|
P-block
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-block, p-block, d-block, and f-block. The block names (s, p, d, and f) are derived from the spectroscopic notation for the value of an electron's azimuthal quantum number: sharp (0), principal (1), diffuse (2), or fundamental (3). Succeeding notations proceed in alphabetical order, as g, h, etc., though elements that would belong in such blocks have not yet been found. Characteristics There is an ''approximate'' correspondence between this nomenclature of blocks, based on electronic configuration, and sets of elements based on chemical properties. The s-block and p-block together are usually considered main-group elements, the d-block corresponds to the transition metals, and the f-block corresponds to the inner transiti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periodic Table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of chemistry. It is a graphic formulation of the periodic law, which states that the properties of the chemical elements exhibit an approximate periodic dependence on their atomic numbers. The table is divided into four roughly rectangular areas called blocks. The rows of the table are called periods, and the columns are called groups. Elements from the same group of the periodic table show similar chemical characteristics. Trends run through the periodic table, with nonmetallic character (keeping their own electrons) increasing from left to right across a period, and from down to up across a group, and metallic character (surrendering electrons to other atoms) increasing in the opposite direction. The underlying reason for these trends is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
S-block
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-block, p-block, d-block, and f-block. The block names (s, p, d, and f) are derived from the spectroscopic notation for the value of an electron's azimuthal quantum number: sharp (0), principal (1), diffuse (2), or fundamental (3). Succeeding notations proceed in alphabetical order, as g, h, etc., though elements that would belong in such blocks have not yet been found. Characteristics There is an ''approximate'' correspondence between this nomenclature of blocks, based on electronic configuration, and sets of elements based on chemical properties. The s-block and p-block together are usually considered main-group elements, the d-block corresponds to the transition metals, and the f-block corresponds to the inner transi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
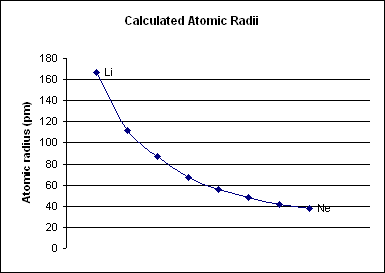
Period 2 Element
A period 2 element is one of the chemical elements in the second row (or period) of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties. The second period contains the elements lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon. In a quantum mechanical description of atomic structure, this period corresponds to the filling of the second () shell, more specifically its 2s and 2p subshells. Period 2 elements (carbon, nitrogen, oxygen, fluorine and neon) obey the octet rule in that they need eight electrons to complete their valence shell (lithium and beryllium obey duet rule, boron is electron deficient.), where at most eight electrons can be accommodated: two in the 2s orbital and si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Period (periodic Table)
A period in the periodic table is a row of chemical elements. All elements in a row have the same number of electron shells. Each next element in a period has one more proton and is less metallic than its predecessor. Arranged this way, elements in the same group (column) have similar chemical and physical properties, reflecting the periodic law. For example, the halogens lie in the second-to-last group ( group 17) and share similar properties, such as high reactivity and the tendency to gain one electron to arrive at a noble-gas electronic configuration. , a total of 118 elements have been discovered and confirmed. Modern quantum mechanics explains these periodic trends in properties in terms of electron shells. As atomic number increases, shells fill with electrons in approximately the order shown in the ordering rule diagram. The filling of each shell corresponds to a row in the table. In the s-block and p-block of the periodic table, elements within the same period generally ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Main-group Element
In chemistry and atomic physics, the main group is the group of elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, and fluorine as arranged in the periodic table of the elements. The main group includes the elements (except hydrogen, which is sometimes not included) in groups 1 and 2 (s-block), and groups 13 to 18 (p-block). The s-block elements are primarily characterised by one main oxidation state, and the p-block elements, when they have multiple oxidation states, often have common oxidation states separated by two units. Main-group elements (with some of the lighter transition metals) are the most abundant elements on Earth, in the Solar System, and in the universe. Group 12 elements are often considered to be transition metals; however, zinc (Zn), cadmium (Cd), and mercury (Hg) share some properties of both groups, and some scientists believe they should be included ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Group 12 Element
Group 12, by modern IUPAC numbering, is a group of chemical elements in the periodic table. It includes zinc (Zn), cadmium (Cd), mercury (Hg), and copernicium (Cn). Formerly this group was named ''IIB'' (pronounced as "group two B", as the "II" is a Roman numeral) by CAS and old IUPAC system. The three group 12 elements that occur naturally are zinc, cadmium and mercury. They are all widely used in electric and electronic applications, as well as in various alloys. The first two members of the group share similar properties as they are solid metals under standard conditions. Mercury is the only metal that is a liquid at room temperature. While zinc is very important in the biochemistry of living organisms, cadmium and mercury are both highly toxic. As copernicium does not occur in nature, it has to be synthesized in the laboratory. Physical and atomic properties Like other groups of the periodic table, the members of group 12 show patterns in its electron configuration, especial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can use d orbitals as valence orbitals to form chemical bonds. The lanthanide and actinide elements (the f-block) are called inner transition metals and are sometimes considered to be transition metals as well. Since they are metals, they are lustrous and have good electrical and thermal conductivity. Most (with the exception of group 11 and group 12) are hard and strong, and have high melting and boiling temperatures. They form compounds in any of two or more different oxidation states and bind to a variety of ligands to form coordination complexes that are often coloured. They form many useful alloys and are often employed as catalysts in elemental form or in compounds such as coordination complexes and oxides. Most are strongly paramag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronic Configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is , meaning that the 1s, 2s and 2p subshells are occupied by 2, 2 and 6 electrons respectively. Electronic configurations describe each electron as moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions. According to the laws of quantum mechanics, for systems with only one electron, a level of energy is associated with each electron configuration and in certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon. Knowledge of the electron configuration of different atoms is useful in understanding the struc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neon
Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered (along with krypton and xenon) in 1898 as one of the three residual rare inert elements remaining in dry air, after nitrogen, oxygen, argon and carbon dioxide were removed. Neon was the second of these three rare gases to be discovered and was immediately recognized as a new element from its bright red emission spectrum. The name neon is derived from the Greek word, , neuter singular form of (), meaning 'new'. Neon is chemically inert, and no uncharged neon compounds are known. The compounds of neon currently known include ionic molecules, molecules held together by van der Waals forces and clathrates. During cosmic nucleogenesis of the elements, large amounts of neon are built up from the alpha-capture fusion process in stars. Although neon is a very ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valence Electrons
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron. The presence of valence electrons can determine the element's chemical properties, such as its valence—whether it may bond with other elements and, if so, how readily and with how many. In this way, a given element's reactivity is highly dependent upon its electronic configuration. For a main-group element, a valence electron can exist only in the outermost electron shell; for a transition metal, a valence electron can also be in an inner shell. An atom with a closed shell of valence electrons (corresponding to a noble gas configuration) tends to be chemically inert. Atoms with one or two valence electrons more than a closed shell are highly reactive due to the re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Orbitals
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term ''atomic orbital'' may also refer to the physical region or space where the electron can be calculated to be present, as predicted by the particular mathematical form of the orbital. Each orbital in an atom is characterized by a set of values of the three quantum numbers , , and , which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component ( magnetic quantum number). Alternative to the magnetic quantum number, the orbitals are often labeled by the associated harmonic polynomials (e.g., ''xy'', ). Each such orbital can be occupied by a maximum of two electrons, each with its own projection of spin m_s. The simple names s orbital, p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term ''atomic orbital'' may also refer to the physical region or space where the electron can be calculated to be present, as predicted by the particular mathematical form of the orbital. Each orbital in an atom is characterized by a set of values of the three quantum numbers , , and , which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (magnetic quantum number). Alternative to the magnetic quantum number, the orbitals are often labeled by the associated harmonic polynomials (e.g., ''xy'', ). Each such orbital can be occupied by a maximum of two electrons, each with its own projection of spin m_s. The simple names s orbital ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |