|
Suboxide
Suboxides are a class of oxides wherein the electropositive element is in excess relative to the “normal” oxides. When the electropositive element is a metal, the compounds are sometimes referred to as “metal-rich”. Thus the normal oxide of caesium is Cs2O, which is described as a Cs+ salt of O2−. A suboxide of caesium is Cs11O3, where the charge on Cs is clearly less than 1+, but the oxide is still described as O2−. Suboxides typically feature extensive bonding between the electropositive element, often leading to clusters. Examples of suboxides other than alkali metal derivatives: * Boron suboxide, BO; * Carbon suboxide, C3O2; * Boron suboxide, B6O; * Phosphorus suboxide, PO Metal-containing suboxides Suboxides are intermediates along the pathway that forms the normal oxide. Suboxides are sometimes visible when certain metals are exposed to small amounts of O2: :22 Cs + 3 O2 → 2 Cs11O3 :4 Cs11O3 + 5 O2 → 22 Cs2O Several suboxides of caesium and rub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
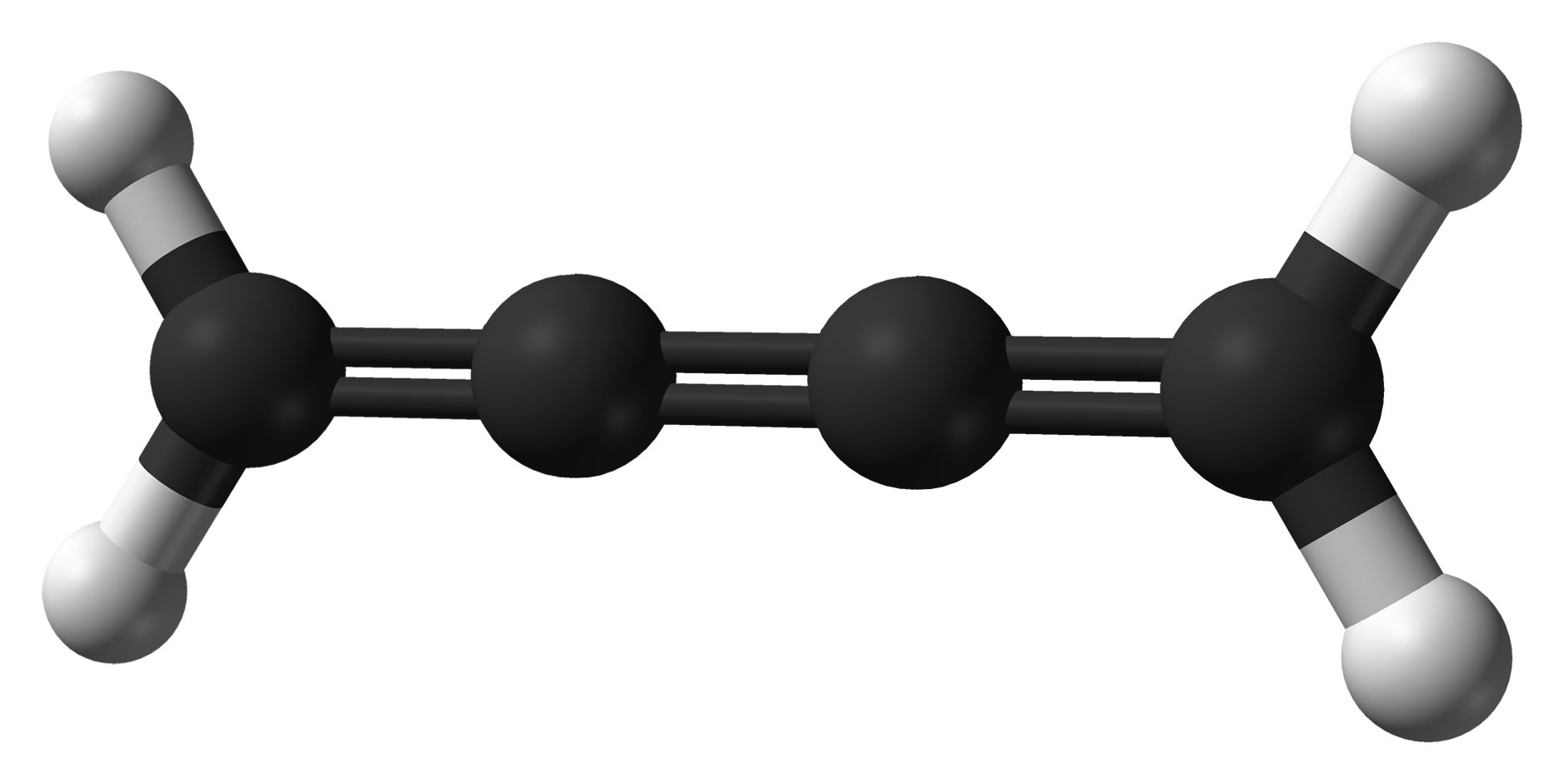
Carbon Suboxide
Carbon suboxide, or tricarbon dioxide, is an organic, oxygen-containing chemical compound with formula and structure . Its four cumulative double bonds make it a cumulene. It is one of the stable members of the series of linear oxocarbons , which also includes carbon dioxide () and pentacarbon dioxide (). Although if carefully purified it can exist at room temperature in the dark without decomposing, it will polymerize under certain conditions. The substance was discovered in 1873 by Benjamin Brodie by subjecting carbon monoxide to an electric current. He claimed that the product was part of a series of "oxycarbons" with formulas , namely , , , , …, and to have identified the last two; however, only is known. In 1891 Marcellin Berthelot observed that heating pure carbon monoxide at about 550 °C created small amounts of carbon dioxide but no trace of carbon, and assumed that a carbon-rich oxide was created instead, which he named "sub-oxide". He assumed it was the same ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Suboxide
Boron suboxide (chemical formula B6O) is a solid compound with a structure built of eight icosahedra at the apexes of the rhombohedral unit cell. Each icosahedron is composed of twelve boron atoms. Two oxygen atoms are located in the interstices along the 11rhombohedral direction. Due to its short interatomic bond lengths and strongly covalent character, B6O displays a range of outstanding physical and chemical properties such as great hardness (close to that of rhenium diboride and boron nitride), low mass density, high thermal conductivity, high chemical inertness, and excellent wear resistance. and references therein B6O can be synthesized by reducing B2O3 with boron or by oxidation of boron with zinc oxide or other oxidants. These boron suboxide materials formed at or near ambient pressure are generally oxygen deficient and non-stoichiometric (B6Ox, x<0.9) and have poor crystallinity and very small grain size (less than 5 μm). High pressure applied during the synthesis o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that are liquid at or near room temperature. Caesium has physical and chemical properties similar to those of rubidium and potassium. It is pyrophoric and reacts with water even at . It is the least electronegative element, with a value of 0.79 on the Pauling scale. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. The element has 40 known isotopes, making it, along with barium and mercury, one of the elements with the most isotopes. Caesium-137, a fission product, is extracted from waste produced by nuclear reactors. The German chemist Robert Bunsen and physicist Gustav Kirchhoff discovered caesium in 1860 by the newly developed method of flame spectroscopy. The first small-scale applications for caesium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cumulene
In organic chemistry, a cumulene is a compound having three or more ''cumulative'' (consecutive) double bonds. They are analogous to allenes, only having a more extensive chain. The simplest molecule in this class is butatriene (), which is also called simply ''cumulene''. Unlike most alkanes and alkenes, cumulenes tend to be rigid, comparable to polyynes. Cumulene carbenes for ''n'' from 3 to 6 have been observed in interstellar molecular clouds and in laboratory experiments by using microwave and infrared spectroscopy. (The more stable cumulenes are difficult to detect optically because they lack an electric dipole moment.) Cumulenes containing heteroatoms are called heterocumulenes; an example is carbon suboxide. Synthesis The first reported synthesis of a butatriene is that of tetraphenylbutatriene in 1921. The most common synthetic method for butatriene synthesis is based on reductive coupling of a geminal dihalovinylidene. Tetraphenylbutatriene was reported synthesized ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cs11O3 Cluster
Caesium oxide (IUPAC name), or cesium oxide, describes inorganic compounds composed of caesium and oxygen. Several binary (containing only Cs and O) oxides of caesium are known.. Caesium oxide may refer to: * Caesium suboxides (Cs7O, Cs4O, and Cs11O3) * Caesium monoxide (Cs2O, the most common oxide) * Caesium peroxide (Cs2O2) * Caesium sesquioxide (Cs2O3) * Caesium superoxide (CsO2) * Caesium ozonide Caesium ozonide (CsO3) is an oxygen-rich compound of caesium. It is an ozonide, meaning it contains the ozonide anion (O3−). It can be formed by reacting ozone with caesium superoxide: :CsO2 + O3 -> CsO3 + O2 The compound will react strongly ... (CsO3) References {{Authority control Caesium compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Stoichiometry (the measurable relationship between reactants and chemical equations of a equation or reaction) Oxides are extraordinarily diverse in terms of stoichiometries and in terms of the structures of each stoichiometry. Most elements form oxides of more than one stoichiometry. A well known example is carbon monoxide and carbon dioxide.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cluster Chemistry
In chemistry, an atom cluster (or simply cluster) is an ensemble of bound atoms or molecules that is intermediate in size between a simple molecule and a nanoparticle; that is, up to a few nanometers (nm) in diameter. The term ''microcluster'' may be used for ensembles with up to couple dozen atoms. Clusters with a definite number and type of atoms in a specific arrangement are often considered a specific chemical compound and are studied as such. For example, fullerene is a cluster of 60 carbon atoms arranged as the vertices of a truncated icosahedron, and decaborane is a cluster of 10 boron atoms forming an incomplete icosahedron, surrounded by 14 hydrogen atoms. The term is most commonly used for ensembles consisting of several atoms of the same element, or of a few different elements, bonded in a three-dimensional arrangement. Transition metals and main group elements form especially robust clusters. Indeed, in some contexts, the term may refer specifically to a metal cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octahedral
In geometry, an octahedron (plural: octahedra, octahedrons) is a polyhedron with eight faces. The term is most commonly used to refer to the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex. A regular octahedron is the dual polyhedron of a cube. It is a rectified tetrahedron. It is a square bipyramid in any of three orthogonal orientations. It is also a triangular antiprism in any of four orientations. An octahedron is the three-dimensional case of the more general concept of a cross polytope. A regular octahedron is a 3-ball in the Manhattan () metric. Regular octahedron Dimensions If the edge length of a regular octahedron is ''a'', the radius of a circumscribed sphere (one that touches the octahedron at all vertices) is :r_u = \frac a \approx 0.707 \cdot a and the radius of an inscribed sphere (tangent to each of the octahedron's faces) is :r_i = \frac a \approx 0.408\cdot a while the midradius, which t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitride
In chemistry, a nitride is an inorganic compound of nitrogen. The "nitride" anion, N3- ion, is very elusive but compounds of nitride are numerous, although rarely naturally occuring. Some nitrides have a find applications, such as wear-resistant coatings (e.g., titanium nitride, TiN), hard ceramic materials (e.g., silicon nitride, Si3N4), and semiconductors (e.g., gallium nitride, GaN). The development of GaN-based light emitting diodes was recognized by the 2014 Nobel Prize in Physics. Metal nitrido complexes are also common. Synthesis of inorganic metal nitrides is challenging because nitrogen gas (N2) is not very reactive at low temperatures, but it becomes more reactive at higher temperatures. Therefore, a balance must be achieved between the low reactivity of nitrogen gas at low temperatures and the entropy driven formation of N2 at high temperatures. However, synthetic methods for nitrides are growing more sophisticated and the materials are of increasing technological re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Subnitride
Subnitrides are a class of nitrides wherein the electropositive element is in excess relative to the “normal” nitrides.Simon, A. ”Group 1 and 2 Suboxides and Subnitrides — Metals with Atomic Size Holes and Tunnels” Coordination Chemistry Reviews 1997, volume 163, Pages 253–270. Examples of subnitrides include * Dicyanoacetylene * Na16Ba6N features a nitride-centered octahedral cluster of six barium Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element. Th ... atoms embedded in a matrix of sodium. * Boron subnitrides (B13N2, B50N2, B6N) References Nitrides {{chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octet Rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The rule is especially applicable to carbon, nitrogen, oxygen, and the halogens; although more generally the rule is applicable for the s-block and p-block of the periodic table. Other rules exist for other elements, such as the duplet rule for hydrogen and helium, or the 18-electron rule for transition metals. The valence electrons can be counted using a Lewis electron dot diagram as shown at the right for carbon dioxide. The electrons shared by the two atoms in a covalent bond are counted twice, once for each atom. In carbon dioxide each oxygen shares four electrons with the central carbon, two (shown in red) from the oxygen itself and two (shown in black) from the carbon. All four of these electrons are counted in both the carbon octet and the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |