|
Stoichiometry
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions. Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products, leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of the products can be empirically determined, then the amount of the other reactants can also be calculated. This is illustrated in the image here, where the balanced equation is: : Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. This particular chemical equation is an example of complete combust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Combustion Reaction Of Methane
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While the activation energy must be overcome to initiate combustion (e.g., using a lit match to light a fire), the heat from a flame may provide enough energy to make the reaction self-sustaining. Combustion is often a complicated sequence of elementary radical reactions. Solid fuels, such as wood and coal, first undergo endothermic pyrolysis to produce gaseous fuels whose combustion then supplies the heat required to produce more of them. Combustion is often hot enough that incandescent light in the form of either glowing or a flame is produced. A sim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mole (unit)
The mole, symbol mol, is the unit of amount of substance in the International System of Units (SI). The quantity amount of substance is a measure of how many elementary entities of a given substance are in an object or sample. The mole is defined as containing exactly elementary entities. Depending on what the substance is, an elementary entity may be an atom, a molecule, an ion, an ion pair, or a subatomic particle such as an electron. For example, 10 moles of water (a chemical compound) and 10 moles of mercury (a chemical element), contain equal amounts of substance and the mercury contains exactly one atom for each molecule of the water, despite the two having different volumes and different masses. The number of elementary entities in one mole is known as the Avogadro number, which is the approximate number of nucleons ( protons or neutrons) in one gram of ordinary matter. The previous definition of a mole was simply the number of elementary entities equal to that of 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Law Of Reciprocal Proportions
The law of reciprocal proportions also called law of equivalent proportions or law of permanent ratios is one of the basic laws of stoichiometry. It relates the proportions in which elements combine across a number of different elements. It was first formulated by Jeremias Richter in 1791. A simple statement of the law is:- :If element A combines with element B and also with C, then, if B and C combine together, the proportion by weight in which they do so will be simply related to the weights of B and C which separately combine with a constant weight of A. :As an example, 1 gram of sodium (Na = A) is observed to combine with either 1.54 grams of chlorine (Cl = B) or 5.52 grams of iodine Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ... (I = C). (These ratios correspond to the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Law Of Multiple Proportions
In chemistry, the law of multiple proportions states that if two elements form more than one compound, then the ratios of the masses of the second element which combine with a fixed mass of the first element will always be ratios of small whole numbers. This law is also known as: ''Dalton's Law'', named after John Dalton, the chemist who first expressed it. For example, Dalton knew that the element carbon forms two oxides by combining with oxygen in different proportions. A fixed mass of carbon, say 100 grams, may react with 133 grams of oxygen to produce one oxide, or with 266 grams of oxygen to produce the other. The ratio of the masses of oxygen that can react with 100 grams of carbon is 266:133 = 2:1, a ratio of small whole numbers. Dalton interpreted this result in his atomic theory by proposing (correctly in this case) that the two oxides have one and two oxygen atoms respectively for each carbon atom. In modern notation the first is CO (carbon monoxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Law Of Constant Composition
In chemistry, the law of definite proportions, sometimes called Proust's law, or law of constant composition states that a given chemical compound always contains its component elements in fixed ratio (by mass) and does not depend on its source and method of preparation. For example, oxygen makes up about 8/9 of the mass of any sample of pure water, while hydrogen makes up the remaining 1/9 of the mass: the mass of two elements in a compound are always in the same ratio. Along with the law of multiple proportions, the law of definite proportions forms the basis of stoichiometry. History The law of constant proportion was given by Joseph Proust in 1797. This observation was first made by the English theologian and chemist Joseph Priestley, and Antoine Lavoisier, a French nobleman and chemist centered on the process of combustion. The law of definite proportions might seem obvious to the modern chemist, inherent in the very definition of a chemical compound. At the end of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jeremias Benjamin Richter
Jeremias Benjamin Richter (; 10 March 1762 – 4 May 1807) was a German chemist. He was born at Hirschberg in Silesia, became a mining official at Breslau in 1794, and in 1800 was appointed assessor to the department of mines and chemist to the royal porcelain factory at Berlin, where he died. He is known for introducing the term ''stoichiometry''. Developer of titration He made some of the earliest known determinations of the quantities by weight in which acids saturate bases and bases acids. He realised that those amounts of different bases which can saturate the same quantity of a particular acid are equivalent to each other (see ''Titration''). He was thus led to conclude that chemistry is a branch of applied mathematics and to endeavour to trace a law according to which the quantities of different bases required to saturate a given acid formed an arithmetical progression, and the quantities of acids saturating a given base a geometric progression. Law of definite propor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Mass
The molecular mass (''m'') is the mass of a given molecule: it is measured in daltons (Da or u). Different molecules of the same compound may have different molecular masses because they contain different isotopes of an element. The related quantity relative molecular mass, as defined by IUPAC, is the ratio of the mass of a molecule to the unified atomic mass unit (also known as the dalton) and is unitless. The molecular mass and relative molecular mass are distinct from but related to the molar mass. The molar mass is defined as the mass of a given substance divided by the amount of a substance and is expressed in g/mol. That makes the molar mass an average of many particles or molecules, and the molecular mass the mass of one specific particle or molecule. The molar mass is usually the more appropriate figure when dealing with macroscopic (weigh-able) quantities of a substance. The definition of molecular weight is most authoritatively synonymous with relative molecular mass; ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Relative Atomic Mass
Relative atomic mass (symbol: ''A''; sometimes abbreviated RAM or r.a.m.), also known by the deprecated synonym atomic weight, is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a given sample to the atomic mass constant. The atomic mass constant (symbol: ''m'') is defined as being of the mass of a carbon-12 atom. Since both quantities in the ratio are masses, the resulting value is dimensionless; hence the value is said to be ''relative''. For a single given sample, the relative atomic mass of a given element is the weighted arithmetic mean of the masses of the individual atoms (including their isotopes) that are present in the sample. This quantity can vary substantially between samples because the sample's origin (and therefore its radioactive history or diffusion history) may have produced unique combinations of isotopic abundances. For example, due to a different mixture of stable carbon-12 and carbon-13 isoto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molar Mass
In chemistry, the molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the amount of substance which is the number of moles in that sample, measured in moles. The molar mass is a bulk, not molecular, property of a substance. The molar mass is an ''average'' of many instances of the compound, which often vary in mass due to the presence of isotopes. Most commonly, the molar mass is computed from the standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on Earth. The molar mass is appropriate for converting between the mass of a substance and the amount of a substance for bulk quantities. The molecular mass and formula mass are commonly used as a synonym of molar mass, particularly for molecular compounds; however, the most authoritative sources define it differently. The difference is that molecular mass is the mass of one specific particle or m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
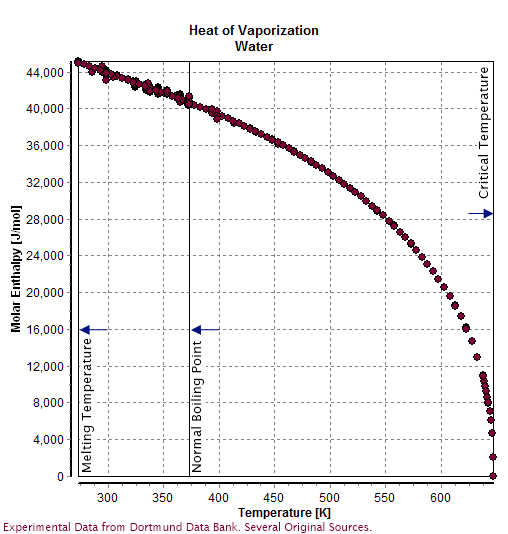
Properties Of Water
Water () is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an inherent hint of blue. It is by far the most studied chemical compound and is described as the "universal solvent" and the "solvent of life". It is the most abundant substance on the surface of Earth and the only common substance to exist as a solid, liquid, and gas on Earth's surface. It is also the third most abundant molecule in the universe (behind molecular hydrogen and carbon monoxide). Water molecules form hydrogen bonds with each other and are strongly polar. This polarity allows it to dissociate ions in salts and bond to other polar substances such as alcohols and acids, thus dissolving them. Its hydrogen bonding causes its many unique properties, such as having a solid form less dense than its liquid form, a relatively high boiling point of 100 °C for its molar mass, and a high heat capacity. Water is amphoteric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biblical Canon
A biblical canon is a set of texts (also called "books") which a particular Jewish or Christian religious community regards as part of the Bible. The English word ''canon'' comes from the Greek , meaning "rule" or " measuring stick". The use of the word "canon" to refer to a set of religious scriptures was first used by David Ruhnken, in the 18th century. Various biblical canons have developed through debate and agreement on the part of the religious authorities of their respective faiths and denominations. Some books, such as the Jewish–Christian gospels, have been excluded from various canons altogether, but many disputed books are considered to be biblical apocrypha or deuterocanonical by many, while some denominations may consider them fully canonical. Differences exist between the Hebrew Bible and Christian biblical canons, although the majority of manuscripts are shared in common. Different religious groups include different books in their biblical canons, in v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |