Caesium on:
[Wikipedia]
[Google]
[Amazon]
Caesium ( IUPAC spelling) (or cesium in
 Of all elements that are solid at room temperature, caesium is the softest: it has a hardness of 0.2 Mohs. It is a very ductile, pale metal, which darkens in the presence of trace amounts of oxygen. When in the presence of mineral oil (where it is best kept during transport), it loses its metallic lustre and takes on a duller, grey appearance. It has a melting point of , making it one of the few elemental metals that are liquid near
Of all elements that are solid at room temperature, caesium is the softest: it has a hardness of 0.2 Mohs. It is a very ductile, pale metal, which darkens in the presence of trace amounts of oxygen. When in the presence of mineral oil (where it is best kept during transport), it loses its metallic lustre and takes on a duller, grey appearance. It has a melting point of , making it one of the few elemental metals that are liquid near  Caesium forms
Caesium forms
 Most caesium compounds contain the element as the cation , which binds ionically to a wide variety of anions. One noteworthy exception is the caeside anion (), and others are the several suboxides (see section on oxides below). More recently, caesium is predicted to behave as a p-block element and capable of forming higher fluorides with higher oxidation states (i.e., CsFn with n > 1) under high pressure. This prediction needs to be validated by further experiments.
Salts of Cs+ are usually colourless unless the anion itself is coloured. Many of the simple salts are hygroscopic, but less so than the corresponding salts of lighter alkali metals. The phosphate, acetate, carbonate,
Most caesium compounds contain the element as the cation , which binds ionically to a wide variety of anions. One noteworthy exception is the caeside anion (), and others are the several suboxides (see section on oxides below). More recently, caesium is predicted to behave as a p-block element and capable of forming higher fluorides with higher oxidation states (i.e., CsFn with n > 1) under high pressure. This prediction needs to be validated by further experiments.
Salts of Cs+ are usually colourless unless the anion itself is coloured. Many of the simple salts are hygroscopic, but less so than the corresponding salts of lighter alkali metals. The phosphate, acetate, carbonate,
 Caesium fluoride (CsF) is a hygroscopic white solid that is widely used in
Caesium fluoride (CsF) is a hygroscopic white solid that is widely used in
 More so than the other alkali metals, caesium forms numerous binary compounds with oxygen. When caesium burns in air, the superoxide is the main product. The "normal" caesium oxide () forms yellow-orange hexagonal crystals, and is the only oxide of the anti- type. It vaporizes at , and decomposes to caesium metal and the peroxide at temperatures above . In addition to the superoxide and the ozonide , several brightly coloured suboxides have also been studied. These include , , , (dark-green), CsO, , as well as . The latter may be heated in a vacuum to generate . Binary compounds with
More so than the other alkali metals, caesium forms numerous binary compounds with oxygen. When caesium burns in air, the superoxide is the main product. The "normal" caesium oxide () forms yellow-orange hexagonal crystals, and is the only oxide of the anti- type. It vaporizes at , and decomposes to caesium metal and the peroxide at temperatures above . In addition to the superoxide and the ozonide , several brightly coloured suboxides have also been studied. These include , , , (dark-green), CsO, , as well as . The latter may be heated in a vacuum to generate . Binary compounds with
 The radioactive 135Cs has a very long half-life of about 2.3 million years, the longest of all radioactive isotopes of caesium. 137Cs and 134Cs have half-lives of 30 and two years, respectively. 137Cs decomposes to a short-lived 137mBa by beta decay, and then to nonradioactive barium, while 134Cs transforms into 134Ba directly. The isotopes with mass numbers of 129, 131, 132 and 136, have half-lives between a day and two weeks, while most of the other isotopes have half-lives from a few seconds to fractions of a second. At least 21 metastable nuclear isomers exist. Other than 134mCs (with a half-life of just under 3 hours), all are very unstable and decay with half-lives of a few minutes or less.
The isotope 135Cs is one of the
The radioactive 135Cs has a very long half-life of about 2.3 million years, the longest of all radioactive isotopes of caesium. 137Cs and 134Cs have half-lives of 30 and two years, respectively. 137Cs decomposes to a short-lived 137mBa by beta decay, and then to nonradioactive barium, while 134Cs transforms into 134Ba directly. The isotopes with mass numbers of 129, 131, 132 and 136, have half-lives between a day and two weeks, while most of the other isotopes have half-lives from a few seconds to fractions of a second. At least 21 metastable nuclear isomers exist. Other than 134mCs (with a half-life of just under 3 hours), all are very unstable and decay with half-lives of a few minutes or less.
The isotope 135Cs is one of the
 Caesium is a relatively rare element, estimated to average 3 parts per million in the
Caesium is a relatively rare element, estimated to average 3 parts per million in the
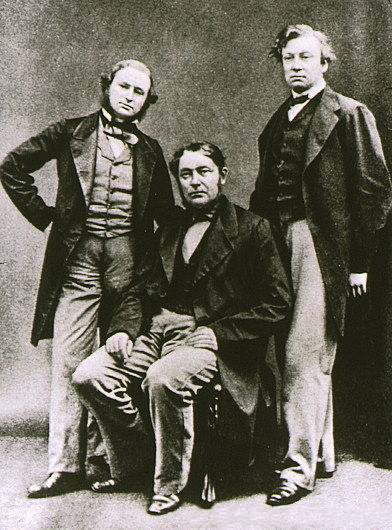 In 1860, Robert Bunsen and Gustav Kirchhoff discovered caesium in the mineral water from Bad Dürkheim, Dürkheim, Germany. Because of the bright blue lines in the
In 1860, Robert Bunsen and Gustav Kirchhoff discovered caesium in the mineral water from Bad Dürkheim, Dürkheim, Germany. Because of the bright blue lines in the
 Caesium-based
Caesium-based
 Relatively few chemical applications use caesium. Doping with caesium compounds enhances the effectiveness of several metal-ion catalysts for chemical synthesis, such as acrylic acid, anthraquinone, ethylene oxide, methanol, phthalic anhydride, styrene, methyl methacrylate monomers, and various alkene, olefins. It is also used in the catalytic conversion of sulfur dioxide into sulfur trioxide in the production of sulfuric acid.
Caesium fluoride enjoys a niche use in organic chemistry as a base (chemistry), base and as an anhydrous source of
Relatively few chemical applications use caesium. Doping with caesium compounds enhances the effectiveness of several metal-ion catalysts for chemical synthesis, such as acrylic acid, anthraquinone, ethylene oxide, methanol, phthalic anhydride, styrene, methyl methacrylate monomers, and various alkene, olefins. It is also used in the catalytic conversion of sulfur dioxide into sulfur trioxide in the production of sulfuric acid.
Caesium fluoride enjoys a niche use in organic chemistry as a base (chemistry), base and as an anhydrous source of
 Caesium and mercury were used as a propellant in early ion thruster, ion engines designed for spacecraft propulsion on very long interplanetary or extraplanetary missions. The fuel was ionized by contact with a charged tungsten electrode. But corrosion by caesium on spacecraft components has pushed development in the direction of inert gas propellants, such as xenon, which are easier to handle in ground-based tests and do less potential damage to the spacecraft. Xenon was used in the experimental spacecraft Deep Space 1 launched in 1998. Nevertheless, field-emission electric propulsion thrusters that accelerate liquid metal ions such as caesium have been built.
Caesium nitrate is used as an oxidizing agent, oxidizer and pyrotechnic colorant to burn silicon in infrared flare (pyrotechnic), flares, such as the LUU-19 flare, because it emits much of its light in the infrared, near infrared spectrum. Caesium compounds may have been used as fuel additives to reduce the radar cross-section, radar signature of exhaust gas, exhaust plumes in the Lockheed A-12 CIA reconnaissance aircraft. Caesium and rubidium have been added as a carbonate to glass because they reduce electrical conductivity and improve stability and durability of optical fiber, fibre optics and night vision devices. Caesium fluoride or caesium aluminium fluoride are used in fluxes formulated for brazing aluminium alloys that contain magnesium.
MHD generator, Magnetohydrodynamic (MHD) power-generating systems were researched, but failed to gain widespread acceptance. Caesium metal has also been considered as the working fluid in high-temperature Rankine cycle turboelectric generators.
Caesium salts have been evaluated as antishock reagents following the administration of arsenic toxicity, arsenical drugs. Because of their effect on heart rhythms, however, they are less likely to be used than potassium or rubidium salts. They have also been used to treat epilepsy.
Caesium-133 can be laser cooling, laser cooled and used to probe fundamental and Quantum technology, technological problems in quantum mechanics, quantum physics. It has a particularly convenient Feshbach resonance, Feshbach spectrum to enable studies of ultracold atoms requiring tunable interactions.
Caesium and mercury were used as a propellant in early ion thruster, ion engines designed for spacecraft propulsion on very long interplanetary or extraplanetary missions. The fuel was ionized by contact with a charged tungsten electrode. But corrosion by caesium on spacecraft components has pushed development in the direction of inert gas propellants, such as xenon, which are easier to handle in ground-based tests and do less potential damage to the spacecraft. Xenon was used in the experimental spacecraft Deep Space 1 launched in 1998. Nevertheless, field-emission electric propulsion thrusters that accelerate liquid metal ions such as caesium have been built.
Caesium nitrate is used as an oxidizing agent, oxidizer and pyrotechnic colorant to burn silicon in infrared flare (pyrotechnic), flares, such as the LUU-19 flare, because it emits much of its light in the infrared, near infrared spectrum. Caesium compounds may have been used as fuel additives to reduce the radar cross-section, radar signature of exhaust gas, exhaust plumes in the Lockheed A-12 CIA reconnaissance aircraft. Caesium and rubidium have been added as a carbonate to glass because they reduce electrical conductivity and improve stability and durability of optical fiber, fibre optics and night vision devices. Caesium fluoride or caesium aluminium fluoride are used in fluxes formulated for brazing aluminium alloys that contain magnesium.
MHD generator, Magnetohydrodynamic (MHD) power-generating systems were researched, but failed to gain widespread acceptance. Caesium metal has also been considered as the working fluid in high-temperature Rankine cycle turboelectric generators.
Caesium salts have been evaluated as antishock reagents following the administration of arsenic toxicity, arsenical drugs. Because of their effect on heart rhythms, however, they are less likely to be used than potassium or rubidium salts. They have also been used to treat epilepsy.
Caesium-133 can be laser cooling, laser cooled and used to probe fundamental and Quantum technology, technological problems in quantum mechanics, quantum physics. It has a particularly convenient Feshbach resonance, Feshbach spectrum to enable studies of ultracold atoms requiring tunable interactions.
 Nonradioactive caesium compounds are only mildly toxic, and nonradioactive caesium is not a significant environmental hazard. Because biochemical processes can confuse and substitute caesium with potassium, excess caesium can lead to hypokalemia, heart arrhythmia, arrhythmia, and acute cardiac arrest, but such amounts would not ordinarily be encountered in natural sources.
The median lethal dose (LD50) for caesium chloride in mice is 2.3 g per kilogram, which is comparable to the LD50 values of potassium chloride and
Nonradioactive caesium compounds are only mildly toxic, and nonradioactive caesium is not a significant environmental hazard. Because biochemical processes can confuse and substitute caesium with potassium, excess caesium can lead to hypokalemia, heart arrhythmia, arrhythmia, and acute cardiac arrest, but such amounts would not ordinarily be encountered in natural sources.
The median lethal dose (LD50) for caesium chloride in mice is 2.3 g per kilogram, which is comparable to the LD50 values of potassium chloride and
Caesium or Cesium
at ''The Periodic Table of Videos'' (University of Nottingham)
View the reaction of Caesium (most reactive metal in the periodic table) with Fluorine (most reactive non-metal)
courtesy of The Royal Institution. * {{Authority control Caesium, Alkali metals Chemical elements with body-centered cubic structure Chemical elements Glycine receptor agonists Reducing agents Articles containing video clips
American English
American English, sometimes called United States English or U.S. English, is the set of varieties of the English language native to the United States. English is the most widely spoken language in the United States and in most circumstances ...
) is a chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
with the symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typi ...
s that are liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, a ...
at or near room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
. Caesium has physical and chemical properties similar to those of rubidium
Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher ...
and potassium. It is pyrophoric and reacts with water even at . It is the least electronegative element, with a value of 0.79 on the Pauling scale
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and ...
. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. The element has 40 known isotopes, making it, along with barium and mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
, one of the elements with the most isotopes. Caesium-137, a fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
, is extracted from waste produced by nuclear reactors
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nu ...
.
The German chemist Robert Bunsen and physicist Gustav Kirchhoff discovered caesium in 1860 by the newly developed method of flame spectroscopy. The first small-scale applications for caesium were as a " getter" in vacuum tubes and in photoelectric cells
A solar cell, or photovoltaic cell, is an electronic device that converts the energy of light directly into electricity by the photovoltaic effect, which is a physical and chemical phenomenon.
. In 1967, acting on Einstein's proof that the speed of light is the most-constant dimension in the universe, the International System of Units
The International System of Units, known by the international abbreviation SI in all languages and sometimes pleonastically as the SI system, is the modern form of the metric system and the world's most widely used system of measurement. ...
used two specific wave counts from an emission spectrum
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a atomic electron transition, transition from a high energy state to a lower energy st ...
of caesium-133 to co-define the second
The second (symbol: s) is the unit of time in the International System of Units (SI), historically defined as of a day – this factor derived from the division of the day first into 24 hours, then to 60 minutes and finally to 60 seconds ...
and the metre. Since then, caesium has been widely used in highly accurate atomic clock
An atomic clock is a clock that measures time by monitoring the resonant frequency of atoms. It is based on atoms having different energy levels. Electron states in an atom are associated with different energy levels, and in transitions betw ...
s.
Since the 1990s, the largest application of the element has been as caesium formate
Formate ( IUPAC name: methanoate) is the conjugate base of formic acid. Formate is an anion () or its derivatives such as ester of formic acid. The salts and esters are generally colorless.Werner Reutemann and Heinz Kieczka "Formic Acid" in ' ...
for drilling fluids, but it has a range of applications in the production of electricity, in electronics, and in chemistry. The radioactive isotope caesium-137 has a half-life of about 30 years and is used in medical applications, industrial gauges, and hydrology. Nonradioactive caesium compounds are only mildly toxic, but the pure metal's tendency to react explosively with water means that caesium is considered a hazardous material, and the radioisotopes present a significant health and ecological hazard in the environment.
Characteristics
Physical properties
room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
. Mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
is the only stable elemental metal with a known melting point lower than caesium. In addition, the metal has a rather low boiling point, , the lowest
Low or LOW or lows, may refer to:
People
* Low (surname), listing people surnamed Low
Places
* Low, Quebec, Canada
* Low, Utah, United States
* Lo Wu station (MTR code LOW), Hong Kong; a rail station
* Salzburg Airport (ICAO airport code: LOW ...
of all metals other than mercury. Its compounds burn with a blue or violet colour.
 Caesium forms
Caesium forms alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductilit ...
s with the other alkali metals, gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile ...
, and mercury ( amalgams). At temperatures below , it does not alloy with cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, ...
, iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
, molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lea ...
, nickel, platinum, tantalum, or tungsten. It forms well-defined intermetallic compounds with antimony, gallium
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group (aluminiu ...
, indium, and thorium, which are photosensitive. It mixes with all the other alkali metals (except lithium); the alloy with a molar distribution of 41% caesium, 47% potassium, and 12% sodium has the lowest melting point of any known metal alloy, at . A few amalgams have been studied: is black with a purple metallic lustre, while CsHg is golden-coloured, also with a metallic lustre.
The golden colour of caesium comes from the decreasing frequency of light required to excite electrons of the alkali metals as the group is descended. For lithium through rubidium this frequency is in the ultraviolet, but for caesium it enters the blue–violet end of the spectrum; in other words, the plasmonic frequency of the alkali metals becomes lower from lithium to caesium. Thus caesium transmits and partially absorbs violet light preferentially while other colours (having lower frequency) are reflected; hence it appears yellowish.
Chemical properties
Caesium metal is highly reactive and very pyrophoric. It ignites spontaneously in air, and reacts explosively with water even at low temperatures, more so than the other alkali metals (first group
FirstGroup plc is a British multi-national transport group, based in Aberdeen, Scotland.periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
). It reacts with ice at temperatures as low as . Because of this high reactivity, caesium metal is classified as a hazardous material
Dangerous goods, abbreviated DG, are substances that when transported are a risk to health, safety, property or the environment. Certain dangerous goods that pose risks even when not being transported are known as hazardous materials ( syllabi ...
. It is stored and shipped in dry, saturated hydrocarbons such as mineral oil. It can be handled only under inert gas, such as argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as a ...
. However, a caesium-water explosion is often less powerful than a sodium-water explosion with a similar amount of sodium. This is because caesium explodes instantly upon contact with water, leaving little time for hydrogen to accumulate. Caesium can be stored in vacuum-sealed borosilicate glass ampoules. In quantities of more than about , caesium is shipped in hermetically sealed, stainless steel containers.
The chemistry of caesium is similar to that of other alkali metals, in particular rubidium
Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher ...
, the element above caesium in the periodic table. As expected for an alkali metal, the only common oxidation state is +1. Some slight differences arise from the fact that it has a higher atomic mass and is more electropositive than other (nonradioactive) alkali metals. Caesium is the most electropositive chemical element. The caesium ion is also larger and less "hard" than those of the lighter alkali metals.
Compounds
 Most caesium compounds contain the element as the cation , which binds ionically to a wide variety of anions. One noteworthy exception is the caeside anion (), and others are the several suboxides (see section on oxides below). More recently, caesium is predicted to behave as a p-block element and capable of forming higher fluorides with higher oxidation states (i.e., CsFn with n > 1) under high pressure. This prediction needs to be validated by further experiments.
Salts of Cs+ are usually colourless unless the anion itself is coloured. Many of the simple salts are hygroscopic, but less so than the corresponding salts of lighter alkali metals. The phosphate, acetate, carbonate,
Most caesium compounds contain the element as the cation , which binds ionically to a wide variety of anions. One noteworthy exception is the caeside anion (), and others are the several suboxides (see section on oxides below). More recently, caesium is predicted to behave as a p-block element and capable of forming higher fluorides with higher oxidation states (i.e., CsFn with n > 1) under high pressure. This prediction needs to be validated by further experiments.
Salts of Cs+ are usually colourless unless the anion itself is coloured. Many of the simple salts are hygroscopic, but less so than the corresponding salts of lighter alkali metals. The phosphate, acetate, carbonate, halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
s, oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
, nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
, and sulfate salts are water-soluble. Its double salts are often less soluble, and the low solubility of caesium aluminium sulfate is exploited in refining Cs from ores. The double salts with antimony (such as ), bismuth, cadmium, copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish ...
, iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
, and lead are also poorly soluble.
Caesium hydroxide (CsOH) is hygroscopic and strongly basic
BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Thomas E. Kurtz at Dartmouth College ...
. It rapidly etches the surface of semiconductors such as silicon. CsOH has been previously regarded by chemists as the "strongest base", reflecting the relatively weak attraction between the large Cs+ ion and OH−; it is indeed the strongest Arrhenius base; however, a number of compounds such as ''n''-butyllithium, sodium amide, sodium hydride, caesium hydride, etc., which cannot be dissolved in water as reacting violently with it but rather only used in some anhydrous polar aprotic solvents A polar aprotic solvent is a solvent that lacks an acidic proton and is polar. Such solvents lack hydroxyl and amine groups. In contrast to protic solvents, these solvents do not serve as proton donors in hydrogen bonding
In chemistry, a hydro ...
, are far more basic on the basis of the Brønsted–Lowry acid–base theory.
A stoichiometric mixture of caesium and gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile ...
will react to form yellow caesium auride (Cs+Au−) upon heating. The auride anion here behaves as a pseudohalogen. The compound reacts violently with water, yielding caesium hydroxide, metallic gold, and hydrogen gas; in liquid ammonia it can be reacted with a caesium-specific ion exchange resin to produce tetramethylammonium auride. The analogous platinum compound, red caesium platinide (Cs2Pt), contains the platinide ion that behaves as a pseudo chalcogen.
Complexes
Like all metal cations, Cs+ forms complexes withLewis base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
s in solution. Because of its large size, Cs+ usually adopts coordination numbers greater than 6, the number typical for the smaller alkali metal cations. This difference is apparent in the 8-coordination of CsCl. This high coordination number and softness (tendency to form covalent bonds) are properties exploited in separating Cs+ from other cations in the remediation of nuclear wastes, where 137Cs+ must be separated from large amounts of nonradioactive K+.
Halides
 Caesium fluoride (CsF) is a hygroscopic white solid that is widely used in
Caesium fluoride (CsF) is a hygroscopic white solid that is widely used in organofluorine chemistry
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from Lipophobicity, oil and hydrophobe, water repellents ...
as a source of fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typ ...
anions. Caesium fluoride has the halite structure, which means that the Cs+ and F− pack in a cubic closest packed
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement (or lattice). Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occu ...
array as do Na+ and Cl− in sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
. Notably, caesium and fluorine have the lowest and highest electronegativities, respectively, among all the known elements.
Caesium chloride (CsCl) crystallizes in the simple cubic crystal system. Also called the "caesium chloride structure", this structural motif is composed of a primitive
Primitive may refer to:
Mathematics
* Primitive element (field theory)
* Primitive element (finite field)
* Primitive cell (crystallography)
* Primitive notion, axiomatic systems
* Primitive polynomial (disambiguation), one of two concepts
* Pr ...
cubic lattice with a two-atom basis, each with an eightfold coordination
Coordination may refer to:
* Coordination (linguistics), a compound grammatical construction
* Coordination complex, consisting of a central atom or ion and a surrounding array of bound molecules or ions
* Coordination number or ligancy of a centr ...
; the chloride atoms lie upon the lattice points at the edges of the cube, while the caesium atoms lie in the holes in the centre of the cubes. This structure is shared with CsBr and CsI, and many other compounds that do not contain Cs. In contrast, most other alkaline halides have the sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
(NaCl) structure. The CsCl structure is preferred because Cs+ has an ionic radius of 174 pm and 181 pm.
Oxides
 More so than the other alkali metals, caesium forms numerous binary compounds with oxygen. When caesium burns in air, the superoxide is the main product. The "normal" caesium oxide () forms yellow-orange hexagonal crystals, and is the only oxide of the anti- type. It vaporizes at , and decomposes to caesium metal and the peroxide at temperatures above . In addition to the superoxide and the ozonide , several brightly coloured suboxides have also been studied. These include , , , (dark-green), CsO, , as well as . The latter may be heated in a vacuum to generate . Binary compounds with
More so than the other alkali metals, caesium forms numerous binary compounds with oxygen. When caesium burns in air, the superoxide is the main product. The "normal" caesium oxide () forms yellow-orange hexagonal crystals, and is the only oxide of the anti- type. It vaporizes at , and decomposes to caesium metal and the peroxide at temperatures above . In addition to the superoxide and the ozonide , several brightly coloured suboxides have also been studied. These include , , , (dark-green), CsO, , as well as . The latter may be heated in a vacuum to generate . Binary compounds with sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
, selenium, and tellurium also exist.
Isotopes
Caesium has 40 known isotopes, ranging in mass number (i.e. number ofnucleon
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number (nucleon number).
Until the 1960s, nucleons w ...
s in the nucleus) from 112 to 151. Several of these are synthesized from lighter elements by the slow neutron capture process (S-process
The slow neutron-capture process, or ''s''-process, is a series of reactions in nuclear astrophysics that occur in stars, particularly asymptotic giant branch stars. The ''s''-process is responsible for the creation (nucleosynthesis) of approximat ...
) inside old stars and by the R-process
In nuclear astrophysics, the rapid neutron-capture process, also known as the ''r''-process, is a set of nuclear reactions that is responsible for the creation of approximately half of the atomic nuclei heavier than iron, the "heavy elements", ...
in supernova
A supernova is a powerful and luminous explosion of a star. It has the plural form supernovae or supernovas, and is abbreviated SN or SNe. This transient astronomical event occurs during the last evolutionary stages of a massive star or when ...
explosions. The only stable
A stable is a building in which livestock, especially horses, are kept. It most commonly means a building that is divided into separate stalls for individual animals and livestock. There are many different types of stables in use today; the ...
caesium isotope is 133Cs, with 78 neutrons. Although it has a large nuclear spin
In atomic physics, the spin quantum number is a quantum number (designated ) which describes the intrinsic angular momentum (or spin angular momentum, or simply spin) of an electron or other particle. The phrase was originally used to describe th ...
(+), nuclear magnetic resonance studies can use this isotope at a resonating frequency of 11.7 MHz.
long-lived fission product
Long-lived fission products (LLFPs) are radioactive materials with a long half-life (more than 200,000 years) produced by nuclear fission of uranium and plutonium. Because of their persistent radiotoxicity it is necessary to isolate them from man ...
s of uranium produced in nuclear reactors
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nu ...
. However, this fission product yield is reduced in most reactors because the predecessor, 135Xe, is a potent neutron poison and frequently transmutes to stable 136Xe before it can decay to 135Cs.
The beta decay from 137Cs to 137mBa is a strong emission of gamma radiation
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically s ...
. 137Cs and 90Sr are the principal medium-lived products of nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radio ...
, and the prime sources of radioactivity from spent nuclear fuel after several years of cooling, lasting several hundred years. Those two isotopes are the largest source of residual radioactivity in the area of the Chernobyl disaster. Because of the low capture rate, disposing of 137Cs through neutron capture is not feasible and the only current solution is to allow it to decay over time.
Almost all caesium produced from nuclear fission comes from the beta decay of originally more neutron-rich fission products, passing through various isotopes of iodine and xenon. Because iodine and xenon are volatile and can diffuse through nuclear fuel or air, radioactive caesium is often created far from the original site of fission. With nuclear weapons testing in the 1950s through the 1980s, 137Cs was released into the atmosphere and returned to the surface of the earth as a component of radioactive fallout. It is a ready marker of the movement of soil and sediment from those times.
Occurrence
 Caesium is a relatively rare element, estimated to average 3 parts per million in the
Caesium is a relatively rare element, estimated to average 3 parts per million in the Earth's crust
Earth's crust is Earth's thin outer shell of rock, referring to less than 1% of Earth's radius and volume. It is the top component of the lithosphere, a division of Earth's layers that includes the crust and the upper part of the mantle. The ...
. It is the 45th most abundant element and the 36th among the metals. Nevertheless, it is more abundant than such elements as antimony, cadmium, tin, and tungsten, and two orders of magnitude more abundant than mercury and silver; it is 3.3% as abundant as rubidium
Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher ...
, with which it is closely associated, chemically.
Due to its large ionic radius, caesium is one of the " incompatible elements". During magma crystallization
Magma () is the molten or semi-molten natural material from which all igneous rocks are formed. Magma is found beneath the surface of the Earth, and evidence of magmatism has also been discovered on other terrestrial planets and some natur ...
, caesium is concentrated in the liquid phase and crystallizes last. Therefore, the largest deposits of caesium are zone pegmatite
A pegmatite is an igneous rock showing a very coarse texture, with large interlocking crystals usually greater in size than and sometimes greater than . Most pegmatites are composed of quartz, feldspar, and mica, having a similar silicic com ...
ore bodies formed by this enrichment process. Because caesium does not substitute for potassium as readily as rubidium does, the alkali evaporite minerals sylvite
Sylvite, or sylvine, is potassium chloride (KCl) in natural mineral form. It forms crystals in the isometric system very similar to normal rock salt, halite ( NaCl). The two are, in fact, isomorphous. Sylvite is colorless to white with shades of ...
(KCl) and carnallite () may contain only 0.002% caesium. Consequently, caesium is found in few minerals. Percentage amounts of caesium may be found in beryl
Beryl ( ) is a mineral composed of beryllium aluminium silicate with the chemical formula Be3Al2Si6O18. Well-known varieties of beryl include emerald and aquamarine. Naturally occurring, hexagonal crystals of beryl can be up to several ...
() and avogadrite
Avogadrite ((K,Cs)BF4) is a potassium- caesium tetrafluoroborate in the halide class. Avogadrite crystallizes in the orthorhombic system (space group ''Pnma'') with cell parameters ''a'' 8.66 Å, ''b'' 5.48 Å and ''c'' Å 7.03.
History
The min ...
(), up to 15 wt% Cs2O in the closely related mineral pezzottaite (), up to 8.4 wt% Cs2O in the rare mineral londonite
The borate minerals are minerals which contain a borate anion group. The borate (BO3) units may be polymerised similar to the SiO4 unit of the silicate mineral class. This results in B2O5, B3O6, B2O4 anions as well as more complex structures whic ...
(), and less in the more widespread rhodizite. The only economically important ore for caesium is pollucite , which is found in a few places around the world in zoned pegmatites, associated with the more commercially important lithium minerals, lepidolite and petalite. Within the pegmatites, the large grain size and the strong separation of the minerals results in high-grade ore for mining.
The world's most significant and richest known source of caesium is the Tanco Mine at Bernic Lake in Manitoba
, image_map = Manitoba in Canada 2.svg
, map_alt = Map showing Manitoba's location in the centre of Southern Canada
, Label_map = yes
, coordinates =
, capital = Win ...
