Hydrogen Pipeline Size 3600 PSI on:
[Wikipedia]
[Google]
[Amazon]
Hydrogen is the chemical element with the
 The
The

 * Gaseous hydrogen
* Liquid hydrogen
*
* Gaseous hydrogen
* Liquid hydrogen
*
 Compounds of hydrogen are often called hydrides, a term that is used fairly loosely. The term "hydride" suggests that the H atom has acquired a negative or anionic character, denoted , and is used when hydrogen forms a compound with a more electropositive element. The existence of the
Compounds of hydrogen are often called hydrides, a term that is used fairly loosely. The term "hydride" suggests that the H atom has acquired a negative or anionic character, denoted , and is used when hydrogen forms a compound with a more electropositive element. The existence of the


 Hydrogen has three naturally occurring isotopes, denoted , and . Other, highly unstable nuclei ( to ) have been synthesized in the laboratory but not observed in nature.
* is the most common hydrogen isotope, with an abundance of more than 99.98%. Because the
Hydrogen has three naturally occurring isotopes, denoted , and . Other, highly unstable nuclei ( to ) have been synthesized in the laboratory but not observed in nature.
* is the most common hydrogen isotope, with an abundance of more than 99.98%. Because the
 Because of its simple atomic structure, consisting only of a proton and an electron, the
Because of its simple atomic structure, consisting only of a proton and an electron, the
 Hydrogen, as atomic H, is the most abundant chemical element in the universe, making up 75 percent of normal matter by mass and more than 90 percent by number of atoms. (Most of the mass of the universe, however, is not in the form of chemical-element type matter, but rather is postulated to occur as yet-undetected forms of mass such as dark matter and dark energy.) This element is found in great abundance in stars and gas giant planets. Molecular clouds of are associated with
Hydrogen, as atomic H, is the most abundant chemical element in the universe, making up 75 percent of normal matter by mass and more than 90 percent by number of atoms. (Most of the mass of the universe, however, is not in the form of chemical-element type matter, but rather is postulated to occur as yet-undetected forms of mass such as dark matter and dark energy.) This element is found in great abundance in stars and gas giant planets. Molecular clouds of are associated with
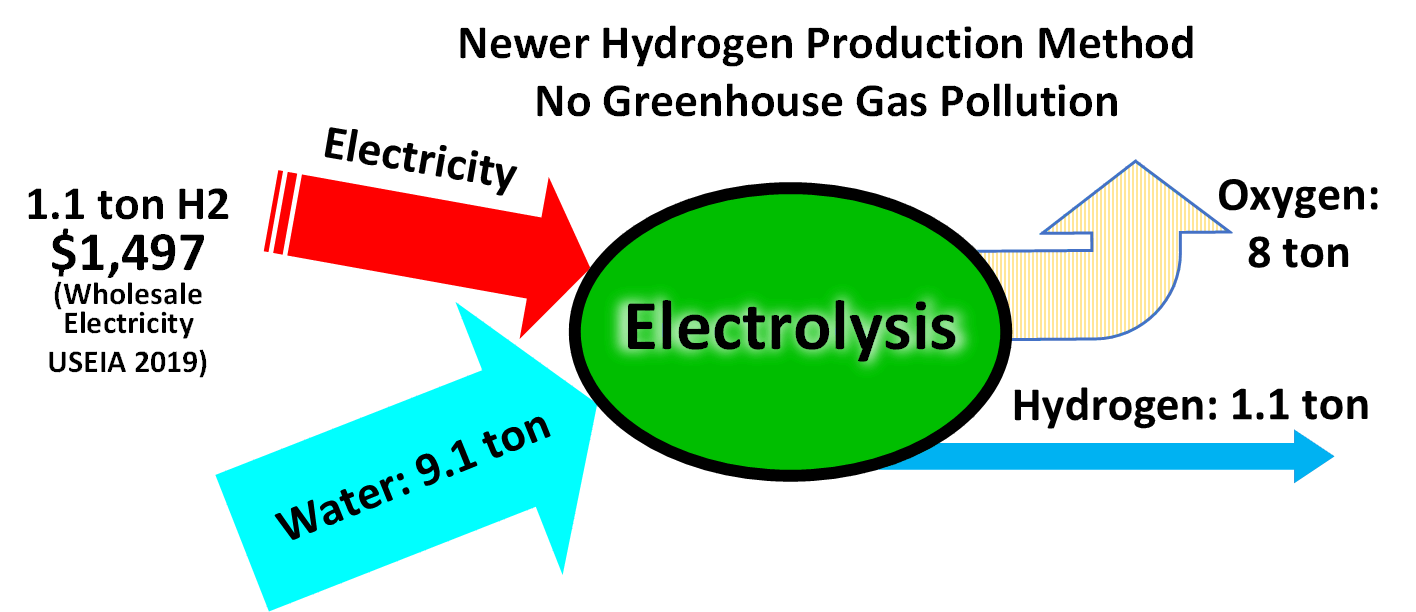 The electrolysis of water is a simple method of producing hydrogen. A current is run through the water, and gaseous oxygen forms at the anode while gaseous hydrogen forms at the cathode. Typically the cathode is made from platinum or another inert metal when producing hydrogen for storage. If, however, the gas is to be burnt on site, oxygen is desirable to assist the combustion, and so both electrodes would be made from inert metals. (Iron, for instance, would oxidize, and thus decrease the amount of oxygen given off.) The theoretical maximum efficiency (electricity used vs. energetic value of hydrogen produced) is in the range 88–94%.
:
The electrolysis of water is a simple method of producing hydrogen. A current is run through the water, and gaseous oxygen forms at the anode while gaseous hydrogen forms at the cathode. Typically the cathode is made from platinum or another inert metal when producing hydrogen for storage. If, however, the gas is to be burnt on site, oxygen is desirable to assist the combustion, and so both electrodes would be made from inert metals. (Iron, for instance, would oxidize, and thus decrease the amount of oxygen given off.) The theoretical maximum efficiency (electricity used vs. energetic value of hydrogen produced) is in the range 88–94%.
