Octet rule on:
[Wikipedia]
[Google]
[Amazon]
 The octet rule is a
The octet rule is a
 In 1864, the English chemist John Newlands classified the sixty-two known elements into eight groups, based on their physical properties.
In the late 19th century, it was known that coordination compounds (formerly called "molecular compounds") were formed by the combination of atoms or molecules in such a manner that the valencies of the atoms involved apparently became satisfied. In 1893,
In 1864, the English chemist John Newlands classified the sixty-two known elements into eight groups, based on their physical properties.
In the late 19th century, it was known that coordination compounds (formerly called "molecular compounds") were formed by the combination of atoms or molecules in such a manner that the valencies of the atoms involved apparently became satisfied. In 1893,
 Some stable molecular radicals (e.g.
Some stable molecular radicals (e.g.
 The octet rule is a
The octet rule is a chemical
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., wi ...
rule of thumb
In English, the phrase ''rule of thumb'' refers to an approximate method for doing something, based on practical experience rather than theory. This usage of the phrase can be traced back to the 17th century and has been associated with various t ...
that reflects the theory that main-group element
In chemistry and atomic physics, the main group is the group of elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, and fluorine as arrange ...
s tend to bond
Bond or bonds may refer to:
Common meanings
* Bond (finance), a type of debt security
* Bail bond, a commercial third-party guarantor of surety bonds in the United States
* Chemical bond, the attraction of atoms, ions or molecules to form chemica ...
in such a way that each atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
has eight electrons
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
in its valence shell
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
, giving it the same electronic configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
as a noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemi ...
. The rule is especially applicable to carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
, nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
, oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
, and the halogens
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group i ...
; although more generally the rule is applicable for the s-block
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-blo ...
and p-block
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-blo ...
of the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
. Other rules exist for other elements, such as the duplet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The rule ...
for hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
and helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
, or the 18-electron rule for transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
s.
The valence electrons can be counted using a Lewis electron dot diagram as shown at the right for carbon dioxide. The electrons shared by the two atoms in a covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
are counted twice, once for each atom. In carbon dioxide each oxygen shares four electrons with the central carbon, two (shown in red) from the oxygen itself and two (shown in black) from the carbon. All four of these electrons are counted in both the carbon octet and the oxygen octet, so that both atoms are considered to obey the octet rule.
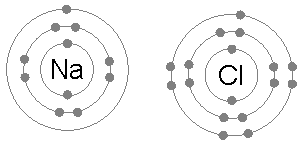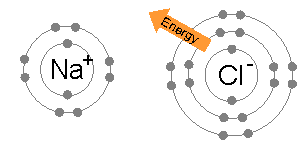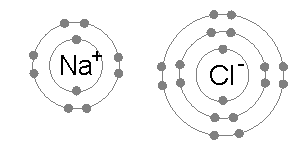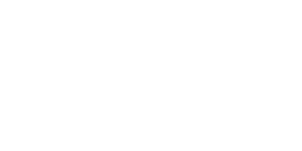
Example: sodium chloride (NaCl)

Ionic bonding
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds ...
is common between pairs of atoms, where one of the pair is a metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
of low electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
(such as sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
) and the second a nonmetal
In chemistry, a nonmetal is a chemical element that generally lacks a predominance of metallic properties; they range from colorless gases (like hydrogen) to shiny solids (like carbon, as graphite). The electrons in nonmetals behave differentl ...
of high electronegativity (such as chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
).
A chlorine atom has seven electrons in its third and outer electron shell, the first and second shells being filled with two and eight electrons respectively. The first electron affinity
The electron affinity (''E''ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.
::X(g) + e− → X−(g) + energy
Note that this is ...
of chlorine (the energy release when chlorine gains an electron to form Cl−) is 349 kJ per mole of chlorine atoms. Adding a second electron to form a hypothetical Cl2- would require energy, energy that cannot be recovered by the formation of a chemical bond. The result is that chlorine will very often form a compound in which it has eight electrons in its outer shell (a complete octet), as in Cl−.
A sodium atom has a single electron in its outermost electron shell, the first and second shells again being full with two and eight electrons respectively. To remove this outer electron requires only the first ionization energy
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule i ...
, which is +495.8 kJ per mole
Mole (or Molé) may refer to:
Animals
* Mole (animal) or "true mole", mammals in the family Talpidae, found in Eurasia and North America
* Golden moles, southern African mammals in the family Chrysochloridae, similar to but unrelated to Talpida ...
of sodium atoms, a small amount of energy. By contrast, the second electron resides in the deeper second electron shell, and the second ionization energy required for its removal is much larger: +4562 kJ per mole. Thus sodium will, in most cases, form a compound in which it has lost a single electron and have a full outer shell of eight electrons, or octet.
The energy required to transfer an electron from a sodium atom to a chlorine atom (the difference of the 1st ionization energy of sodium and the electron affinity of chlorine) is small: +495.8 − 349 = +147 kJ mol−1. This energy is easily offset by the lattice energy
In chemistry, the lattice energy is the energy change upon formation of one mole of a crystalline ionic compound from its constituent ions, which are assumed to initially be in the gaseous state. It is a measure of the cohesive forces that bind ...
of sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
: −783 kJ mol−1. This completes the explanation of the octet rule in this case.
History
 In 1864, the English chemist John Newlands classified the sixty-two known elements into eight groups, based on their physical properties.
In the late 19th century, it was known that coordination compounds (formerly called "molecular compounds") were formed by the combination of atoms or molecules in such a manner that the valencies of the atoms involved apparently became satisfied. In 1893,
In 1864, the English chemist John Newlands classified the sixty-two known elements into eight groups, based on their physical properties.
In the late 19th century, it was known that coordination compounds (formerly called "molecular compounds") were formed by the combination of atoms or molecules in such a manner that the valencies of the atoms involved apparently became satisfied. In 1893, Alfred Werner
Alfred Werner (12 December 1866 – 15 November 1919) was a Swiss chemist who was a student at ETH Zurich and a professor at the University of Zurich. He won the Nobel Prize in Chemistry in 1913 for proposing the octahedral configuration of ...
showed that the number of atoms or groups associated with a central atom (the "coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central i ...
") is often 4 or 6; other coordination numbers up to a maximum of 8 were known, but less frequent. In 1904, Richard Abegg
Richard Wilhelm Heinrich Abegg (9 January 1869 – 3 April 1910) was a German chemist and pioneer of valence theory. He proposed that the difference of the maximum positive and negative valence of an element tends to be eight. This has come to be ...
was one of the first to extend the concept of coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central i ...
to a concept of valence in which he distinguished atoms as electron donors or acceptors, leading to positive and negative valence states that greatly resemble the modern concept of oxidation states
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
. Abegg noted that the difference between the maximum positive and negative valences of an element under his model is frequently eight. In 1916, Gilbert N. Lewis referred to this insight as Abegg's rule In chemistry, Abegg’s rule states that the difference between the maximum positive and negative valence of an element is frequently eight. The rule used a historic meaning of valence which resembles the modern concept of oxidation state in whic ...
and used it to help formulate his cubical atom
The cubical atom was an early atomic model in which electrons were positioned at the eight corners of a cube in a non-polar atom or molecule. This theory was developed in 1902 by Gilbert N. Lewis and published in 1916 in the article "The Atom and ...
model and the "rule of eight", which began to distinguish between valence and valence electron
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
s. In 1919, Irving Langmuir
Irving Langmuir (; January 31, 1881 – August 16, 1957) was an American chemist, physicist, and engineer. He was awarded the Nobel Prize in Chemistry in 1932 for his work in surface chemistry.
Langmuir's most famous publication is the 1919 art ...
refined these concepts further and renamed them the "cubical octet atom" and "octet theory". The "octet theory" evolved into what is now known as the "octet rule".
Walther Kossel
Walther Ludwig Julius Kossel (4 January 1888 – 22 May 1956) was a German physicist known for his theory of the chemical bond (ionic bond/octet rule), Sommerfeld–Kossel displacement law of atomic spectra, the Kossel-Stranski model for crysta ...
and Gilbert N. Lewis saw that noble gases did not have the tendency of taking part in chemical reactions under ordinary conditions. On the basis of this observation, they concluded that atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s of noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemi ...
es are stable and on the basis of this conclusion they proposed a theory of valency known as "electronic theory of valency" in 1916:
Explanation in quantum theory
The quantum theory of the atom explains the eight electrons as aclosed shell
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
with an s2p6 electron configuration. A closed-shell configuration is one in which low-lying energy levels are full and higher energy levels are empty. For example, the neon
Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered (along with krypton ...
atom ground state has a full shell (2s2 2p6) and an empty shell. According to the octet rule, the atoms immediately before and after neon in the periodic table (i.e. C, N, O, F, Na, Mg and Al), tend to attain a similar configuration by gaining, losing, or sharing electrons.
The argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
atom has an analogous 3s2 3p6 configuration. There is also an empty 3d level, but it is at considerably higher energy than 3s and 3p (unlike in the hydrogen atom), so that 3s2 3p6 is still considered a closed shell for chemical purposes. The atoms immediately before and after argon tend to attain this configuration in compounds. There are, however, some hypervalent molecule
In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. Phosphorus p ...
s in which the 3d level may play a part in the bonding, although this is controversial (see below).
For helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
there is no 1p level according to the quantum theory, so that 1s2 is a closed shell with no p electrons. The atoms before and after helium (H and Li) follow a duet rule and tend to have the same 1s2 configuration as helium.
Exceptions
Manyreactive intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these comp ...
s are unstable and do not obey the octet rule. This includes species such as carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
s, as well as free radicals
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spont ...
and the methyl radical
Methyl (also systematically named trihydridocarbon) is an organic compound with the chemical formula (also written as •). It is a metastable colourless gas, which is mainly produced ''in situ'' as a precursor to other hydrocarbons in the petrol ...
(CH3) which has an unpaired electron in a non-bonding orbital
A non-bonding orbital, also known as ''non-bonding molecular orbital'' (NBMO), is a molecular orbital whose occupation by electrons neither increases nor decreases the bond order between the involved atoms. Non-bonding orbitals are often designat ...
on the carbon atom and no electron of opposite spin in the same orbital. Another example is the radical chlorine monoxide
Chlorine monoxide is a chemical radical with the chemical formula ClO•. It plays an important role in the process of ozone depletion. In the stratosphere, chlorine atoms react with ozone molecules to form chlorine monoxide and oxygen.
:Cl� ...
(ClO•) which is involved in ozone depletion
Ozone depletion consists of two related events observed since the late 1970s: a steady lowering of about four percent in the total amount of ozone in Earth's atmosphere, and a much larger springtime decrease in stratospheric ozone (the ozone l ...
. These molecules often react so as to complete their octet. Electron deficient molecules such as boranes
Boranes is the name given to compounds with the formula BxHy and related anions. Many such boranes are known. Most common are those with 1 to 12 boron atoms. Although they have few practical applications, the boranes exhibit structures and bond ...
also do not obey the octet rule but share delocalized electron
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly di ...
s in a manner similar to metallic bonding
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be descri ...
.
Although stable odd-electron molecules and hypervalent molecules are commonly taught as violating the octet rule, ab initio molecular orbital calculations show that they largely obey the octet rule (see three-electron bonds and hypervalent molecules sections below).
Three-electron bonds
nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its che ...
, NO) obtain octet configurations by means of a three-electron bond which contributes one shared and one unshared electron to the octet of each bonded atom. In NO, the octet on each atom consists of two electrons from the three-electron bond, plus four electrons from two two-electron bonds and two electrons from a lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
of non-bonding electrons on that atom alone. The bond order is 2.5, since each two-electron bond counts as one bond while the three-electron bond has only one shared electron and therefore corresponds to a half-bond.
Dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (O2), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (O3). Others are:
*A ...
is sometimes represented as obeying the octet rule with a double bond (O=O) containing two pairs of shared electrons. However the ground state of this molecule is paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, d ...
, indicating the presence of unpaired electrons. Pauling proposed that this molecule actually contains two three-electron bonds and one normal covalent (two-electron) bond. The octet on each atom then consists of two electrons from each three-electron bond, plus the two electrons of the covalent bond, plus one lone pair of non-bonding electrons. The bond order is 1+0.5+0.5=2.
Hypervalent molecules
Main-group elements in the third and later rows of the periodic table can form hypercoordinate orhypervalent molecule
In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. Phosphorus p ...
s in which the central main-group atom is bonded to more than four other atoms, such as phosphorus pentafluoride
Phosphorus pentafluoride, P F5, is a phosphorus halide. It is a colourless, toxic gas that fumes in air.
Preparation
Phosphorus pentafluoride was first prepared in 1876 by the fluorination of phosphorus pentachloride using arsenic trifluoride, w ...
, PF5, and sulfur hexafluoride
Sulfur hexafluoride or sulphur hexafluoride (British spelling) is an inorganic compound with the formula SF6. It is a colorless, odorless, non- flammable, and non-toxic gas. has an octahedral geometry, consisting of six fluorine atoms attached ...
, SF6. For example, in PF5, if it is supposed that there are five true covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
s in which five distinct electron pairs are shared, then the phosphorus would be surrounded by 10 valence electrons in violation of the octet rule. In the early days of quantum mechanics, Pauling proposed that third-row atoms can form five bonds by using one s, three p and one d orbitals, or six bonds by using one s, three p and two d orbitals. To form five bonds, the one s, three p and one d orbitals combine to form five sp3d hybrid orbitals which each share an electron pair with a halogen atom, for a total of 10 shared electrons, two more than the octet rule predicts. Similarly to form six bonds, the six sp3d2 hybrid orbitals form six bonds with 12 shared electrons. In this model the availability of empty d orbitals is used to explain the fact that third-row atoms such as phosphorus and sulfur can form more than four covalent bonds, whereas second-row atoms such as nitrogen and oxygen are strictly limited by the octet rule.
500px , center , 5 resonance structures of phosphorus pentafluoride
However other models describe the bonding using only s and p orbitals in agreement with the octet rule. A valence bond
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
description of PF5 uses resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillatin ...
between different PF4+ F− structures, so that each F is bonded by a covalent bond in four structures and an ionic bond in one structure. Each resonance structure has eight valence electrons on P. A molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century.
In molecular orbital theory, electrons in a molecule ...
description considers the highest occupied molecular orbital
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
to be a non-bonding orbital localized on the five fluorine atoms, in addition to four occupied bonding orbitals, so again there are only eight valence electrons on the phosphorus. The validity of the octet rule for hypervalent molecules is further supported by ab initio molecular orbital calculations, which show that the contribution of d functions to the bonding orbitals is small.
Nevertheless, for historical reasons, structures implying more than eight electrons around elements like P, S, Se, or I are still common in textbooks and research articles. In spite of the unimportance of d shell expansion in chemical bonding, this practice allows structures to be shown without using a large number of formal charges or using partial bonds and is recommended by the IUPAC as a convenient formalism in preference to depictions that better reflect the bonding. On the other hand, showing more than eight electrons around Be, B, C, N, O, or F (or more than two around H, He, or Li) is considered an error by most authorities.
Other rules
The octet rule is only applicable tomain-group element
In chemistry and atomic physics, the main group is the group of elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, and fluorine as arrange ...
s. Other elements follow other electron counting rules as their valence electron
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
configurations are different from main-group elements. These other rules are shown below:
* The duet rule or duplet rule of the first shell applies to H, He and Li—the noble gas helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
has two electrons in its outer shell, which is very stable. (Since there is no 1''p'' subshell, 1''s'' is followed immediately by 2''s'', and thus shell 1 can only have at most 2 valence electrons). Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
only needs one additional electron to attain this stable configuration, while lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid el ...
needs to lose one.
* For transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
s, molecules tend to obey the 18-electron rule which corresponds to the utilization of valence ''d'', ''s'' and ''p'' orbitals to form bonding and non-bonding orbitals. However, unlike the octet rule for main-group elements, transition metals do not strictly obey the 18-electron rule and the valence electron count can vary between 12 to 18.
See also
*Lewis structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the chemical bonding, bonding between atoms of a molecule, as well as the lone pairs ...
* Electron counting
References
{{Electron configuration navbox Chemical bonding Rules of thumb