, Canada, estimated to contain 350,000 metric tons
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United States ...
of pollucite ore, representing more than two-thirds of the world's reserve base. Although the stoichiometric content of caesium in pollucite is 42.6%, pure pollucite samples from this deposit contain only about 34% caesium, while the average content is 24 wt%. Commercial pollucite contains more than 19% caesium. The Bikita pegmatite deposit in Zimbabwe is mined for its petalite, but it also contains a significant amount of pollucite. Another notable source of pollucite is in the Karibib Desert, Namibia. At the present rate of world mine production of 5 to 10 metric tons per year, reserves will last for thousands of years.
Production
Mining and refining pollucite ore is a selective process and is conducted on a smaller scale than for most other metals. The ore is crushed, hand-sorted, but not usually concentrated, and then ground. Caesium is then extracted from pollucite primarily by three methods: acid digestion, alkaline decomposition, and direct reduction. In the acid digestion, thesilicate
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is al ...
pollucite rock is dissolved with strong acids, such as hydrochloric (HCl), sulfuric
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
(), hydrobromic acid, hydrobromic (HBr), or hydrofluoric acid, hydrofluoric (HF) acids. With hydrochloric acid, a mixture of soluble chlorides is produced, and the insoluble chloride double salts of caesium are precipitated as caesium antimony chloride (), caesium iodine chloride (), or caesium hexachlorocerate (). After separation, the pure precipitated double salt is decomposed, and pure CsCl is precipitated by evaporating the water.
The sulfuric acid method yields the insoluble double salt directly as caesium alum (). The aluminium sulfate component is converted to insoluble aluminium oxide by roasting the alum with carbon, and the resulting product is leaching (metallurgy), leached with water to yield a solution.
Roasting pollucite with calcium carbonate and calcium chloride yields insoluble calcium silicates and soluble caesium chloride. Leaching with water or dilute ammonia () yields a dilute chloride (CsCl) solution. This solution can be evaporated to produce caesium chloride or transformed into caesium alum or caesium carbonate. Though not commercially feasible, the ore can be directly reduced with potassium, sodium, or calcium in vacuum to produce caesium metal directly.
Most of the mined caesium (as salts) is directly converted into formate, caesium formate (HCOO−Cs+) for applications such as oil drilling. To supply the developing market, Cabot Corporation built a production plant in 1997 at the Tanco mine near Bernic Lake in Manitoba
, image_map = Manitoba in Canada 2.svg
, map_alt = Map showing Manitoba's location in the centre of Southern Canada
, Label_map = yes
, coordinates =
, capital = Win ...
, with a capacity of per year of caesium formate solution. The primary smaller-scale commercial compounds of caesium are caesium chloride and caesium nitrate, nitrate.
Alternatively, caesium metal may be obtained from the purified compounds derived from the ore. Caesium chloride and the other caesium halides can be reduced at with calcium or barium, and caesium metal distilled from the result. In the same way, the aluminate, carbonate, or hydroxide may be reduced by magnesium.
The metal can also be isolated by electrolysis of fused caesium cyanide (CsCN). Exceptionally pure and gas-free caesium can be produced by thermal decomposition of caesium azide , which can be produced from aqueous caesium sulfate and barium azide. In vacuum applications, caesium dichromate can be reacted with zirconium to produce pure caesium metal without other gaseous products.
: + 2 → 2 + 2 +
The price of 99.8% pure caesium (metal basis) in 2009 was about , but the compounds are significantly cheaper.
History
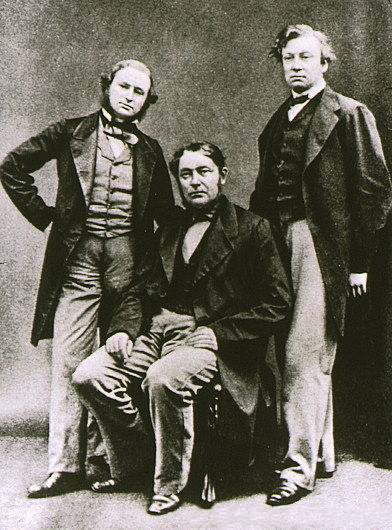 In 1860, Robert Bunsen and Gustav Kirchhoff discovered caesium in the mineral water from Bad Dürkheim, Dürkheim, Germany. Because of the bright blue lines in the
In 1860, Robert Bunsen and Gustav Kirchhoff discovered caesium in the mineral water from Bad Dürkheim, Dürkheim, Germany. Because of the bright blue lines in the emission spectrum
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a atomic electron transition, transition from a high energy state to a lower energy st ...
, they derived the name from the Latin word ''caesius'', meaning sky-blue.Bunsen quotes Aulus Gellius, Aulus Gellius Noctes Atticae II, 26 by Nigidius Figulus: ''Nostris autem veteribus caesia dicts est quae Graecis, ut Nigidus ait, de colore coeli quasi coelia.'' Caesium was the first element to be discovered with a spectroscopy, spectroscope, which had been invented by Bunsen and Kirchhoff only a year previously.
To obtain a pure sample of caesium, of mineral water had to be evaporated to yield of concentrated salt solution. The alkaline earth metals were precipitated either as sulfates or oxalates, leaving the alkali metal in the solution. After conversion to the nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
s and extraction with ethanol, a sodium-free mixture was obtained. From this mixture, the lithium was precipitated by ammonium carbonate. Potassium, rubidium, and caesium form insoluble salts with chloroplatinic acid, but these salts show a slight difference in solubility in hot water, and the less-soluble caesium and rubidium hexachloroplatinate ((Cs,Rb)2PtCl6) were obtained by fractional crystallization (chemistry), fractional crystallization. After reduction of the hexachloroplatinate with hydrogen, caesium and rubidium were separated by the difference in solubility of their carbonates in alcohol. The process yielded of rubidium chloride and of caesium chloride from the initial 44,000 litres of mineral water.
From the caesium chloride, the two scientists estimated the atomic weight of the new element at 123.35 (compared to the currently accepted one of 132.9). They tried to generate elemental caesium by electrolysis of molten caesium chloride, but instead of a metal, they obtained a blue homogeneous substance which "neither under the naked eye nor under the microscope showed the slightest trace of metallic substance"; as a result, they assigned it as a non-stoichiometric compound, subchloride (). In reality, the product was probably a colloidal mixture of the metal and caesium chloride. The electrolysis of the aqueous solution of chloride with a mercury cathode produced a caesium amalgam which readily decomposed under the aqueous conditions. The pure metal was eventually isolated by the Swedish chemist Carl Setterberg while working on his doctorate with Friedrich August Kekulé von Stradonitz, Kekulé and Bunsen. In 1882, he produced caesium metal by electrolysing caesium cyanide, avoiding the problems with the chloride.
Historically, the most important use for caesium has been in research and development, primarily in chemical and electrical fields. Very few applications existed for caesium until the 1920s, when it came into use in radio vacuum tubes, where it had two functions; as a getter, it removed excess oxygen after manufacture, and as a coating on the heated cathode, it increased the electrical conductivity. Caesium was not recognized as a high-performance industrial metal until the 1950s. Applications for nonradioactive caesium included photoelectric cells
A solar cell, or photovoltaic cell, is an electronic device that converts the energy of light directly into electricity by the photovoltaic effect, which is a physical and chemical phenomenon.
, photomultiplier tubes, optical components of infrared spectroscopy, infrared spectrophotometers, catalysts for several organic reactions, crystals for scintillation counters, and in MHD generator, magnetohydrodynamic power generators. Caesium is also used as a source of positive ions in secondary ion mass spectrometry (SIMS).
Since 1967, the International System of Units, International System of Measurements has based the primary unit of time, the second, on the properties of caesium. The International System of Units (SI) defines the second as the duration of 9,192,631,770 cycles at the microwave frequency of the spectral line corresponding to the transition between two hyperfine structure, hyperfine energy levels of the ground state of caesium-133. The 13th General Conference on Weights and Measures of 1967 defined a second as: "the duration of 9,192,631,770 cycles of microwave light absorbed or emitted by the hyperfine transition of caesium-133 atoms in their ground state undisturbed by external fields".
Applications
Petroleum exploration
The largest present-day use of nonradioactive caesium is in formate, caesium formate drilling fluids for the extractive oil industry. Aqueous solutions of caesium formate (HCOO−Cs+)—made by reacting caesium hydroxide with formic acid—were developed in the mid-1990s for use as oil well drilling and completion (oil and gas wells), completion fluids. The function of a drilling fluid is to lubricate drill bits, to bring rock cuttings to the surface, and to maintain pressure on the formation during drilling of the well. Completion fluids assist the emplacement of control hardware after drilling but prior to production by maintaining the pressure. The high density of the caesium formate brine (up to 2.3 g/cm3, or 19.2 pounds per gallon), coupled with the relatively benign nature of most caesium compounds, reduces the requirement for toxic high-density suspended solids in the drilling fluid—a significant technological, engineering and environmental advantage. Unlike the components of many other heavy liquids, caesium formate is relatively environment-friendly. Caesium formate brine can be blended with potassium and sodium formates to decrease the density of the fluids to that of water (1.0 g/cm3, or 8.3 pounds per gallon). Furthermore, it is biodegradable and may be recycled, which is important in view of its high cost (about $4,000 per barrel (volume)#Oil barrel, barrel in 2001). Alkali formates are safe to handle and do not damage the producing formation or downhole metals as corrosive alternative, high-density brines (such as zinc bromide solutions) sometimes do; they also require less cleanup and reduce disposal costs.Atomic clocks
 Caesium-based
Caesium-based atomic clock
An atomic clock is a clock that measures time by monitoring the resonant frequency of atoms. It is based on atoms having different energy levels. Electron states in an atom are associated with different energy levels, and in transitions betw ...
s use the electromagnetic radiation, electromagnetic transitions in the hyperfine structure of caesium-133 atoms as a reference point. The first accurate caesium clock was built by Louis Essen in 1955 at the National Physical Laboratory, UK, National Physical Laboratory in the UK. Caesium clocks have improved over the past half-century and are regarded as "the most accurate realization of a unit that mankind has yet achieved." These clocks measure frequency with an error of 2 to 3 parts in 1014, which corresponds to an accuracy of 2 nanoseconds per day, or one second in 1.4 million years. The latest versions are more accurate than 1 part in 1015, about 1 second in 20 million years. The caesium standard is the primary standard for standards-compliant time and frequency measurements. Caesium clocks regulate the timing of cell phone networks and the Internet.
Definition of the second
The second, symbol ''s'', is the SI unit of time. It is defined by taking the fixed numerical value of the caesium frequency , the unperturbed ground-state hyperfine transition frequency of the caesium-133 atom, to be when expressed in the unit Hz, which is equal to s−1.Electric power and electronics
Caesium vapour thermionic converter, thermionic generators are low-power devices that convert heat energy to electrical energy. In the two-electrode vacuum tube converter, caesium neutralizes the space charge near the cathode and enhances the current flow. Caesium is also important for its photoelectric effect, photoemissive properties, converting light to electron flow. It is used inphotoelectric cells
A solar cell, or photovoltaic cell, is an electronic device that converts the energy of light directly into electricity by the photovoltaic effect, which is a physical and chemical phenomenon.
because caesium-based cathodes, such as the intermetallic compound , have a low threshold voltage for emission of electrons. The range of photoemissive devices using caesium include optical character recognition devices, photomultiplier, photomultiplier tubes, and video camera tubes. Nevertheless, germanium, rubidium, selenium, silicon, tellurium, and several other elements can be substituted for caesium in photosensitive materials.
Caesium iodide (CsI), caesium bromide, bromide (CsBr) and caesium fluoride (CsF) crystals are employed for scintillators in scintillation counters widely used in mineral exploration and particle physics research to detect gamma ray, gamma and X-ray radiation. Being a heavy element, caesium provides good stopping power with better detection. Caesium compounds may provide a faster response (CsF) and be less hygroscopic (CsI).
Caesium vapour is used in many common magnetometers.
The element is used as an internal standard in spectrophotometry. Like other alkali metals, caesium has a great affinity for oxygen and is used as a " getter" in vacuum tubes. Other uses of the metal include high-energy lasers, fluorescent lamp, vapour glow lamps, and vapour rectifiers.
Centrifugation fluids
The high density of the caesium ion makes solutions of caesium chloride, caesium sulfate, and caesium trifluoroacetic acid, trifluoroacetate () useful in molecular biology for density gradient differential centrifugation, ultracentrifugation. This technology is used primarily in the isolation of virus, viral particles, subcellular organelles and fractions, and nucleic acids from biological samples.Chemical and medical use
 Relatively few chemical applications use caesium. Doping with caesium compounds enhances the effectiveness of several metal-ion catalysts for chemical synthesis, such as acrylic acid, anthraquinone, ethylene oxide, methanol, phthalic anhydride, styrene, methyl methacrylate monomers, and various alkene, olefins. It is also used in the catalytic conversion of sulfur dioxide into sulfur trioxide in the production of sulfuric acid.
Caesium fluoride enjoys a niche use in organic chemistry as a base (chemistry), base and as an anhydrous source of
Relatively few chemical applications use caesium. Doping with caesium compounds enhances the effectiveness of several metal-ion catalysts for chemical synthesis, such as acrylic acid, anthraquinone, ethylene oxide, methanol, phthalic anhydride, styrene, methyl methacrylate monomers, and various alkene, olefins. It is also used in the catalytic conversion of sulfur dioxide into sulfur trioxide in the production of sulfuric acid.
Caesium fluoride enjoys a niche use in organic chemistry as a base (chemistry), base and as an anhydrous source of fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typ ...
ion. Caesium salts sometimes replace potassium or sodium salts in organic synthesis, such as cyclic compound, cyclization, esterification, and polymerization. Caesium has also been used in thermoluminescent radiation dosimetry (TLD): When exposed to radiation, it acquires crystal defects that, when heated, revert with emission of light proportionate to the received dose. Thus, measuring the light pulse with a photomultiplier tube can allow the accumulated radiation dose to be quantified.
Nuclear and isotope applications
Caesium-137 is a radionuclide, radioisotope commonly used as a gamma ray, gamma-emitter in industrial applications. Its advantages include a half-life of roughly 30 years, its availability from the nuclear fuel cycle, and having Isotopes of barium, 137Ba as a stable end product. The high water solubility is a disadvantage which makes it incompatible with large pool irradiators for food and medical supplies. It has been used in agriculture, cancer treatment, and the sterilization (microbiology), sterilization of food, sewage sludge, and surgical equipment. Radioactive isotopes of caesium in radiation therapy, radiation devices were used in the medical field to treat certain types of cancer, but emergence of better alternatives and the use of water-soluble caesium chloride in the sources, which could create wide-ranging contamination, gradually put some of these caesium sources out of use. Caesium-137 has been employed in a variety of industrial measurement gauges, including moisture, density, levelling, and thickness gauges. It has also been used in well logging devices for measuring the electron density of the rock formations, which is analogous to the bulk density of the formations. Caesium-137 has been used in hydrology, hydrologic studies analogous to those with tritium. As a daughter product of fission bomb testing from the 1950s through the mid-1980s, caesium-137 was released into the atmosphere, where it was absorbed readily into solution. Known year-to-year variation within that period allows correlation with soil and sediment layers. Caesium-134, and to a lesser extent caesium-135, have also been used in hydrology to measure the caesium output by the nuclear power industry. While they are less prevalent than either caesium-133 or caesium-137, these bellwether isotopes are produced solely from anthropogenic sources.Other uses
Health and safety hazards
sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
. The principal use of nonradioactive caesium is as caesium formate in petroleum drilling fluids because it is much less toxic than alternatives, though it is more costly.
Caesium metal is one of the most reactive elements and is highly explosive material, explosive in the presence of water. The hydrogen gas produced by the reaction is heated by the thermal energy released at the same time, causing ignition and a violent explosion. This can occur with other alkali metals, but caesium is so potent that this explosive reaction can be triggered even by cold water.
It is highly pyrophoric: the autoignition temperature of caesium is , and it ignites explosively in air to form caesium hydroxide and various oxides. Caesium hydroxide is a very strong base (chemistry), base, and will rapidly corrode glass.
The isotopes Caesium-134, 134 and 137 are present in the biosphere in small amounts from human activities, differing by location. Radiocaesium does not accumulate in the body as readily as other fission products (such as radioiodine and radiostrontium). About 10% of absorbed radiocaesium washes out of the body relatively quickly in sweat and urine. The remaining 90% has a biological half-life between 50 and 150 days. Radiocaesium follows potassium and tends to accumulate in plant tissues, including fruits and vegetables. Plants vary widely in the absorption of caesium, sometimes displaying great resistance to it. It is also well-documented that mushrooms from contaminated forests accumulate radiocaesium (caesium-137) in the fungal sporocarp (fungi), sporocarps. Accumulation of caesium-137 in lakes has been a great concern after the Chernobyl disaster. Experiments with dogs showed that a single dose of 3.8 curie (unit), millicuries (140 Becquerel, MBq, 4.1 μg of caesium-137) per kilogram is lethal within three weeks; smaller amounts may cause infertility and cancer. The International Atomic Energy Agency and other sources have warned that radioactive materials, such as caesium-137, could be used in radiological dispersion devices, or "dirty bombs".
See also
* * Acerinox accident, a caesium-137 contamination accident in 1998 * Goiânia accident, a major radioactive contamination incident in 1987 involving caesium-137 * Kramatorsk radiological accident, a 137Cs lost-source incident between 1980 and 1989Notes
References
External links
Caesium or Cesium
at ''The Periodic Table of Videos'' (University of Nottingham)
View the reaction of Caesium (most reactive metal in the periodic table) with Fluorine (most reactive non-metal)
courtesy of The Royal Institution. * {{Authority control Caesium, Alkali metals Chemical elements with body-centered cubic structure Chemical elements Glycine receptor agonists Reducing agents Articles containing video clips