|
Shielding Effect
In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an electron and the nucleus in any atom with more than one electron. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces on the electrons in the atom. It is a special case of electric-field screening. This effect also has some significance in many projects in material sciences. Strength per electron shell The wider the electron shells are in space, the weaker is the electric interaction between the electrons and the nucleus due to screening. In general we can order the electron shells (s,p,d,f) as such S(\mathrm) > S(\mathrm) > S(\mathrm) > S(\mathrm) , where ''S'' is the screening strength that a given orbital provides to the rest of the electrons. Description In hydrogen, or any other atom in group 1A of the periodic table (those with only one v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schrödinger Equation
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of the subject. The equation is named after Erwin Schrödinger, who postulated the equation in 1925, and published it in 1926, forming the basis for the work that resulted in his Nobel Prize in Physics in 1933. Conceptually, the Schrödinger equation is the quantum counterpart of Newton's second law in classical mechanics. Given a set of known initial conditions, Newton's second law makes a mathematical prediction as to what path a given physical system will take over time. The Schrödinger equation gives the evolution over time of a wave function, the quantum-mechanical characterization of an isolated physical system. The equation can be derived from the fact that the time-evolution operator must be unitary, and must therefore be generated by t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Guelph
, mottoeng = "to learn the reasons of realities" , established = May 8, 1964 ()As constituents: OAC: (1874) Macdonald Institute: (1903) OVC: (1922) , type = Public university , chancellor = Mary Anne Chambers (not yet installed) , president = Charlotte A.B. Yates , city = Guelph, Ontario , country = Canada , students = 29,923 , undergrad = 23,926 , postgrad = 3,035 , faculty = 830 , administrative_staff = 3,100 , campus = Urban , athletics_affiliations = CIS, OUA , sports_nickname = Gryphons , colours = , , affiliations = AUCC, CARL, IAU, COU, CIS, CUSID, Fields Institute, OUA, Ontario Network of Women in engineering, CBIE , endowment = CA$418 million (2021) , website = , logo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
D-block Contraction
The d-block contraction (sometimes called scandide contraction) is a term used in chemistry to describe the effect of having full d orbitals on the period 4 elements. The elements in question are gallium, germanium, arsenic, selenium, bromine, and krypton. Their electronic configurations include completely filled d orbitals (d10). The d-block contraction is best illustrated by comparing some properties of the group 13 elements to highlight the effect on gallium. Gallium can be seen to be anomalous. The most obvious effect is that the sum of the first three ionization potentials of gallium is higher than that of aluminium, whereas the trend in the group would be for it to be lower. The second table below shows the trend in the sum of the first three ionization potentials for the elements B, Al, Sc, Y, and La. Sc, Y, and La have three valence electrons above a noble gas electron core. In contrast to the group 13 elements, this sequence shows a smooth reduction. Oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanide Contraction
The lanthanide contraction is the greater-than-expected decrease in atomic radii/ionic radii of the elements in the lanthanide series from atomic number 57, lanthanum, to 71, lutetium, which results in smaller than otherwise expected atomic radii/ionic radii for the subsequent elements starting with 72, hafnium. Jolly, William L. ''Modern Inorganic Chemistry'', McGraw-Hill 1984, p. 22 The term was coined by the Norwegian geochemist Victor Goldschmidt in his series "Geochemische Verteilungsgesetze der Elemente" (Geochemical distribution laws of the elements). Goldschmidt, Victor M. "Geochemische Verteilungsgesetze der Elemente", Part V "Isomorphie und Polymorphie der Sesquioxyde. Die Lanthaniden-Kontraktion und ihre Konsequenzen", Oslo, 1925 Cause The effect results from poor shielding of nuclear charge (nuclear attractive force on electrons) by 4f electrons; the 6s electrons are drawn towards the nucleus, thus resulting in a smaller atomic radius. In single-electron atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steric Effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel. Steric hindrance Steric hindrance is a consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk. It is usually manifested in ''intermolecular reactions'', whereas discussion of steric effects often focus on ''intramolecular interactions''. Steric hindrance is often exploited to control selectivity, such as slowing unwanted side-reactions. Steric hindrance between adjacent groups can also affect torsional ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
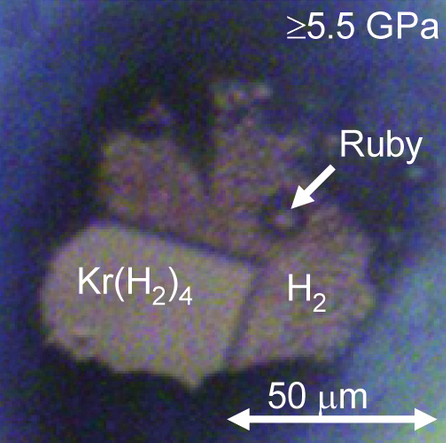
Noble Gas Compound
In chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon. From the standpoint of chemistry, the noble gases may be divided into two groups: the relatively reactive krypton (ionisation energy 14.0 eV), xenon (12.1 eV), and radon (10.7 eV) on one side, and the very unreactive argon (15.8 eV), neon (21.6 eV), and helium (24.6 eV) on the other. Consistent with this classification, Kr, Xe, and Rn form compounds that can be isolated in bulk at or near standard temperature and pressure, whereas He, Ne, Ar have been observed to form true chemical bonds using spectroscopic techniques, but only when frozen into a noble gas matrix at temperatures of 40 K or lower, in supersonic jets of noble gas, or under extremely high pressures with metals. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Effective Nuclear Charge
In atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an electron in a multi-electron atom. The term "effective" is used because the shielding effect of negatively charged electrons prevent higher energy electrons from experiencing the full nuclear charge of the nucleus due to the repelling effect of inner layer. The effective nuclear charge experienced by an electron is also called the core charge. It is possible to determine the strength of the nuclear charge by the oxidation number of the atom. Most of the physical and chemical properties of the elements can be explained on the basis of electronic configuration. Consider the behavior of ionization energies in the periodic table. It is known that the magnitude of ionization potential depends upon the following factors: # Size of atom; # The nuclear charge; # The screening effect of the inner shells, and # The extent to which the outermost electron penetrates into the charge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Core Charge
Core charge is the effective nuclear charge experienced by an outer shell electron. In other words, core charge is an expression of the attractive force experienced by the valence electrons to the core of an atom which takes into account the shielding effect of core electrons. Core charge can be calculated by taking the number of protons in the nucleus minus the number of core electrons, also called inner shell electrons, and is always a positive value in neutral atoms. Core charge is a convenient way of explaining trends in the periodic table. Since the core charge increases as you move across a row of the periodic table, the outer-shell electrons are pulled more and more strongly towards the nucleus and the atomic radius decreases. This can be used to explain a number of periodic trends such as atomic radius, first ionization energy (IE), electronegativity, and oxidizing. Core charge can also be calculated as 'atomic number' minus 'all electrons except those in the outer shell' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements. In an ordinary uncharged atom, the atomic number is also equal to the number of electrons. For an ordinary atom, the sum of the atomic number ''Z'' and the neutron number ''N'' gives the atom's atomic mass number ''A''. Since protons and neutrons have approximately the same mass (and the mass of the electrons is negligible for many purposes) and the mass defect of the nucleon binding is always small compared to the nucleon mass, the atomic mass of any atom, when expressed in unified atomic mass units (making a quantity called the "relative isotopic mass"), is within 1% of the whole number ''A''. Atoms with the same atomic number but dif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rutherford Backscattering Spectroscopy
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample. Geiger–Marsden experiment Rutherford backscattering spectrometry is named after Ernest Rutherford, Lord Rutherford, a physicist sometimes referred to as the father of nuclear physics. Rutherford supervised a series of experiments carried out by Hans Geiger and Ernest Marsden between 1909 and 1914 studying the scattering of alpha particles through metal foils. While attempting to eliminate "stray particles" they believed to be caused by an imperfection in their alpha source, Rutherford suggested that Marsden attempt to measure backscattering from a gold foil sample. According to the then-dominant Plum pudding model, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Slater's Rules
In quantum chemistry, Slater's rules provide numerical values for the effective nuclear charge in a many-electron atom. Each electron is said to experience less than the actual nuclear charge, because of shielding or screening by the other electrons. For each electron in an atom, Slater's rules provide a value for the screening constant, denoted by ''s'', ''S'', or ''σ'', which relates the effective and actual nuclear charges as :Z_= Z - s.\, The rules were devised semi-empirically by John C. Slater and published in 1930. Revised values of screening constants based on computations of atomic structure by the Hartree–Fock method were obtained by Enrico Clementi et al. in the 1960s. Rules Firstly, the electrons are arranged into a sequence of groups in order of increasing principal quantum number n, and for equal n in order of increasing azimuthal quantum number l, except that s- and p- orbitals are kept together. : s s,2p s,3p d s,4p d f s, 5p detc. Each group is given a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.png)