:
 Hydrogen production using natural gas methane pyrolysis is a one-step process that produces no
Hydrogen production using natural gas methane pyrolysis is a one-step process that produces no
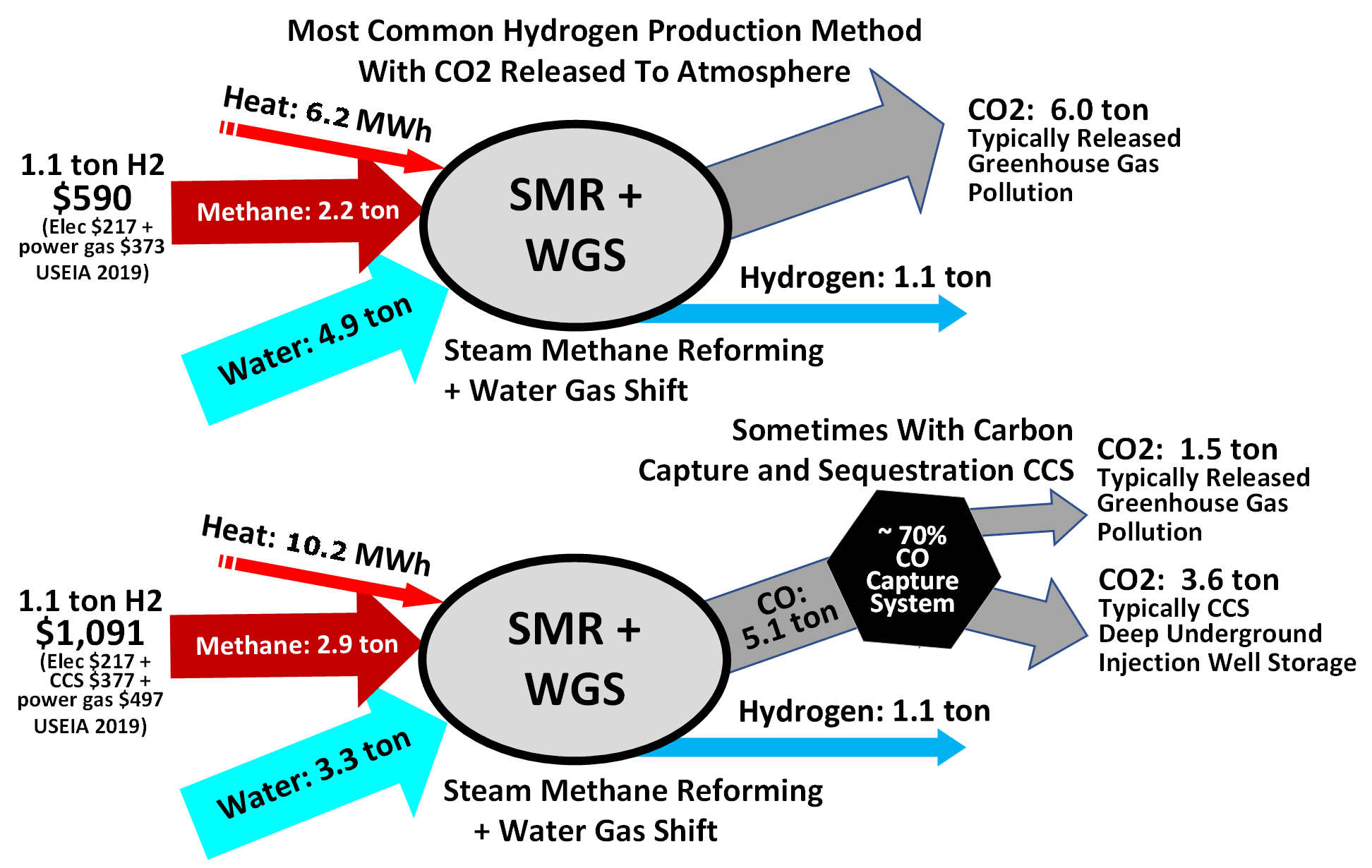 Hydrogen is often produced by reacting water with methane and carbon monoxide, which causes the removal of hydrogen from hydrocarbons at very high temperatures, with 48% of hydrogen production coming from steam reforming. The water vapor is then reacted with the carbon monoxide produced by steam reforming to oxidize it to carbon dioxide and turn the water into hydrogen. Commercial bulk hydrogen is usually produced by the steam reforming of natural gas with release of atmospheric greenhouse gas or with capture using
Hydrogen is often produced by reacting water with methane and carbon monoxide, which causes the removal of hydrogen from hydrocarbons at very high temperatures, with 48% of hydrogen production coming from steam reforming. The water vapor is then reacted with the carbon monoxide produced by steam reforming to oxidize it to carbon dioxide and turn the water into hydrogen. Commercial bulk hydrogen is usually produced by the steam reforming of natural gas with release of atmospheric greenhouse gas or with capture using
''carrier'' of energy rather than an energy resource, because there is no naturally occurring source of hydrogen in useful quantities.
Hydrogen can be burned to produce heat or combined with oxygen in
Basic Hydrogen Calculations of Quantum Mechanics
at '' The Periodic Table of Videos'' (University of Nottingham)
{{Authority control Chemical elements Reactive nonmetals Diatomic nonmetals Nuclear fusion fuels Airship technology Reducing agents Refrigerants Gaseous signaling molecules E-number additives
symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwee ...
. It is colorless, odorless
The sense of smell, or olfaction, is the special sense through which smells (or odors) are perceived. The sense of smell has many functions, including detecting desirable foods, hazards, and pheromones, and plays a role in taste.
In humans, it ...
, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Star
A star is an astronomical object comprising a luminous spheroid of plasma (physics), plasma held together by its gravity. The List of nearest stars and brown dwarfs, nearest star to Earth is the Sun. Many other stars are visible to the naked ...
s such as the Sun are mainly composed of hydrogen in the . Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
, one electron, and no neutrons.
In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang
The Big Bang event is a physical theory that describes how the universe expanded from an initial state of high density and temperature. Various cosmological models of the Big Bang explain the evolution of the observable universe from the ...
. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 years later during the recombination epoch, when the plasma
Plasma or plasm may refer to:
Science
* Plasma (physics), one of the four fundamental states of matter
* Plasma (mineral), a green translucent silica mineral
* Quark–gluon plasma, a state of matter in quantum chromodynamics
Biology
* Blood pla ...
had cooled enough for electrons to remain bound to protons.
Hydrogen is nonmetallic
In chemistry, a nonmetal is a chemical element that generally lacks a predominance of metallic properties; they range from colorless gases (like hydrogen) to shiny solids (like carbon, as graphite). The electrons in nonmetals behave differentl ...
(except it becomes metallic at extremely high pressures) and readily forms a single covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
with most nonmetallic elements, forming compounds such as water and nearly all organic compounds. Hydrogen plays a particularly important role in acid–base reactions because these reactions usually involve the exchange of protons between soluble molecules. In ionic compound
In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negatively charged i ...
s, hydrogen can take the form of a negative charge (i.e., anion) where it is known as a hydride, or as a positively charged (i.e., cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
) species denoted by the symbol . The cation is simply a proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
(symbol p) but its behavior in aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be re ...
s and in ionic compound
In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negatively charged i ...
s involves screening
Screening may refer to:
* Screening cultures, a type a medical test that is done to find an infection
* Screening (economics), a strategy of combating adverse selection (includes sorting resumes to select employees)
* Screening (environmental), a ...
of its electric charge by nearby polar
Polar may refer to:
Geography
Polar may refer to:
* Geographical pole, either of two fixed points on the surface of a rotating body or planet, at 90 degrees from the equator, based on the axis around which a body rotates
* Polar climate, the c ...
molecules or anions. Because hydrogen is the only neutral atom for which the Schrödinger equation can be solved analytically, the study of its energetics and chemical bonding has played a key role in the development of quantum mechanics.
Hydrogen gas was first artificially produced in the early 16th century by the reaction of acids on metals. In 1766–1781, Henry Cavendish was the first to recognize that hydrogen gas was a discrete substance, and that it produces water when burned, the property for which it was later named: in Greek, hydrogen means "water-former".
Industrial production
Industrial production is a measure of output of the industrial sector of the economy. The industrial sector includes manufacturing, mining, and utilities. Although these sectors contribute only a small portion of gross domestic product (GDP), the ...
is mainly from steam reforming of natural gas, oil reforming, or coal gasification Coal gasification is the process of producing syngas—a mixture consisting primarily of carbon monoxide (CO), hydrogen (H2), carbon dioxide (CO2), methane (CH4), and water vapour (H2O)—from coal and water, air and/or oxygen.
Historically, coal ...
. A small percentage is also produced using more energy-intensive methods such as the electrolysis of water. Most hydrogen is used near the site of its production, the two largest uses being fossil fuel
A fossil fuel is a hydrocarbon-containing material formed naturally in the Earth's crust from the remains of dead plants and animals that is extracted and burned as a fuel. The main fossil fuels are coal, oil, and natural gas. Fossil fuels m ...
processing (e.g., hydrocracking
In petrochemistry, petroleum geology and organic chemistry, cracking is the process whereby complex organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of ...
) and ammonia production, mostly for the fertilizer market. It can be burned to produce heat or combined with oxygen in fuel cells
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen fuel, hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most bat ...
to generate electricity directly, with water being the only emissions at the point of usage. Hydrogen atoms (but not gaseous molecules) are problematic in metallurgy
Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys.
Metallurgy encompasses both the sc ...
because they can embrittle many metals.
Properties
Combustion
Hydrogen gas (dihydrogen or molecular hydrogen) is highly flammable: : (572 kJ/2 mol = 286 kJ/mol = 141.865 MJ/kg)286 kJ/mol: energy per mole of the combustible material (molecular hydrogen). Theenthalpy of combustion
The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of heat released during the combustion of a specified amount of it.
The ''calorific value'' is the total energy relea ...
is −286 kJ/mol.
Hydrogen gas forms explosive mixtures with air in concentrations from 4–74% and with chlorine at 5–95%. The explosive reactions may be triggered by spark, heat, or sunlight. The hydrogen autoignition temperature
The autoignition temperature or kindling point of a substance is the lowest temperature in which it spontaneously ignites in a normal atmosphere without an external source of ignition, such as a flame or spark. This temperature is required to su ...
, the temperature of spontaneous ignition in air, is .
Flame
Pure hydrogen-oxygen flames emit ultraviolet light and with high oxygen mix are nearly invisible to the naked eye, as illustrated by the faint plume of theSpace Shuttle Main Engine
The Aerojet Rocketdyne RS-25, also known as the Space Shuttle Main Engine (SSME), is a liquid-fuel cryogenic rocket engine that was used on NASA's Space Shuttle and is currently used on the Space Launch System (SLS).
Designed and manufacture ...
, compared to the highly visible plume of a Space Shuttle Solid Rocket Booster, which uses an ammonium perchlorate composite. The detection of a burning hydrogen leak may require a flame detector; such leaks can be very dangerous. Hydrogen flames in other conditions are blue, resembling blue natural gas flames. The destruction of the Hindenburg airship was a notorious example of hydrogen combustion and the cause is still debated. The visible flames in the photographs were the result of carbon compounds in the airship skin burning.
Reactants
is unreactive compared to diatomic elements such as halogens or oxygen. The thermodynamic basis of this low reactivity is the very strong H–H bond, with a bond dissociation energy of 435.7 kJ/mol. The kinetic basis of the low reactivity is the nonpolar nature of and its weak polarizability. It spontaneously reacts with chlorine andfluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
to form hydrogen chloride and hydrogen fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock i ...
, respectively. The reactivity of is strongly affected by the presence of metal catalysts. Thus, while mixtures of with or air combust readily when heated to at least 500 °C by a spark or flame, they do not react at room temperature in the absence of a catalyst.
Electron energy levels
ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. ...
energy level of the electron in a hydrogen atom is −13.6 eV, which is equivalent to an ultraviolet photon of roughly 91 nm wavelength.
The energy levels of hydrogen can be calculated fairly accurately using the Bohr model of the atom, which conceptualizes the electron as "orbiting" the proton in analogy to the Earth's orbit of the Sun. However, the atomic electron and proton are held together by electromagnetic force, while planets and celestial objects are held by gravity. Because of the discretization of angular momentum postulated in early quantum mechanics by Bohr, the electron in the Bohr model can only occupy certain allowed distances from the proton, and therefore only certain allowed energies.
A more accurate description of the hydrogen atom comes from a purely quantum mechanical treatment that uses the Schrödinger equation, Dirac equation or Feynman
Richard Phillips Feynman (; May 11, 1918 – February 15, 1988) was an American theoretical physicist, known for his work in the path integral formulation of quantum mechanics, the theory of quantum electrodynamics, the physics of the superflu ...
path integral formulation to calculate the probability density of the electron around the proton. The most complicated treatments allow for the small effects of special relativity and vacuum polarization. In the quantum mechanical treatment, the electron in a ground state hydrogen atom has no angular momentum at all—illustrating how the "planetary orbit" differs from electron motion.
Spin isomers
Molecular exists as two spin isomers, i.e. compounds that differ only in the spin states of their nuclei. In the orthohydrogen form, the spins of the two nuclei are parallel, forming a spin triplet state having a total molecular spin ; in the parahydrogen form the spins are antiparallel and form a spin singlet state having spin . The equilibrium ratio of ortho- to para-hydrogen depends on temperature. At room temperature or warmer, equilibrium hydrogen gas contains about 25% of the para form and 75% of the ortho form. The ortho form is anexcited state
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to a ...
, having higher energy than the para form by 1.455 kJ/mol, and it converts to the para form over the course of several minutes when cooled to low temperature. The thermal properties of the forms differ because they differ in their allowed rotational quantum states, resulting in different thermal properties such as the heat capacity.
The ortho-to-para ratio in is an important consideration in the liquefaction
In materials science, liquefaction is a process that generates a liquid from a solid or a gas or that generates a non-liquid phase which behaves in accordance with fluid dynamics.
It occurs both naturally and artificially. As an example of the ...
and storage of liquid hydrogen: the conversion from ortho to para is exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
and produces enough heat to evaporate a most of the liquid if not converted first to parahydrogen during the cooling process. Catalysts for the ortho-para interconversion, such as ferric oxide
Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturally a ...
and activated carbon
Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that increase the surface area avail ...
compounds, are used during hydrogen cooling to avoid this loss of liquid.
Phases

 * Gaseous hydrogen
* Liquid hydrogen
*
* Gaseous hydrogen
* Liquid hydrogen
* Slush hydrogen Slush hydrogen is a combination of liquid hydrogen and solid hydrogen at the triple point with a lower temperature and a higher density than liquid hydrogen. It is commonly formed by repeating a freeze-thaw process. This is most easily done by bring ...
* Solid hydrogen
* Metallic hydrogen
* Plasma
Plasma or plasm may refer to:
Science
* Plasma (physics), one of the four fundamental states of matter
* Plasma (mineral), a green translucent silica mineral
* Quark–gluon plasma, a state of matter in quantum chromodynamics
Biology
* Blood pla ...
hydrogen
Compounds
Covalent and organic compounds
While is not very reactive under standard conditions, it does form compounds with most elements. Hydrogen can form compounds with elements that are more electronegative, such ashalogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
s (F, Cl, Br, I), or oxygen; in these compounds hydrogen takes on a partial positive charge. When bonded to a more electronegative element, particularly fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
, oxygen, or nitrogen, hydrogen can participate in a form of medium-strength noncovalent bonding with another electronegative element with a lone pair, a phenomenon called hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
ing that is critical to the stability of many biological molecules. Hydrogen also forms compounds with less electronegative elements, such as metals and metalloid
A metalloid is a type of chemical element which has a preponderance of material property, properties in between, or that are a mixture of, those of metals and nonmetals. There is no standard definition of a metalloid and no complete agreement on ...
s, where it takes on a partial negative charge. These compounds are often known as hydrides.
Hydrogen forms a vast array of compounds with carbon called the hydrocarbons, and an even vaster array with heteroatoms
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is usually used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecula ...
that, because of their general association with living things, are called organic compounds. The study of their properties is known as organic chemistry and their study in the context of living organisms is known as biochemistry. By some definitions, "organic" compounds are only required to contain carbon. However, most of them also contain hydrogen, and because it is the carbon-hydrogen bond that gives this class of compounds most of its particular chemical characteristics, carbon-hydrogen bonds are required in some definitions of the word "organic" in chemistry. Millions of hydrocarbons are known, and they are usually formed by complicated pathways that seldom involve elemental hydrogen.
Hydrogen is highly soluble in many rare earth and transition metals and is soluble in both nanocrystalline and amorphous metals. Hydrogen solubility in metals is influenced by local distortions or impurities in the crystal lattice
In geometry and crystallography, a Bravais lattice, named after , is an infinite array of discrete points generated by a set of discrete translation operations described in three dimensional space by
: \mathbf = n_1 \mathbf_1 + n_2 \mathbf_2 + n ...
. These properties may be useful when hydrogen is purified by passage through hot palladium disks, but the gas's high solubility is a metallurgical problem, contributing to the embrittlement of many metals, complicating the design of pipelines and storage tanks.
Hydrides
 Compounds of hydrogen are often called hydrides, a term that is used fairly loosely. The term "hydride" suggests that the H atom has acquired a negative or anionic character, denoted , and is used when hydrogen forms a compound with a more electropositive element. The existence of the
Compounds of hydrogen are often called hydrides, a term that is used fairly loosely. The term "hydride" suggests that the H atom has acquired a negative or anionic character, denoted , and is used when hydrogen forms a compound with a more electropositive element. The existence of the hydride anion
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of ...
, suggested by Gilbert N. Lewis
Gilbert Newton Lewis (October 23 or October 25, 1875 – March 23, 1946) was an American physical chemist and a Dean of the College of Chemistry at University of California, Berkeley. Lewis was best known for his discovery of the covalent bond a ...
in 1916 for group 1 and 2 salt-like hydrides, was demonstrated by Moers in 1920 by the electrolysis of molten lithium hydride (LiH), producing a stoichiometric quantity of hydrogen at the anode. For hydrides other than group 1 and 2 metals, the term is quite misleading, considering the low electronegativity of hydrogen. An exception in group 2 hydrides is , which is polymeric. In lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li Al H4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic ...
, the anion carries hydridic centers firmly attached to the Al(III).
Although hydrides can be formed with almost all main-group elements, the number and combination of possible compounds varies widely; for example, more than 100 binary borane hydrides are known, but only one binary aluminium hydride. Binary indium hydride has not yet been identified, although larger complexes exist.
In inorganic chemistry
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disci ...
, hydrides can also serve as bridging ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually r ...
s that link two metal centers in a coordination complex. This function is particularly common in group 13 element
The Group 13 network ( pl, Trzynastka, Yiddish: ''דאָס דרײַצענטל'') was a Jewish Nazi collaborationist organization in the Warsaw Ghetto during the German occupation of Poland in World War II. The rise and fall of the Group ...
s, especially in boranes (boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
hydrides) and aluminium complexes, as well as in clustered carborane
Carboranes are electron-delocalized (non-classically bonded) clusters composed of boron, carbon and hydrogen atoms.Grimes, R. N., ''Carboranes 3rd Ed.'', Elsevier, Amsterdam and New York (2016), . Like many of the related boron hydrides, these cl ...
s.
Protons and acids
Oxidation of hydrogen removes its electron and gives , which contains no electrons and anucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
*Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucle ...
which is usually composed of one proton. That is why is often called a proton. This species is central to discussion of acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
s. Under the Brønsted–Lowry acid–base theory, acids are proton donors, while bases are proton acceptors.
A bare proton, , cannot exist in solution or in ionic crystals because of its unstoppable attraction to other atoms or molecules with electrons. Except at the high temperatures associated with plasmas, such protons cannot be removed from the electron clouds of atoms and molecules, and will remain attached to them. However, the term 'proton' is sometimes used loosely and metaphorically to refer to positively charged or cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
ic hydrogen attached to other species in this fashion, and as such is denoted "" without any implication that any single protons exist freely as a species.
To avoid the implication of the naked "solvated proton" in solution, acidic aqueous solutions are sometimes considered to contain a less unlikely fictitious species, termed the " hydronium ion" (). However, even in this case, such solvated hydrogen cations are more realistically conceived as being organized into clusters that form species closer to . Other oxonium ions are found when water is in acidic solution with other solvents.
Although exotic on Earth, one of the most common ions in the universe is the ion, known as protonated molecular hydrogen
The trihydrogen cation or protonated molecular hydrogen is a cation (positive ion) with chemical formula, formula , consisting of three hydrogen nuclei (protons) sharing two electrons.
The trihydrogen cation is one of the most abundant ions in t ...
or the trihydrogen cation.
Isotopes


 Hydrogen has three naturally occurring isotopes, denoted , and . Other, highly unstable nuclei ( to ) have been synthesized in the laboratory but not observed in nature.
* is the most common hydrogen isotope, with an abundance of more than 99.98%. Because the
Hydrogen has three naturally occurring isotopes, denoted , and . Other, highly unstable nuclei ( to ) have been synthesized in the laboratory but not observed in nature.
* is the most common hydrogen isotope, with an abundance of more than 99.98%. Because the nucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
*Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucle ...
of this isotope consists of only a single proton, it is given the descriptive but rarely used formal name '' protium''. It is unique among all stable isotopes in having no neutrons; see diproton for a discussion of why others do not exist.
* , the other stable hydrogen isotope, is known as '' deuterium'' and contains one proton and one neutron in the nucleus. All deuterium in the universe is thought to have been produced at the time of the Big Bang
The Big Bang event is a physical theory that describes how the universe expanded from an initial state of high density and temperature. Various cosmological models of the Big Bang explain the evolution of the observable universe from the ...
, and has endured since that time. Deuterium is not radioactive, and does not represent a significant toxicity hazard. Water enriched in molecules that include deuterium instead of normal hydrogen is called heavy water. Deuterium and its compounds are used as a non-radioactive label in chemical experiments and in solvents for - NMR spectroscopy. Heavy water is used as a neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, ideally without capturing any, leaving them as thermal neutrons with only minimal (thermal) kinetic energy. These thermal neutrons are immensely mo ...
and coolant for nuclear reactors. Deuterium is also a potential fuel for commercial nuclear fusion.
* is known as '' tritium'' and contains one proton and two neutrons in its nucleus. It is radioactive, decaying into helium-3
Helium-3 (3He see also helion) is a light, stable isotope of helium with two protons and one neutron (the most common isotope, helium-4, having two protons and two neutrons in contrast). Other than protium (ordinary hydrogen), helium-3 is the ...
through beta decay with a half-life of 12.32 years. It is so radioactive that it can be used in luminous paint
Luminous paint or luminescent paint is paint that exhibits luminescence. In other words, it gives off Visible spectrum, visible light through fluorescence, phosphorescence, or radioluminescence. There are three types of luminous paints: fluoresc ...
, making it useful in such things as watches. The glass prevents the small amount of radiation from getting out. Small amounts of tritium are produced naturally by the interaction of cosmic rays with atmospheric gases; tritium has also been released during nuclear weapons tests. It is used in nuclear fusion reactions, as a tracer in isotope geochemistry, and in specialized self-powered lighting
Tritium radioluminescence is the use of gaseous tritium, a radioactive isotope of hydrogen, to create visible light. Tritium emits electrons through beta decay and, when they interact with a phosphor material, light is emitted through the proces ...
devices. Tritium has also been used in chemical and biological labeling experiments as a radiolabel.
Unique among the elements, distinct names are assigned to its isotopes in common use today. During the early study of radioactivity, various heavy radioactive isotopes were given their own names, but such names are no longer used, except for deuterium and tritium. The symbols D and T (instead of and ) are sometimes used for deuterium and tritium, but the symbol P is already in use for phosphorus and thus is not available for protium. In its nomenclatural
Nomenclature (, ) is a system of names or terms, or the rules for forming these terms in a particular field of arts or sciences. The principles of naming vary from the relatively informal conventions of everyday speech to the internationally ag ...
guidelines, the International Union of Pure and Applied Chemistry (IUPAC) allows any of D, T, , and to be used, although and are preferred.
The exotic atom muonium
Muonium is an exotic atom made up of an antimuon and an electron,
which was discovered in 1960 by Vernon W. Hughes
and is given the chemical symbol Mu. During the muon's lifetime, muonium can undergo chemical reactions. Due to the mass diffe ...
(symbol Mu), composed of an antimuon and an electron, can also be considered a light radioisotope of hydrogen. Because muons decay with lifetime , muonium is too unstable to exhibit observable chemistry. Nevertheless, muonium compounds are important test cases for quantum simulation
Quantum simulators permit the study of a quantum system in a programmable fashion. In this instance, simulators are special purpose devices designed to provide insight about specific physics problems.
Note: This manuscript is a contribution o ...
, due to the mass difference between the antimuon and the proton, and IUPAC nomenclature incorporates such hypothetical compounds as muonium chloride (MuCl) and sodium muonide (NaMu), analogous to hydrogen chloride and sodium hydride respectively.
Thermal and physical properties
Table of thermal and physical properties of hydrogen (H2) at atmospheric pressure:History
Discovery and use
In 1671, Robert Boyle discovered and described the reaction between iron filings and diluteacid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
s, which results in the production of hydrogen gas.
In 1766, Henry Cavendish was the first to recognize hydrogen gas as a discrete substance, by naming the gas from a metal-acid reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
"inflammable air". He speculated that "inflammable air" was in fact identical to the hypothetical substance called "phlogiston
The phlogiston theory is a superseded scientific theory that postulated the existence of a fire-like element called phlogiston () contained within combustible bodies and released during combustion. The name comes from the Ancient Greek (''burni ...
" and further finding in 1781 that the gas produces water when burned. He is usually given credit for the discovery of hydrogen as an element. In 1783, Antoine Lavoisier gave the element the name hydrogen (from the Greek ὑδρο- ''hydro'' meaning "water" and -γενής ''genes'' meaning "former") when he and Laplace
Pierre-Simon, marquis de Laplace (; ; 23 March 1749 – 5 March 1827) was a French scholar and polymath whose work was important to the development of engineering, mathematics, statistics, physics, astronomy, and philosophy. He summarized ...
reproduced Cavendish's finding that water is produced when hydrogen is burned.
Lavoisier produced hydrogen for his experiments on mass conservation by reacting a flux of steam with metallic iron through an incandescent iron tube heated in a fire. Anaerobic oxidation of iron by the protons of water at high temperature can be schematically represented by the set of following reactions:
:1)
:2)
:3)
Many metals such as zirconium undergo a similar reaction with water leading to the production of hydrogen.
Hydrogen was liquefied for the first time by James Dewar in 1898 by using regenerative cooling
Regenerative cooling is a method of cooling gases in which compressed gas is cooled by allowing it to expand and thereby take heat from the surroundings. The cooled expanded gas then passes through a heat exchanger where it cools the incoming comp ...
and his invention, the vacuum flask. He produced solid hydrogen the next year. Deuterium was discovered in December 1931 by Harold Urey, and tritium was prepared in 1934 by Ernest Rutherford, Mark Oliphant, and Paul Harteck. Heavy water, which consists of deuterium in the place of regular hydrogen, was discovered by Urey's group in 1932. François Isaac de Rivaz
François Isaac de Rivaz (Paris, December 19, 1752 – Sion, July 30, 1828) was a French-born Swiss inventor and a politician. He invented a hydrogen-powered internal combustion engine with electric ignition and described it in a French patent ...
built the first de Rivaz engine, an internal combustion engine powered by a mixture of hydrogen and oxygen in 1806. Edward Daniel Clarke
Edward Daniel Clarke (5 June 17699 March 1822) was an English clergyman, naturalist, mineralogist, and traveller.
Life
Edward Daniel Clarke was born at Willingdon, Sussex, and educated first at Uckfield School"Anthony Saunders, D.D." in Mark ...
invented the hydrogen gas blowpipe in 1819. The Döbereiner's lamp and limelight
Limelight (also known as Drummond light or calcium light)James R. Smith (2004). ''San Francisco's Lost Landmarks'', Quill Driver Books. is a type of stage lighting once used in theatres and music halls. An intense illumination is created when ...
were invented in 1823.
The first hydrogen-filled balloon was invented by Jacques Charles in 1783. Hydrogen provided the lift for the first reliable form of air-travel following the 1852 invention of the first hydrogen-lifted airship by Henri Giffard. German count Ferdinand von Zeppelin promoted the idea of rigid airships lifted by hydrogen that later were called Zeppelins; the first of which had its maiden flight in 1900. Regularly scheduled flights started in 1910 and by the outbreak of World War I in August 1914, they had carried 35,000 passengers without a serious incident. Hydrogen-lifted airships were used as observation platforms and bombers during the war.
The first non-stop transatlantic crossing was made by the British airship ''R34 R34 may refer to:
* R34 (New York City Subway car)
* R34 (South Africa)
* HM Airship ''R.34'', a rigid airship of the Royal Air Force
* , a destroyer of the Royal Navy
* Nissan Skyline (R34), a mid-size car
* Nissan Skyline GT-R (R34), a sports ca ...
'' in 1919. Regular passenger service resumed in the 1920s and the discovery of helium reserves in the United States promised increased safety, but the U.S. government refused to sell the gas for this purpose. Therefore, was used in the ''Hindenburg'' airship, which was destroyed in a midair fire over New Jersey on 6 May 1937. The incident was broadcast live on radio and filmed. Ignition of leaking hydrogen is widely assumed to be the cause, but later investigations pointed to the ignition of the aluminized
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ...
fabric coating by static electricity
Static electricity is an imbalance of electric charges within or on the surface of a material or between materials. The charge remains until it is able to move away by means of an electric current or electrical discharge. Static electricity is na ...
. But the damage to hydrogen's reputation as a lifting gas was already done and commercial hydrogen airship travel ceased. Hydrogen is still used, in preference to non-flammable but more expensive helium, as a lifting gas for weather balloons
A weather balloon, also known as sounding balloon, is a balloon (specifically a type of high-altitude balloon) that carries instruments aloft to send back information on atmospheric pressure, temperature, humidity and wind speed by means of a ...
.
In the same year, the first hydrogen-cooled turbogenerator
A hydrogen-cooled turbo generator is a turbo generator with gaseous hydrogen as a coolant. Hydrogen-cooled turbo generators are designed to provide a low- drag atmosphere and cooling for single-shaft and combined-cycle applications in combination ...
went into service with gaseous hydrogen as a coolant in the rotor and the stator in 1937 at Dayton
Dayton () is the sixth-largest city in the U.S. state of Ohio and the county seat of Montgomery County. A small part of the city extends into Greene County. The 2020 U.S. census estimate put the city population at 137,644, while Greater Da ...
, Ohio, by the Dayton Power & Light Co.; because of the thermal conductivity and very low viscosity of hydrogen gas, thus lower drag than air, this is the most common type in its field today for large generators (typically 60 MW and bigger; smaller generators are usually air-cooled).
The nickel hydrogen battery
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to r ...
was used for the first time in 1977 aboard the U.S. Navy's Navigation technology satellite-2 (NTS-2). For example, the ISS, Mars Odyssey and the Mars Global Surveyor are equipped with nickel-hydrogen batteries. In the dark part of its orbit, the Hubble Space Telescope is also powered by nickel-hydrogen batteries, which were finally replaced in May 2009, more than 19 years after launch and 13 years beyond their design life.
Role in quantum theory
hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen consti ...
, together with the spectrum of light produced from it or absorbed by it, has been central to the development of the theory of atomic structure. Furthermore, study of the corresponding simplicity of the hydrogen molecule and the corresponding cation brought understanding of the nature of the chemical bond, which followed shortly after the quantum mechanical treatment of the hydrogen atom had been developed in the mid-1920s.
One of the first quantum effects to be explicitly noticed (but not understood at the time) was a Maxwell observation involving hydrogen, half a century before full quantum mechanical theory arrived. Maxwell observed that the specific heat capacity of unaccountably departs from that of a diatomic
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear. Ot ...
gas below room temperature and begins to increasingly resemble that of a monatomic gas at cryogenic temperatures. According to quantum theory, this behavior arises from the spacing of the (quantized) rotational energy levels, which are particularly wide-spaced in because of its low mass. These widely spaced levels inhibit equal partition of heat energy into rotational motion in hydrogen at low temperatures. Diatomic gases composed of heavier atoms do not have such widely spaced levels and do not exhibit the same effect.
Antihydrogen
Antihydrogen () is the antimatter counterpart of hydrogen. Whereas the common hydrogen atom is composed of an electron and proton, the antihydrogen atom is made up of a positron and antiproton. Scientists hope that studying antihydrogen may shed l ...
() is the antimatter counterpart to hydrogen. It consists of an antiproton with a positron
The positron or antielectron is the antiparticle or the antimatter counterpart of the electron. It has an electric charge of +1 '' e'', a spin of 1/2 (the same as the electron), and the same mass as an electron. When a positron collides ...
. Antihydrogen is the only type of antimatter atom to have been produced .
Cosmic prevalence and distribution
 Hydrogen, as atomic H, is the most abundant chemical element in the universe, making up 75 percent of normal matter by mass and more than 90 percent by number of atoms. (Most of the mass of the universe, however, is not in the form of chemical-element type matter, but rather is postulated to occur as yet-undetected forms of mass such as dark matter and dark energy.) This element is found in great abundance in stars and gas giant planets. Molecular clouds of are associated with
Hydrogen, as atomic H, is the most abundant chemical element in the universe, making up 75 percent of normal matter by mass and more than 90 percent by number of atoms. (Most of the mass of the universe, however, is not in the form of chemical-element type matter, but rather is postulated to occur as yet-undetected forms of mass such as dark matter and dark energy.) This element is found in great abundance in stars and gas giant planets. Molecular clouds of are associated with star formation
Star formation is the process by which dense regions within molecular clouds in The "medium" is present further soon.-->interstellar space
. Hydrogen plays a vital role in powering star
A star is an astronomical object comprising a luminous spheroid of plasma (physics), plasma held together by its gravity. The List of nearest stars and brown dwarfs, nearest star to Earth is the Sun. Many other stars are visible to the naked ...
s through the proton-proton reaction in case of stars with very low to approximately 1 mass of the Sun and the CNO cycle of nuclear fusion in case of stars more massive than our Sun.
States
Throughout the universe, hydrogen is mostly found in the atomic andplasma
Plasma or plasm may refer to:
Science
* Plasma (physics), one of the four fundamental states of matter
* Plasma (mineral), a green translucent silica mineral
* Quark–gluon plasma, a state of matter in quantum chromodynamics
Biology
* Blood pla ...
states, with properties quite distinct from those of molecular hydrogen. As a plasma, hydrogen's electron and proton are not bound together, resulting in very high electrical conductivity and high emissivity (producing the light from the Sun and other stars). The charged particles are highly influenced by magnetic and electric fields. For example, in the solar wind they interact with the Earth's magnetosphere
In astronomy and planetary science, a magnetosphere is a region of space surrounding an astronomical object in which charged particles are affected by that object's magnetic field. It is created by a celestial body with an active interior dynam ...
giving rise to Birkeland currents and the aurora.
Hydrogen is found in the neutral atomic state in the interstellar medium
In astronomy, the interstellar medium is the matter and radiation that exist in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as dust and cosmic rays. It fills interstella ...
because the atoms seldom collide and combine. They are the source of the 21-cm hydrogen line at 1420 MHz that is detected in order to probe primordial hydrogen. The large amount of neutral hydrogen found in the damped Lyman-alpha system
Damped Lyman alpha systems or Damped Lyman alpha absorption systems is a term used by astronomers for concentrations of neutral hydrogen gas that are detected in the spectra of quasars – a class of distant Active Galactic Nuclei. They are defin ...
s is thought to dominate the cosmological baryonic density of the universe up to a redshift
In physics, a redshift is an increase in the wavelength, and corresponding decrease in the frequency and photon energy, of electromagnetic radiation (such as light). The opposite change, a decrease in wavelength and simultaneous increase in f ...
of ''z'' = 4.
Under ordinary conditions on Earth, elemental hydrogen exists as the diatomic gas, . Hydrogen gas is very rare in the Earth's atmosphere (1 ppm by volume) because of its light weight, which enables it to escape from the atmosphere more rapidly than heavier gases. However, hydrogen is the third most abundant element on the Earth's surface, mostly in the form of chemical compounds such as hydrocarbons and water.
A molecular form called protonated molecular hydrogen
The trihydrogen cation or protonated molecular hydrogen is a cation (positive ion) with chemical formula, formula , consisting of three hydrogen nuclei (protons) sharing two electrons.
The trihydrogen cation is one of the most abundant ions in t ...
() is found in the interstellar medium, where it is generated by ionization of molecular hydrogen from cosmic rays. This ion has also been observed in the upper atmosphere of the planet Jupiter. The ion is relatively stable in the environment of outer space due to the low temperature and density. is one of the most abundant ions in the universe, and it plays a notable role in the chemistry of the interstellar medium. Neutral triatomic hydrogen
Triatomic hydrogen or H3 is an unstable triatomic molecule containing only hydrogen. Since this molecule contains only three atoms of hydrogen it is the simplest triatomic molecule and it is relatively simple to numerically solve the quantum mechan ...
can exist only in an excited form and is unstable. By contrast, the positive hydrogen molecular ion () is a rare molecule in the universe.
Production
is produced in chemistry and biology laboratories, often as a by-product of other reactions; in industry for the hydrogenation of unsaturated substrates; and in nature as a means of expelling reducing equivalents in biochemical reactions.Water electrolysis
 The electrolysis of water is a simple method of producing hydrogen. A current is run through the water, and gaseous oxygen forms at the anode while gaseous hydrogen forms at the cathode. Typically the cathode is made from platinum or another inert metal when producing hydrogen for storage. If, however, the gas is to be burnt on site, oxygen is desirable to assist the combustion, and so both electrodes would be made from inert metals. (Iron, for instance, would oxidize, and thus decrease the amount of oxygen given off.) The theoretical maximum efficiency (electricity used vs. energetic value of hydrogen produced) is in the range 88–94%.
:
The electrolysis of water is a simple method of producing hydrogen. A current is run through the water, and gaseous oxygen forms at the anode while gaseous hydrogen forms at the cathode. Typically the cathode is made from platinum or another inert metal when producing hydrogen for storage. If, however, the gas is to be burnt on site, oxygen is desirable to assist the combustion, and so both electrodes would be made from inert metals. (Iron, for instance, would oxidize, and thus decrease the amount of oxygen given off.) The theoretical maximum efficiency (electricity used vs. energetic value of hydrogen produced) is in the range 88–94%.
:
Methane pyrolysis
 Hydrogen production using natural gas methane pyrolysis is a one-step process that produces no
Hydrogen production using natural gas methane pyrolysis is a one-step process that produces no greenhouse gas
A greenhouse gas (GHG or GhG) is a gas that Absorption (electromagnetic radiation), absorbs and Emission (electromagnetic radiation), emits radiant energy within the thermal infrared range, causing the greenhouse effect. The primary greenhouse ...
es. Developing volume production using this method is the key to enabling faster carbon reduction by using hydrogen in industrial processes, fuel cell
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requ ...
electric heavy truck transportation, and in gas turbine electric power generation. Methane pyrolysis is performed by having methane bubbled up through a molten metal catalyst containing dissolved nickel at . This causes the methane to break down into hydrogen gas and solid carbon, with no other byproducts.
: (ΔH° = 74 kJ/mol)
The industrial quality solid carbon may be sold as manufacturing feedstock or permanently landfilled; it is not released into the atmosphere and does not cause ground water pollution in landfill. Methane pyrolysis is in development and considered suitable for commercial bulk hydrogen production. Volume production is being evaluated in the BASF "methane pyrolysis at scale" pilot plant. Further research continues in several laboratories, including at Karlsruhe Liquid-metal Laboratory (KALLA) and the chemical engineering laboratory at University of California – Santa Barbara
Other industrial methods
 Hydrogen is often produced by reacting water with methane and carbon monoxide, which causes the removal of hydrogen from hydrocarbons at very high temperatures, with 48% of hydrogen production coming from steam reforming. The water vapor is then reacted with the carbon monoxide produced by steam reforming to oxidize it to carbon dioxide and turn the water into hydrogen. Commercial bulk hydrogen is usually produced by the steam reforming of natural gas with release of atmospheric greenhouse gas or with capture using
Hydrogen is often produced by reacting water with methane and carbon monoxide, which causes the removal of hydrogen from hydrocarbons at very high temperatures, with 48% of hydrogen production coming from steam reforming. The water vapor is then reacted with the carbon monoxide produced by steam reforming to oxidize it to carbon dioxide and turn the water into hydrogen. Commercial bulk hydrogen is usually produced by the steam reforming of natural gas with release of atmospheric greenhouse gas or with capture using CCS and climate change mitigation
Carbon capture and storage (CCS) or carbon capture and sequestration is the process of capturing carbon dioxide (CO2) before it enters the atmosphere, transporting it, and storing it (carbon sequestration) for centuries or millennia. Usually th ...
. Steam reforming is also known as the Bosch process and is widely used for the industrial preparation of hydrogen.
At high temperatures (1000–1400 K, 700–1100 °C or 1300–2000 °F), steam (water vapor) reacts with methane to yield carbon monoxide and .
:
This reaction is favored at low pressures but is nonetheless conducted at high pressures (2.0 MPa, 20 atm or 600 inHg
Inch of mercury (inHg and ″Hg) is a non- SI unit of measurement for pressure. It is used for barometric pressure in weather reports, refrigeration and aviation in the United States.
It is the pressure exerted by a column of mercury in heigh ...
). This is because high-pressure is the most marketable product, and pressure swing adsorption (PSA) purification systems work better at higher pressures. The product mixture is known as " synthesis gas" because it is often used directly for the production of methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
and related compounds. Hydrocarbons other than methane can be used to produce synthesis gas with varying product ratios. One of the many complications to this highly optimized technology is the formation of coke or carbon:
:
Consequently, steam reforming typically employs an excess of . Additional hydrogen can be recovered from the steam by use of carbon monoxide through the water gas shift reaction, especially with an iron oxide
Iron oxides are chemical compounds composed of iron and oxygen. Several iron oxides are recognized. All are black magnetic solids. Often they are non-stoichiometric. Oxyhydroxides are a related class of compounds, perhaps the best known of whic ...
catalyst. This reaction is also a common industrial source of carbon dioxide:
:
Other important methods for CO and production include partial oxidation of hydrocarbons:
:
and the coal reaction, which can serve as a prelude to the shift reaction above:
:
Hydrogen is sometimes produced and consumed in the same industrial process, without being separated. In the Haber process for the production of ammonia, hydrogen is generated from natural gas. Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
of brine
Brine is a high-concentration solution of salt (NaCl) in water (H2O). In diverse contexts, ''brine'' may refer to the salt solutions ranging from about 3.5% (a typical concentration of seawater, on the lower end of that of solutions used for br ...
to yield chlorine also produces hydrogen as a co-product.
Olefin production units may produce substantial quantities of byproduct hydrogen particularly from cracking light feedstocks like ethane or propane
Propane () is a three-carbon alkane with the molecular formula . It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used a ...
.
Metal-acid
Many metals react with water to produce , but the rate of hydrogen evolution depends on the metal, the pH, and the presence alloying agents. Most commonly, hydrogen evolution is induced by acids. The alkali and alkaline earth metals, aluminium, zinc, manganese, and iron react readily with aqueous acids. This reaction is the basis of theKipp's apparatus
Kipp's apparatus, also called Kipp generator, is an apparatus designed for preparation of small volumes of gases. It was invented around 1844 by the Dutch pharmacist Petrus Jacobus Kipp and widely used in chemical laboratories and for demonstration ...
, which once was used as a laboratory gas source:
:
In the absence of acid, the evolution of is slower. Because iron is widely used structural material, its anaerobic corrosion
Anaerobic corrosion (also known as hydrogen corrosion) is a form of metal corrosion occurring in anoxic water. Typically following aerobic corrosion, anaerobic corrosion involves a redox reaction that reduces hydrogen ions and oxidizes a solid met ...
is of technological significance:
:
Many metals, such as aluminium, are slow to react with water because they form passivated coatings of oxides. An alloy of aluminium and gallium
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group (aluminiu ...
, however, does react with water. At high pH, aluminium can produce :
:
Some metal-containing compounds react with acids to evolve . Under anaerobic conditions, ferrous hydroxide
Iron(II) hydroxide or ferrous hydroxide is an inorganic compound with the formula Fe(OH)2. It is produced when iron(II) salts, from a compound such as iron(II) sulfate, are treated with hydroxide ions. Iron(II) hydroxide is a white solid, but even ...
() can be oxidized by the protons of water to form magnetite and . This process is described by the Schikorr reaction
The Schikorr reaction formally describes the conversion of the iron(II) hydroxide (Fe(OH)2) into iron(II,III) oxide (Fe3O4). This transformation reaction was first studied by Gerhard Schikorr. The global reaction follows:
:\underset -> \underset + ...
:
:
This process occurs during the anaerobic corrosion of iron and steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant ty ...
in oxygen-free groundwater and in reducing soils below the water table.
Thermochemical
More than 200 thermochemical cycles can be used for water splitting. Many of these cycles such as the iron oxide cycle, cerium(IV) oxide–cerium(III) oxide cycle,zinc zinc-oxide cycle
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic ta ...
, sulfur-iodine cycle, copper-chlorine cycle and hybrid sulfur cycle have been evaluated for their commercial potential to produce hydrogen and oxygen from water and heat without using electricity. A number of laboratories (including in France, Germany, Greece, Japan
Japan ( ja, 日本, or , and formally , ''Nihonkoku'') is an island country in East Asia. It is situated in the northwest Pacific Ocean, and is bordered on the west by the Sea of Japan, while extending from the Sea of Okhotsk in the north ...
, and the United States) are developing thermochemical methods to produce hydrogen from solar energy and water.
Serpentinization reaction
In deep geological conditions prevailing far away from the Earth's atmosphere, hydrogen () is produced during the process of serpentinization. In this process, water protons () are reduced by ferrous () ions provided by fayalite (). The reaction forms magnetite (), quartz (), and hydrogen (): : :''fayalite + water → magnetite + quartz + hydrogen'' This reaction closely resembles theSchikorr reaction
The Schikorr reaction formally describes the conversion of the iron(II) hydroxide (Fe(OH)2) into iron(II,III) oxide (Fe3O4). This transformation reaction was first studied by Gerhard Schikorr. The global reaction follows:
:\underset -> \underset + ...
observed in anaerobic oxidation of ferrous hydroxide
Iron(II) hydroxide or ferrous hydroxide is an inorganic compound with the formula Fe(OH)2. It is produced when iron(II) salts, from a compound such as iron(II) sulfate, are treated with hydroxide ions. Iron(II) hydroxide is a white solid, but even ...
in contact with water.
Applications
Petrochemical industry
Large quantities of are used in the "upgrading" of fossil fuels. Key consumers of includehydrodealkylation
Hydrodealkylation is a chemical reaction that often involves reacting an aromatic hydrocarbon, such as toluene, in the presence of hydrogen gas to form a simpler aromatic hydrocarbon devoid of functional groups. An example is the conversion of 1,2, ...
, hydrodesulfurization
Hydrodesulfurization (HDS) is a catalytic chemical process widely used to remove sulfur (S) from natural gas and from refined petroleum products, such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils. The purpose of remov ...
, and hydrocracking
In petrochemistry, petroleum geology and organic chemistry, cracking is the process whereby complex organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of ...
. Many of these reactions can be classified as hydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes lysis (breakdown) by hydrogen.Ralph Connor, Homer Adkins. Hydrogenolysis Of Oxygenated Organic Compounds. J. Am. Chem. Soc. ...
, i.e., the cleavage of bonds to carbon. Illustrative is the separation of sulfur from liquid fossil fuels:
:
Hydrogenation
Hydrogenation, the addition of to various substrates is conducted on a large scale. The hydrogenation of to produce ammonia by the Haber–Bosch process consumes a few percent of the energy budget in the entire industry. The resulting ammonia is used to supply the majority of the protein consumed by humans. Hydrogenation is used to convertunsaturated fat
An unsaturated fat is a fat or fatty acid in which there is at least one double bond within the fatty acid chain. A fatty acid chain is monounsaturated if it contains one double bond, and polyunsaturated if it contains more than one double bond.
...
s and oils to saturated fats and oils. The major application is the production of margarine
Margarine (, also , ) is a spread used for flavoring, baking, and cooking. It is most often used as a substitute for butter. Although originally made from animal fats, most margarine consumed today is made from vegetable oil. The spread was orig ...
. Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
is produced by hydrogenation of carbon dioxide. It is similarly the source of hydrogen in the manufacture of hydrochloric acid. is also used as a reducing agent for the conversion of some ores to the metals.
Coolant
Hydrogen is commonly used in power stations as a coolant in generators due to a number of favorable properties that are a direct result of its light diatomic molecules. These include low density, low viscosity, and the highest specific heat and thermal conductivity of all gases.Energy carrier
Elemental hydrogen has been widely discussed in the context of energy, as a possible future carrier of energy on an economy-wide scale. Hydrogen is afuel cells
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen fuel, hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most bat ...
to generate electricity directly, with water being the only emissions at the point of usage. The overall lifecycle emissions of hydrogen depend on how it is produced. Nearly all of the world's current supply of hydrogen is created from fossil fuels. The main method is steam methane reforming
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is hydrogen produc ...
, in which hydrogen is produced from a chemical reaction between steam and methane, the main component of natural gas. Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide. While carbon capture and storage can remove a large fraction of these emissions, the overall carbon footprint of hydrogen from natural gas is difficult to assess , in part because of emissions created in the production of the natural gas itself.
Electricity can be used to split water molecules, producing sustainable hydrogen provided the electricity was generated sustainably. However, this electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
process is currently more expensive than creating hydrogen from methane and the efficiency of energy conversion is inherently low. Hydrogen can be produced when there is a surplus of variable renewable electricity, then stored and used to generate heat or to re-generate electricity. It can be further transformed into synthetic fuels such as ammonia and methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
.
Innovation in hydrogen electrolysers could make large-scale production of hydrogen from electricity more cost-competitive. There is potential for hydrogen to play a significant role in decarbonising energy systems because in certain sectors, replacing fossil fuels with direct use of electricity would be very difficult. Hydrogen fuel can produce the intense heat required for industrial production of steel, cement, glass, and chemicals. For steelmaking, hydrogen can function as a clean energy carrier and simultaneously as a low-carbon catalyst replacing coal-derived coke. Hydrogen used in transportation would burn relatively cleanly, with some emissions, but without carbon emissions. Disadvantages of hydrogen as an energy carrier include high costs of storage and distribution due to hydrogen's explosivity, its large volume compared to other fuels, and its tendency to make pipes brittle. The infrastructure costs associated with full conversion to a hydrogen economy would be substantial.
Semiconductor industry
Hydrogen is employed to saturate broken ("dangling") bonds of amorphous silicon and amorphous carbon that helps stabilizing material properties. It is also a potential electron donor in various oxide materials, including ZnO, , CdO,MgO
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions ...
, , , , , , , , , , , , and .
Aerospace
Liquid hydrogen and liquid oxygen together serve as cryogenic fuel inliquid-propellant rocket
A liquid-propellant rocket or liquid rocket utilizes a rocket engine that uses liquid rocket propellant, liquid propellants. Liquids are desirable because they have a reasonably high density and high Specific impulse, specific impulse (''I''sp). T ...
s, as in the Space Shuttle main engines
The Aerojet Rocketdyne RS-25, also known as the Space Shuttle Main Engine (SSME), is a liquid-fuel cryogenic rocket engine that was used on NASA's Space Shuttle and is currently used on the Space Launch System (SLS).
Designed and manufacture ...
.
Niche and evolving uses
*Shielding gas: Hydrogen is used as ashielding gas
Shielding gases are inert or semi-inert gases that are commonly used in several welding processes, most notably gas metal arc welding and gas tungsten arc welding (GMAW and GTAW, more popularly known as MIG (Metal Inert Gas) and TIG (Tungsten Iner ...
in welding methods such as atomic hydrogen welding
Atomic hydrogen welding (AHW) is an arc welding process that uses an arc between two tungsten electrodes in a shielding atmosphere of hydrogen. The process was invented by Irving Langmuir in the course of his studies of atomic hydrogen. The elec ...
.
*Cryogenic research: Liquid is used in cryogenic
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of “cryogenics” and “cr ...
research, including superconductivity
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike ...
studies.
*Buoyant lifting: Because is lighter than air, having only 7% of the density of air, it was once widely used as a lifting gas in balloons and airships.
*Leak detection: Pure or mixed with nitrogen (sometimes called forming gas
Forming gas is a mixture of hydrogen (mole fraction varies) and nitrogen. It is sometimes called a "dissociated ammonia atmosphere" due to the reaction which generates it:
:2 NH3 → 3 H2 + N2
It can also be manufactured by thermal cra ...
), hydrogen is a tracer gas for detection of minute leaks. Applications can be found in the automotive, chemical, power generation, aerospace, and telecommunications industries. Hydrogen is an authorized food additive (E 949) that allows food package leak testing, as well as having anti-oxidizing properties.
*Neutron moderation: Deuterium (hydrogen-2) is used in nuclear fission applications as a moderator to slow neutrons.
*Nuclear fusion fuel: Deuterium is used in nuclear fusion reactions.
*Isotopic labeling: Deuterium compounds have applications in chemistry and biology in studies of isotope effects on reaction rates.
*Rocket propellant: NASA has investigated the use of rocket propellant
Rocket propellant is the reaction mass of a rocket. This reaction mass is ejected at the highest achievable velocity from a rocket engine
A rocket engine uses stored rocket propellants as the reaction mass for forming a high-speed propuls ...
made from atomic hydrogen, boron or carbon that is frozen into solid molecular hydrogen particles that are suspended in liquid helium. Upon warming, the mixture vaporizes to allow the atomic species to recombine, heating the mixture to high temperature.
*Tritium uses: Tritium (hydrogen-3), produced in nuclear reactors, is used in the production of hydrogen bomb
A thermonuclear weapon, fusion weapon or hydrogen bomb (H bomb) is a second-generation nuclear weapon design. Its greater sophistication affords it vastly greater destructive power than first-generation nuclear bombs, a more compact size, a lowe ...
s, as an isotopic label in the biosciences, and as a source of beta radiation in radioluminescent paint
Luminous paint or luminescent paint is paint that exhibits luminescence. In other words, it gives off visible light through fluorescence, phosphorescence, or radioluminescence. There are three types of luminous paints: fluorescent paint, phospho ...
for instrument dials and emergency signage.
Biological reactions
is a product of some types of anaerobic metabolism and is produced by several microorganisms, usually via reactions catalyzed by iron- or nickel-containing enzymes called hydrogenases. These enzymes catalyze the reversible redox reaction between and its component two protons and two electrons. Creation of hydrogen gas occurs in the transfer of reducing equivalents produced duringpyruvate
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic aci ...
fermentation
Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food ...
to water. The natural cycle of hydrogen production and consumption by organisms is called the hydrogen cycle
The hydrogen cycle consists of hydrogen exchanges between biotic (living) and abiotic (non-living) sources and sinks of hydrogen-containing compounds.
Hydrogen (H) is the most abundant element in the universe. On Earth, common H-containing inorg ...
. Hydrogen is the most abundant element in the human body in terms of numbers of atoms of the element but, it is the 3rd most abundant element by mass, because hydrogen is so light. occurs in the breath of humans due to the metabolic activity of hydrogenase-containing microorganisms in the large intestine. The concentration in fasted people at rest is typically less than 5 parts per million (ppm) but can be 50 ppm when people with intestinal disorders consume molecules they cannot absorb during diagnostic hydrogen breath tests.
Hydrogen gas is produced by some bacteria and algae
Algae (; singular alga ) is an informal term for a large and diverse group of photosynthetic eukaryotic organisms. It is a polyphyletic grouping that includes species from multiple distinct clades. Included organisms range from unicellular mic ...
and is a natural component of flatus, as is methane, itself a hydrogen source of increasing importance.
Water splitting, in which water is decomposed into its component protons, electrons, and oxygen, occurs in the light reactions
Light-dependent reactions is jargon for certain photochemical reactions that are involved in photosynthesis, the main process by which plants acquire energy. There are two light dependent reactions, the first occurs at photosystem II (PSII) and ...
in all photosynthetic
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored in c ...
organisms. Some such organisms, including the alga '' Chlamydomonas reinhardtii'' and cyanobacteria
Cyanobacteria (), also known as Cyanophyta, are a phylum of gram-negative bacteria that obtain energy via photosynthesis. The name ''cyanobacteria'' refers to their color (), which similarly forms the basis of cyanobacteria's common name, blu ...
, have evolved a second step in the dark reaction
The Calvin cycle, light-independent reactions, bio synthetic phase, dark reactions, or photosynthetic carbon reduction (PCR) cycle of photosynthesis is a series of chemical reactions that convert carbon dioxide and hydrogen-carrier compounds into ...
s in which protons and electrons are reduced to form gas by specialized hydrogenases in the chloroplast
A chloroplast () is a type of membrane-bound organelle known as a plastid that conducts photosynthesis mostly in plant and algal cells. The photosynthetic pigment chlorophyll captures the energy from sunlight, converts it, and stores it in ...
. Efforts have been undertaken to genetically modify cyanobacterial hydrogenases to efficiently synthesize gas even in the presence of oxygen. Efforts have also been undertaken with genetically modified alga in a bioreactor.
Safety and precautions
Hydrogen poses a number of hazards to human safety, from potential detonations and fires when mixed with air to being an asphyxiant in its pure, oxygen-free form. In addition, liquid hydrogen is a cryogen and presents dangers (such as frostbite) associated with very cold liquids. Hydrogen dissolves in many metals and in addition to leaking out, may have adverse effects on them, such as hydrogen embrittlement, leading to cracks and explosions. Hydrogen gas leaking into external air may spontaneously ignite. Moreover, hydrogen fire, while being extremely hot, is almost invisible, and thus can lead to accidental burns. Even interpreting the hydrogen data (including safety data) is confounded by a number of phenomena. Many physical and chemical properties of hydrogen depend on the parahydrogen/orthohydrogen ratio (it often takes days or weeks at a given temperature to reach the equilibrium ratio, for which the data is usually given). Hydrogen detonation parameters, such as critical detonation pressure and temperature, strongly depend on the container geometry.See also
* * * * * * * (for hydrogen) * *Notes
References
Further reading
* * * * * * *Hydrogen safety
Hydrogen safety covers the safe production, handling and use of hydrogen, particularly hydrogen gas fuel and liquid hydrogen.
Hydrogen possesses the NFPA 704's highest rating of 4 on the flammability scale because it is flammable when mixed even i ...
covers the safe production, handling and use
External links
Basic Hydrogen Calculations of Quantum Mechanics
at '' The Periodic Table of Videos'' (University of Nottingham)
{{Authority control Chemical elements Reactive nonmetals Diatomic nonmetals Nuclear fusion fuels Airship technology Reducing agents Refrigerants Gaseous signaling molecules E-number additives