|
Forming Gas
Forming gas is a mixture of hydrogen (mole fraction varies) and nitrogen. It is sometimes called a "dissociated ammonia atmosphere" due to the reaction which generates it: :2 NH3 → 3 H2 + N2 It can also be manufactured by thermal cracking of ammonia, in an ammonia cracker or forming gas generator. Forming gas is used as an atmosphere for processes that need the properties of hydrogen gas. Typical forming gas formulations (5% H2 in N2) are not explosive. It is used in chambers for gas hypersensitization, a process in which photographic film is heated in forming gas to drive out moisture and oxygen and to increase the base fog of the film. Hypersensitization is used particularly in deep-sky astrophotography, which deals with low-intensity incoming light, requires long exposure times, and is thus particularly sensitive to contaminants in the film. Forming gas is also used to regenerate catalysts in glove boxes and as an atmosphere for annealing processes. It can be p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reducing Agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ). Examples of substances that are commonly reducing agents include the Earth metals, formic acid, oxalic acid, and sulfite compounds. In their pre-reaction states, reducers have extra electrons (that is, they are by themselves reduced) and oxidizers lack electrons (that is, they are by themselves oxidized). This is commonly expressed in terms of their oxidation states. An agent's oxidation state describes its degree of loss of electrons, where the higher the oxidation state then the fewer electrons it has. So initially, prior to the reaction, a reducing agent is typically in one of its lower possible oxidation states; its oxidation state increases during the reaction while that of the oxidizer decreases. Thus in a redox reaction, the agent whose oxidation state increases, that "loses/ donates electrons", that "i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brazing And Soldering
Brazing is a metal-joining process in which two or more metal items are joined together by melting and flowing a filler metal into the joint, with the filler metal having a lower melting point than the adjoining metal. Brazing differs from welding in that it does not involve melting the work pieces. Brazing differs from soldering through the use of a higher temperature and much more closely fitted parts than when soldering. During the brazing process, the filler metal flows into the gap between close-fitting parts by capillary action. The filler metal is brought slightly above its melting ( liquidus) temperature while protected by a suitable atmosphere, usually a flux. It then flows over the base metal (in a process known as wetting) and is then cooled to join the work pieces together. A major advantage of brazing is the ability to join the same or different metals with considerable strength. Basics High-quality brazed joints require that parts be closely fitted with base ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Welding
Welding is a fabrication process that joins materials, usually metals or thermoplastics, by using high heat to melt the parts together and allowing them to cool, causing fusion. Welding is distinct from lower temperature techniques such as brazing and soldering, which do not melt the base metal (parent metal). In addition to melting the base metal, a filler material is typically added to the joint to form a pool of molten material (the weld pool) that cools to form a joint that, based on weld configuration (butt, full penetration, fillet, etc.), can be stronger than the base material. Pressure may also be used in conjunction with heat or by itself to produce a weld. Welding also requires a form of shield to protect the filler metals or melted metals from being contaminated or oxidized. Many different energy sources can be used for welding, including a gas flame (chemical), an electric arc (electrical), a laser, an electron beam, friction, and ultrasound. While often a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gases
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma). A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or compound molecules made from a variety of atoms (e.g. carbon dioxide). A gas mixture, such as air, contains a variety of pure gases. What distinguishes a gas from liquids and solids is the vast separation of the individual gas particles. This separation usually makes a colourless gas invisible to the human observer. The gaseous state of matter occurs between the liquid and plasma states, the latter of which provides the upper temperature boundary for gases. Bounding the lower end of the temperature scale lie degenerative quantum gases which are gaining increasing attention. High-density atomic gases super-cooled to very low temperatures are classified by their statistical behavior as either Bose gases or Fermi gases. For a comprehensive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
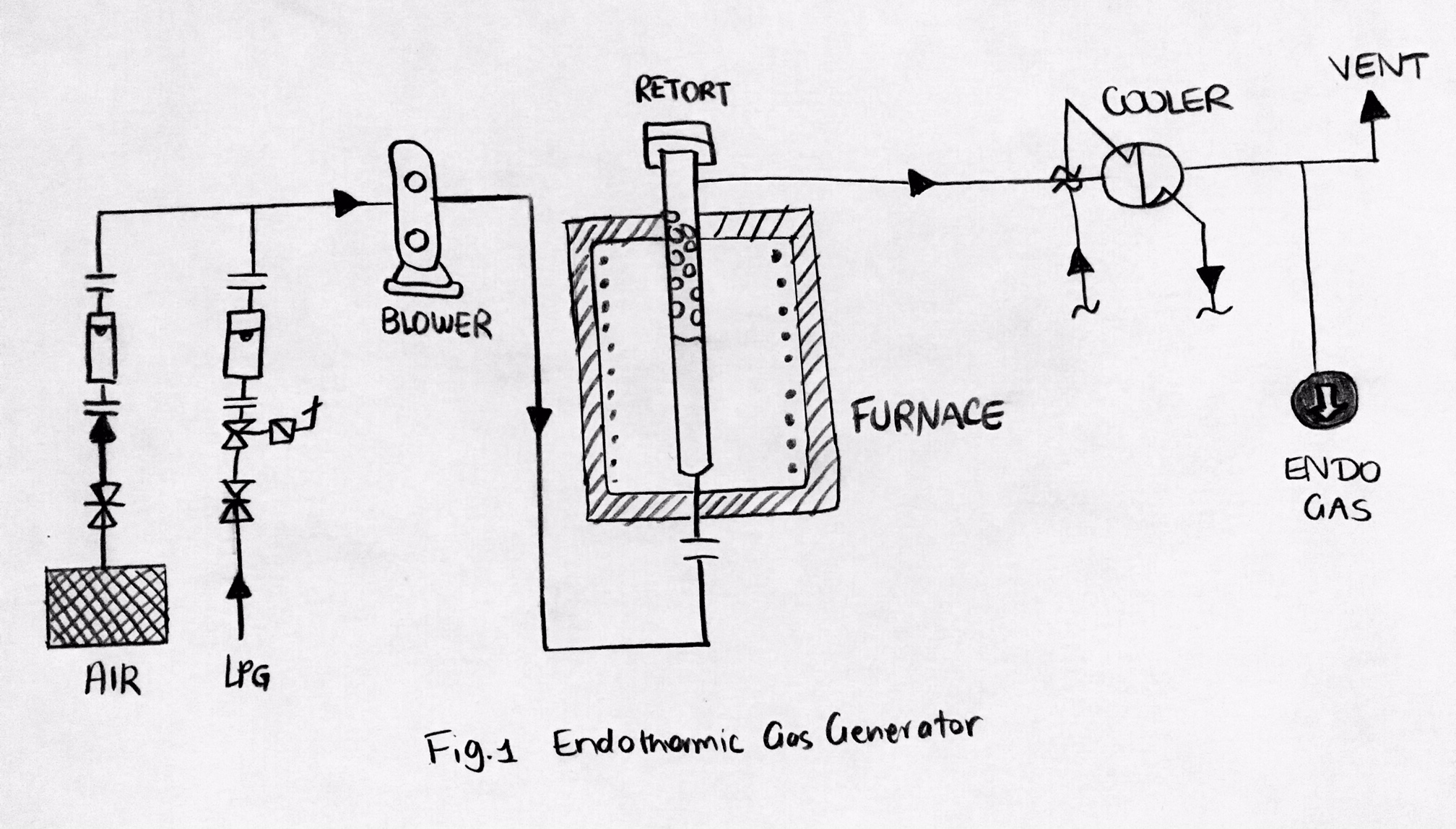
Endothermic Gas
Endothermic gas is a gas that inhibits or reverses oxidation on the surfaces it is in contact with. This gas is the product of incomplete combustion in a controlled environment. An example mixture is hydrogen gas (H2), nitrogen gas (N2), and carbon monoxide (CO). The hydrogen and carbon monoxide are reducing agents, so they work together to shield surfaces from oxidation. Endothermic gas is often used as a carrier gas for gas carburizing and carbonitriding. An endothermic gas generator could be used to supply heat to form an endothermic reaction. Synthesised in the catalytic retort(s) of endothermic generators, the gas in the endothermic atmosphere is combined with an additive gas including natural gas, propane (C3H8) or air and is then used to improve the surface chemistry work positioned in the furnace. Purposes There are two common purposes of the atmospheres in the heat treating industry: # Protect the processed material from surface reactions (chemically inert) # Allo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sintering
Clinker nodules produced by sintering Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction. Sintering happens as part of a manufacturing process used with metals, ceramics, plastics, and other materials. The atoms in the materials diffuse across the boundaries of the particles, fusing the particles together and creating one solid piece. Because the sintering temperature does not have to reach the melting point of the material, sintering is often chosen as the shaping process for materials with extremely high melting points such as tungsten and molybdenum. The study of sintering in metallurgical powder-related processes is known as powder metallurgy. An example of sintering can be observed when ice cubes in a glass of water adhere to each other, which is driven by the temperature difference between the water and the ice. Examples of pressure-driven sintering are the compact ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flux (metallurgy)
In metallurgy, a flux () is a chemical cleaning agent, flowing agent, or purifying agent. Fluxes may have more than one function at a time. They are used in both extractive metallurgy and metal joining. Some of the earliest known fluxes were sodium carbonate, potash, charcoal, coke, borax, lime, lead sulfide and certain minerals containing phosphorus. Iron ore was also used as a flux in the smelting of copper. These agents served various functions, the simplest being a reducing agent, which prevented oxides from forming on the surface of the molten metal, while others absorbed impurities into the slag, which could be scraped off the molten metal. Fluxes are also used in foundries for removing impurities from molten nonferrous metals such as aluminium, or for adding desirable trace elements such as titanium. As cleaning agents, fluxes facilitate soldering, brazing, and welding by removing oxidation from the metals to be joined. In some applications molten flux a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogenation, C=C (and other) bonds a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brazing
Brazing is a metal-joining process in which two or more metal items are joined together by melting and flowing a filler metal into the joint, with the filler metal having a lower melting point than the adjoining metal. Brazing differs from welding in that it does not involve melting the work pieces. Brazing differs from soldering through the use of a higher temperature and much more closely fitted parts than when soldering. During the brazing process, the filler metal flows into the gap between close-fitting parts by capillary action. The filler metal is brought slightly above its melting ( liquidus) temperature while protected by a suitable atmosphere, usually a flux. It then flows over the base metal (in a process known as wetting) and is then cooled to join the work pieces together. A major advantage of brazing is the ability to join the same or different metals with considerable strength. Basics High-quality brazed joints require that parts be closely fitted with base ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Soldering
Soldering (; ) is a process in which two or more items are joined by melting and putting a filler metal (solder) into the joint, the filler metal having a lower melting point than the adjoining metal. Unlike welding, soldering does not involve melting the work pieces. In brazing, the work piece metal also does not melt, but the filler metal is one that melts at a higher temperature than in soldering. In the past, nearly all solders contained lead, but environmental and health concerns have increasingly dictated use of lead-free alloys for electronics and plumbing purposes. Origins There is evidence that soldering was employed as early as 5,000 years ago in Mesopotamia. Soldering and brazing are thought to have originated very early in the history of metal-working, probably before 4000 BC. Sumerian swords from were assembled using hard soldering. Soldering was historically used to make jewelry, cookware and cooking tools, assembling stained glass, as well as other use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Welding
Welding is a fabrication process that joins materials, usually metals or thermoplastics, by using high heat to melt the parts together and allowing them to cool, causing fusion. Welding is distinct from lower temperature techniques such as brazing and soldering, which do not melt the base metal (parent metal). In addition to melting the base metal, a filler material is typically added to the joint to form a pool of molten material (the weld pool) that cools to form a joint that, based on weld configuration (butt, full penetration, fillet, etc.), can be stronger than the base material. Pressure may also be used in conjunction with heat or by itself to produce a weld. Welding also requires a form of shield to protect the filler metals or melted metals from being contaminated or oxidized. Many different energy sources can be used for welding, including a gas flame (chemical), an electric arc (electrical), a laser, an electron beam, friction, and ultrasound. While often a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |