na2CO3·H2O. Also known as crystal carbonate. * anhydrous sodium carbonate ( n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limestone
Limestone ( calcium carbonate ) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of . Limestone forms when these minerals precipitate out of water containing dissolved calcium. This can take place through both biological and nonbiological processes, though biological processes, such as the accumulation of corals and shells in the sea, have likely been more important for the last 540 million years. Limestone often contains fossils which provide scientists with information on ancient environments and on the evolution of life. About 20% to 25% of sedimentary rock is carbonate rock, and most of this is limestone. The remaining carbonate rock is mostly dolomite, a closely related rock, which contains a high percentage of the mineral dolomite, . ''Magnesian limestone'' is an obsolete and poorly-defined term used variously for dolomite, for lime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
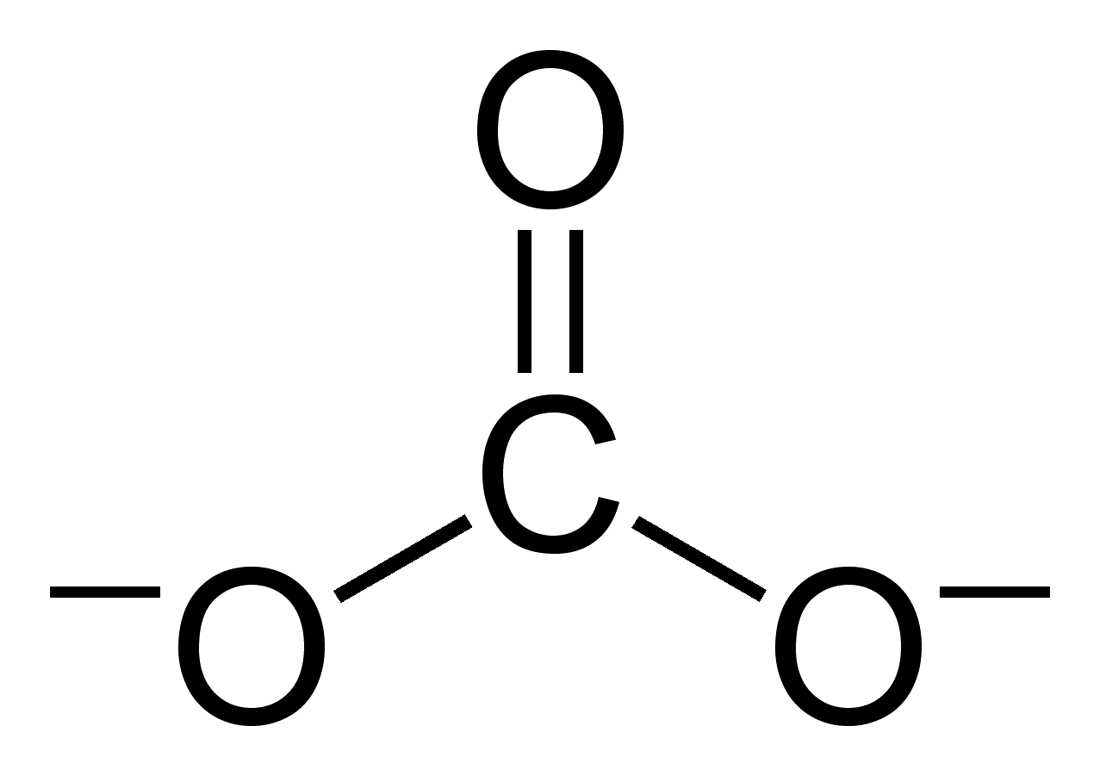
Carbonate Ester
In organic chemistry, a carbonate ester (organic carbonate or organocarbonate) is an ester of carbonic acid. This functional group consists of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is and they are related to esters (), ethers () and also to the inorganic carbonates. Monomers of polycarbonate (e.g. Makrolon or Lexan) are linked by carbonate groups. These polycarbonates are used in eyeglass lenses, compact discs, and bulletproof glass. Small carbonate esters like dimethyl carbonate, ethylene carbonate, propylene carbonate are used as solvents, dimethyl carbonate is also a mild methylating agent. Structures Carbonate esters have planar OC(OC)2 cores, which confers rigidity. The unique O=C bond is short (1.173 Å in the depicted example), while the C-O bonds are more ether-like (the bond distances of 1.326 Å for the example depicted). Carbonate esters can be divided into three structural classes: acyclic, cyclic, and pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicarbonate
In inorganic chemistry, bicarbonate ( IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula . Bicarbonate serves a crucial biochemical role in the physiological pH buffering system. The term "bicarbonate" was coined in 1814 by the English chemist William Hyde Wollaston. The name lives on as a trivial name. Chemical properties The bicarbonate ion (hydrogencarbonate ion) is an anion with the empirical formula and a molecular mass of 61.01 daltons; it consists of one central carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement, with a hydrogen atom attached to one of the oxygens. It is isoelectronic with nitric acid . The bicarbonate ion carries a negative one formal charge and is an amphiprotic species which has both acidic and basic properties. It is both the conjugate base of carbonic acid ; and the conjugate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonated Water
Carbonated water (also known as soda water, sparkling water, fizzy water, club soda, water with gas, in many places as mineral water, or especially in the United States as seltzer or seltzer water) is water containing dissolved carbon dioxide gas, either artificially injected under pressure or occurring due to natural geological processes. Carbonation causes small bubbles to form, giving the water an effervescent quality. Common forms include sparkling natural mineral water, club soda, and commercially-produced sparkling water. Club soda and sparkling mineral water and some other sparkling waters contain added or dissolved minerals such as potassium bicarbonate, sodium bicarbonate, sodium citrate, or potassium sulfate. These occur naturally in some mineral waters but are also commonly added artificially to manufactured waters to mimic a natural flavor profile and offset the acidity of introducing carbon dioxide gas. Various carbonated waters are sold in bottles and cans, with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxocarbon Anion
In chemistry, an oxocarbon anion is a negative ion consisting solely of carbon and oxygen atoms, and therefore having the general formula for some integers ''x'', ''y'', and ''n''. The most common oxocarbon anions are carbonate, , and oxalate, . There is however a large number of stable anions in this class, including several ones that have research or industrial use. There are also many unstable anions, like and , that have a fleeting existence during some chemical reactions; and many hypothetical species, like , that have been the subject of theoretical studies but have yet to be observed. Stable oxocarbon anions form salts with a large variety of cations. Unstable anions may persist in very rarefied gaseous state, such as in interstellar clouds. Most oxocarbon anions have corresponding moieties in organic chemistry, whose compounds are usually esters. Thus, for example, the oxalate moiety occurs in the ester dimethyl oxalate . Electronic structure of the carbo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Carbonate
Potassium carbonate is the inorganic compound with the formula K2 CO3. It is a white salt, which is soluble in water. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used in the production of soap and glass. History Potassium carbonate is the primary component of potash and the more refined pearl ash or salts of tartar. Historically, pearl ash was created by baking potash in a kiln to remove impurities. The fine, white powder remaining was the pearl ash. The first patent issued by the US Patent Office was awarded to Samuel Hopkins in 1790 for an improved method of making potash and pearl ash. In late 18th-century North America, before the development of baking powder, pearl ash was used as a leavening agent for quick breads. Production Potassium carbonate is prepared commercially by the reaction potassium hydroxide with carbon dioxide: : 2 KOH + CO2 → K2CO3 + H2O From the solution crystallizes the sesquihydrate K2CO3·H2O ("po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ceramic Glaze
Ceramic glaze is an impervious layer or coating of a vitreous substance which has been fused to a pottery body through firing. Glaze can serve to color, decorate or waterproof an item. Glazing renders earthenware vessels suitable for holding liquids, sealing the inherent porosity of unglazed biscuit earthenware. It also gives a tougher surface. Glaze is also used on stoneware and porcelain. In addition to their functionality, glazes can form a variety of surface finishes, including degrees of glossy or matte finish and color. Glazes may also enhance the underlying design or texture either unmodified or inscribed, carved or painted. Most pottery produced in recent centuries has been glazed, other than pieces in unglazed biscuit porcelain, terracotta, or some other types. Tiles are almost always glazed on the surface face, and modern architectural terracotta is very often glazed. Glazed brick is also common. Domestic sanitary ware is invariably glazed, as are many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate Minerals
Carbonate minerals are those minerals containing the carbonate ion, . Carbonate divisions Anhydrous carbonates *Calcite group: trigonal **Calcite CaCO3 **Gaspéite (Ni,Mg,Fe2+)CO3 **Magnesite MgCO3 **Otavite CdCO3 **Rhodochrosite MnCO3 **Siderite FeCO3 **Smithsonite ZnCO3 **Spherocobaltite CoCO3 *Aragonite group: orthorhombic **Aragonite CaCO3 **Cerussite PbCO3 **Strontianite SrCO3 **Witherite BaCO3 **Rutherfordine UO2CO3 ** Natrite Na2CO3 Anhydrous carbonates with compound formulas *Dolomite group: trigonal **Ankerite CaFe(CO3)2 **Dolomite CaMg(CO3)2 **Huntite Mg3Ca(CO3)4 **Minrecordite CaZn(CO3)2 **Barytocalcite BaCa(CO3)2 Carbonates with hydroxyl or halogen *Carbonate with hydroxide: monoclinic **Azurite Cu3(CO3)2(OH)2 **Hydrocerussite Pb3(CO3)2(OH)2 **Malachite Cu2CO3(OH)2 **Rosasite (Cu,Zn)2CO3(OH)2 **Phosgenite Pb2(CO3)Cl2 **Hydrozincite Zn5(CO3)2(OH)6 **Aurichalcite (Zn,Cu)5(CO3)2(OH)6 Hydrated carbonates *Hydromagnesite Mg5(CO3)4(OH)2.4H2O *Ikaite CaCO3·6(H2O) *Lan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcite
Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses. Other polymorphs of calcium carbonate are the minerals aragonite and vaterite. Aragonite will change to calcite over timescales of days or less at temperatures exceeding 300 °C, and vaterite is even less stable. Etymology Calcite is derived from the German ''Calcit'', a term from the 19th century that came from the Latin word for lime, ''calx'' (genitive calcis) with the suffix "-ite" used to name minerals. It is thus etymologically related to chalk. When applied by archaeologists and stone trade professionals, the term alabaster is used not just as in geology and mineralogy, where it is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |