Ammonia is an
inorganic
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemist ...
compound
Compound may refer to:
Architecture and built environments
* Compound (enclosure), a cluster of buildings having a shared purpose, usually inside a fence or wall
** Compound (fortification), a version of the above fortified with defensive struct ...
of
nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
and
hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
with the
formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwee ...
. A
stable binary hydride, and the simplest
pnictogen hydride Pnictogen hydrides or hydrogen pnictides are binary compounds of hydrogen with pnictogen ( or ; from grc, πνῑ́γω "to choke" and -gen, "generator") atoms (elements of group 15: nitrogen, phosphorus, arsenic, antimony, and bismuth) covalently ...
, ammonia is a colourless
gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
with a distinct pungent smell. Biologically, it is a common
nitrogenous waste
Metabolic wastes or excrements are substances left over from metabolic processes (such as cellular respiration) which cannot be used by the organism (they are surplus or toxic), and must therefore be excreted. This includes nitrogen compounds, ...
, particularly among aquatic organisms, and it contributes significantly to the
nutrition
Nutrition is the biochemical and physiological process by which an organism uses food to support its life. It provides organisms with nutrients, which can be metabolized to create energy and chemical structures. Failure to obtain sufficient n ...
al needs of terrestrial organisms by serving as a precursor to 45% of the world's
food
Food is any substance consumed by an organism for nutritional support. Food is usually of plant, animal, or fungal origin, and contains essential nutrients, such as carbohydrates, fats, proteins, vitamins, or minerals. The substance is inge ...
and
fertilizer
A fertilizer (American English) or fertiliser (British English; see spelling differences) is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from ...
s. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as
urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important r ...
and
Diammonium phosphate
Diammonium phosphate (DAP; IUPAC name diammonium hydrogen phosphate; chemical formula (NH4)2(HPO4) is one of a series of water-soluble ammonium phosphate salts that can be produced when ammonia reacts with phosphoric acid.
Solid diammonium phosp ...
. Ammonia in pure form is also applied directly into the soil.
Ammonia, either directly or indirectly, is also a building block for the synthesis of many
pharmaceutical products
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and rel ...
and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water.
Although common in nature—both terrestrially and in the
outer planets
The Solar SystemCapitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar S ...
of the
Solar System
The Solar SystemCapitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar S ...
—and in wide use, ammonia is both
caustic
Caustic most commonly refers to:
* Causticity, a property of various corrosive substances
** Sodium hydroxide, sometimes called ''caustic soda''
** Potassium hydroxide, sometimes called ''caustic potash''
** Calcium oxide, sometimes called ''caus ...
and
hazard
A hazard is a potential source of harm
Harm is a moral and legal concept.
Bernard Gert construes harm as any of the following:
* pain
* death
* disability
* mortality
* loss of abil ity or freedom
* loss of pleasure.
Joel Feinberg giv ...
ous in its concentrated form. In many countries it is classified as an
extremely hazardous substance, and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.
The global industrial production of ammonia in 2018 was 175 million tonnes,
with no significant change relative to the 2013 global industrial production of 175 million tonnes. In 2021 this was 235 million tonnes, with very little being made within the United States. Industrial ammonia is sold either as
ammonia liquor (usually 28% ammonia in water) or as pressurized or refrigerated anhydrous liquid ammonia transported in tank cars or cylinders.
For fundamental reasons, the production of ammonia from the elements hydrogen and nitrogen is difficult, requiring high pressures and high temperatures. The
Haber process
The Haber process, also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. It is named after its inventors, the German chemists Fritz Haber and C ...
that enabled industrial production was invented at the beginning of the 20th century, revolutionizing agriculture.
boils at at a pressure of one
atmosphere
An atmosphere () is a layer of gas or layers of gases that envelop a planet, and is held in place by the gravity of the planetary body. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A s ...
, so the liquid must be stored under pressure or at low temperature. Household ammonia or
ammonium hydroxide
Ammonia solution, also known as ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or (inaccurately) ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3(aq). Although ...
is a solution of in water. The concentration of such solutions is measured in units of the
Baumé scale
The Baumé scale is a pair of hydrometer scales developed by French pharmacist Antoine Baumé in 1768 to measure density of various liquids. The unit of the Baumé scale has been notated variously as ''degrees Baumé'', ''B°'', ''Bé°'' and simp ...
(
density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematical ...
), with 26 degrees Baumé (about 30% of ammonia by weight at ) being the typical high-concentration commercial product.
Etymology
Pliny
Pliny may refer to:
People
* Pliny the Elder (23–79 CE), ancient Roman nobleman, scientist, historian, and author of ''Naturalis Historia'' (''Pliny's Natural History'')
* Pliny the Younger (died 113), ancient Roman statesman, orator, w ...
, in Book XXXI of his
Natural History, refers to a salt named ''hammoniacum'', so called because of its proximity to the nearby Temple of
Jupiter Amun (
Greek
Greek may refer to:
Greece
Anything of, from, or related to Greece, a country in Southern Europe:
*Greeks, an ethnic group.
*Greek language, a branch of the Indo-European language family.
**Proto-Greek language, the assumed last common ancestor ...
Ἄμμων ''Ammon'') in the Roman province of
Cyrenaica
Cyrenaica ( ) or Kyrenaika ( ar, برقة, Barqah, grc-koi, Κυρηναϊκή ��παρχίαKurēnaïkḗ parkhíā}, after the city of Cyrene), is the eastern region of Libya. Cyrenaica includes all of the eastern part of Libya between ...
. However, the description Pliny gives of the salt does not conform to the properties of ammonium chloride. According to
Herbert Hoover's commentary in his English translation of
Georgius Agricola's ''
De re metallica'', it is likely to have been common sea salt. In any case, that salt ultimately gave ammonia and
ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
compounds their name. Roman visitors to oracle temple of
Amun
Amun (; also ''Amon'', ''Ammon'', ''Amen''; egy, jmn, reconstructed as (Old Egyptian and early Middle Egyptian) → (later Middle Egyptian) → (Late Egyptian), cop, Ⲁⲙⲟⲩⲛ, Amoun) romanized: ʾmn) was a major ancient Egyptian ...
in
Siwa oasis
The Siwa Oasis ( ar, واحة سيوة, ''Wāḥat Sīwah,'' ) is an urban oasis in Egypt; between the Qattara Depression and the Great Sand Sea in the Western Desert (Egypt), Western Desert, 50 km (30 mi) east of the Libyan Egypt–Li ...
collected a white crystalline material from the ceiling and walls caused by various pollutants. This white crystalline salt was called "salt of Ammon" (
sal ammoniac
Salammoniac, also sal ammoniac or salmiac, is a rare naturally occurring mineral composed of ammonium chloride, NH4Cl. It forms colorless, white, or yellow-brown crystals in the isometric-hexoctahedral class. It has very poor cleavage and is ...
).
Joseph Priestley
Joseph Priestley (; 24 March 1733 – 6 February 1804) was an English chemist, natural philosopher, separatist theologian, grammarian, multi-subject educator, and liberal political theorist. He published over 150 works, and conducted exp ...
noticed that when this salt reacted with lime, a vapor was released, which he termed as Ammonia.
Natural occurrence
Ammonia is a chemical found in trace quantities in nature, being produced from nitrogenous animal and vegetable matter. Ammonia and ammonium salts are also found in small quantities in rainwater, whereas
ammonium chloride (
sal ammoniac
Salammoniac, also sal ammoniac or salmiac, is a rare naturally occurring mineral composed of ammonium chloride, NH4Cl. It forms colorless, white, or yellow-brown crystals in the isometric-hexoctahedral class. It has very poor cleavage and is ...
), and
ammonium sulfate
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen a ...
are found in volcanic districts. Crystals of
ammonium bicarbonate
Ammonium bicarbonate is an inorganic compound with formula (NH4)HCO3. The compound has many names, reflecting its long history. Chemically speaking, it is the bicarbonate salt of the ammonium ion. It is a colourless solid that degrades readily to ...
have been found in
Patagonia
Patagonia () refers to a geographical region that encompasses the southern end of South America, governed by Argentina and Chile. The region comprises the southern section of the Andes Mountains with lakes, fjords, temperate rainforests, and gl ...
guano
Guano (Spanish from qu, wanu) is the accumulated excrement of seabirds or bats. As a manure, guano is a highly effective fertilizer due to the high content of nitrogen, phosphate, and potassium, all key nutrients essential for plant growth. G ...
.
Ammonia is also found throughout the
Solar System
The Solar SystemCapitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar S ...
on
Mars
Mars is the fourth planet from the Sun and the second-smallest planet in the Solar System, only being larger than Mercury (planet), Mercury. In the English language, Mars is named for the Mars (mythology), Roman god of war. Mars is a terr ...
,
Jupiter
Jupiter is the fifth planet from the Sun and the List of Solar System objects by size, largest in the Solar System. It is a gas giant with a mass more than two and a half times that of all the other planets in the Solar System combined, but ...
,
Saturn
Saturn is the sixth planet from the Sun and the second-largest in the Solar System, after Jupiter. It is a gas giant with an average radius of about nine and a half times that of Earth. It has only one-eighth the average density of Earth; h ...
,
Uranus
Uranus is the seventh planet from the Sun. Its name is a reference to the Greek god of the sky, Uranus (mythology), Uranus (Caelus), who, according to Greek mythology, was the great-grandfather of Ares (Mars (mythology), Mars), grandfather ...
,
Neptune
Neptune is the eighth planet from the Sun and the farthest known planet in the Solar System. It is the fourth-largest planet in the Solar System by diameter, the third-most-massive planet, and the densest giant planet. It is 17 times ...
, and
Pluto
Pluto (minor-planet designation: 134340 Pluto) is a dwarf planet in the Kuiper belt, a ring of trans-Neptunian object, bodies beyond the orbit of Neptune. It is the ninth-largest and tenth-most-massive known object to directly orbit the S ...
, among other places: on smaller, icy
bodies
Bodies may refer to:
* The plural of body
* ''Bodies'' (2004 TV series), BBC television programme
* Bodies (upcoming TV series), an upcoming British crime thriller limited series
* "Bodies" (''Law & Order''), 2003 episode of ''Law & Order''
* ...
such as Pluto, ammonia can act as a geologically important antifreeze, as a mixture of water and ammonia can have a melting point as low as if the ammonia concentration is high enough and thus allow such bodies to retain internal oceans and active geology at a far lower temperature than would be possible with water alone. Substances containing ammonia, or those that are similar to it, are called ''ammoniacal''.
Properties
Ammonia is a colourless
gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
with a characteristically
pungent smell. It is
lighter than air
A lifting gas or lighter-than-air gas is a gas that has a density lower than normal atmospheric gases and rises above them as a result. It is required for aerostats to create buoyancy, particularly in lighter-than-air aircraft, which include free ...
, its density being 0.589 times that of
air
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing f ...
. It is easily liquefied due to the strong
hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
ing between molecules. Gaseous ammonia turns to the colourless
liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, a ...
which has
boils
A boil, also called a furuncle, is a deep folliculitis, which is an infection of the hair follicle. It is most commonly caused by infection by the bacterium '' Staphylococcus aureus'', resulting in a painful swollen area on the skin caused by ...
at , and
freezes
Freezing is a phase transition where a liquid turns into a solid when its temperature is lowered below its freezing point. In accordance with the internationally established definition, freezing means the solidification phase change of a liquid o ...
to colourless crystals at . Few data are available at very high temperatures and pressures, such as
supercritical conditions.
Solid
The crystal symmetry is cubic,
Pearson symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure, and was originated by W. B. Pearson. The symbol is made up of two letters followed by a number. For example:
* Diamond structure ...
cP16,
space group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchan ...
P2
13 No.198, lattice constant 0.5125
nm.
Liquid
Liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, a ...
ammonia possesses strong
ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
ising powers reflecting its high
ε of 22. Liquid ammonia has a very high
standard enthalpy change of vaporization (23.35
kJ/mol, for comparison
water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
40.65 kJ/mol, methane 8.19 kJ/mol,
phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
14.6 kJ/mol) and can therefore be used in laboratories in uninsulated vessels without additional refrigeration. See
liquid ammonia as a solvent.
Solvent properties
Ammonia readily
dissolves in water. In an aqueous solution, it can be expelled by boiling. The
aqueous
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be rep ...
solution of ammonia is
basic
BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Thomas E. Kurtz at Dartmouth College ...
. The maximum concentration of ammonia in water (a
saturated solution
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
) has a
density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematical ...
of 0.880 g/cm
3 and is often known as '.880 ammonia'.
Combustion
Ammonia does not burn readily or sustain
combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
, except under narrow fuel-to-air mixtures of 15–25% air. When mixed with
oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
, it burns with a pale yellowish-green flame. Ignition occurs when
chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
is passed into ammonia, forming nitrogen and
hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride ga ...
; if chlorine is present in excess, then the highly explosive
nitrogen trichloride
Nitrogen trichloride, also known as trichloramine, is the chemical compound with the formula NCl3. This yellow, oily, pungent-smelling and explosive liquid is most commonly encountered as a byproduct of chemical reactions between ammonia-derivative ...
() is also formed.
Decomposition
At high temperature and in the presence of a suitable catalyst or in a pressurized vessel with constant volume and high temperature (e.g. ), ammonia is decomposed into its constituent elements.
Decomposition of ammonia is a slightly endothermic process requiring 23 kJ/mol (5.5
kcal/mol) of ammonia, and yields
hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
and
nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
gas. Ammonia can also be used as a source of hydrogen for acid
fuel cell
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requ ...
s if the unreacted ammonia can be removed.
Ruthenium
Ruthenium is a chemical element with the Symbol (chemistry), symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to ...
and
platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
catalysts
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
were found to be the most active, whereas supported
Ni catalysts were less active.
Table of thermal and physical properties of saturated liquid ammonia:
Table of thermal and physical properties of ammonia () at atmospheric pressure:
Structure
The ammonia molecule has a
trigonal pyramidal
In chemistry, a trigonal pyramid is a molecular geometry with one atom at the apex and three atoms at the corners of a trigonal base, resembling a tetrahedron (not to be confused with the tetrahedral geometry). When all three atoms at the corner ...
shape as predicted by the
valence shell electron pair repulsion theory
Valence shell electron pair repulsion (VSEPR) theory ( , ), is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm the ...
(VSEPR theory) with an experimentally determined bond angle of 106.7°.
The central nitrogen atom has five outer electrons with an additional electron from each hydrogen atom. This gives a total of eight electrons, or four electron pairs that are arranged
tetrahedrally. Three of these
electron pair
In chemistry, an electron pair or Lewis pair consists of two electrons that occupy the same molecular orbital but have opposite spins. Gilbert N. Lewis introduced the concepts of both the electron pair and the covalent bond in a landmark paper he ...
s are used as bond pairs, which leaves one
lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
of electrons. The lone pair repels more strongly than bond pairs, therefore the bond angle is not 109.5°, as expected for a regular tetrahedral arrangement, but 106.8°.
This shape gives the molecule a
dipole
In physics, a dipole () is an electromagnetic phenomenon which occurs in two ways:
*An electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple example of this system i ...
moment and makes it
polar
Polar may refer to:
Geography
Polar may refer to:
* Geographical pole, either of two fixed points on the surface of a rotating body or planet, at 90 degrees from the equator, based on the axis around which a body rotates
* Polar climate, the c ...
. The molecule's polarity, and especially, its ability to form
hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
s, makes ammonia highly miscible with water. The lone pair makes ammonia a
base, a proton acceptor. Ammonia is moderately basic; a 1.0
M aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be re ...
has a
pH of 11.6, and if a strong acid is added to such a solution until the solution is neutral (pH = 7), 99.4% of the ammonia molecules are
protonated
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid, ...
. Temperature and
salinity
Salinity () is the saltiness or amount of salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal ...
also affect the proportion of . The latter has the shape of a regular
tetrahedron
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the o ...
and is
isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in the ...
with
methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Eart ...
.
The ammonia molecule readily undergoes
nitrogen inversion In chemistry, pyramidal inversion (also umbrella inversion) is a fluxional process in compounds with a pyramidal molecule, such as ammonia (NH3) "turns inside out". It is a rapid oscillation of the atom and substituents, the molecule or ion passi ...
at room temperature; a useful analogy is an
umbrella
An umbrella or parasol is a folding canopy supported by wooden or metal ribs that is usually mounted on a wooden, metal, or plastic pole. It is designed to protect a person against rain or sunlight. The term ''umbrella'' is traditionally used ...
turning itself inside out in a strong wind. The
energy barrier
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
to this inversion is 24.7 kJ/mol, and the
resonance frequency
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillat ...
is 23.79
GHz
The hertz (symbol: Hz) is the unit of frequency in the International System of Units (SI), equivalent to one event (or cycle) per second. The hertz is an SI derived unit whose expression in terms of SI base units is s−1, meaning that one he ...
, corresponding to
microwave
Microwave is a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter corresponding to frequencies between 300 MHz and 300 GHz respectively. Different sources define different frequency ran ...
radiation of a
wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tro ...
of 1.260 cm. The absorption at this frequency was the first
microwave spectrum to be observed
and was used in the first
maser
A maser (, an acronym for microwave amplification by stimulated emission of radiation) is a device that produces coherent electromagnetic waves through amplification by stimulated emission. The first maser was built by Charles H. Townes, Ja ...
.
Amphotericity
One of the most characteristic properties of ammonia is its
basicity
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. ...
. Ammonia is considered to be a weak base. It combines with
acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
s to form
ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
s; thus with
hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
it forms
ammonium chloride (sal ammoniac); with
nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
,
ammonium nitrate
Ammonium nitrate is a chemical compound with the chemical formula . It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is ...
, etc. Perfectly dry ammonia gas will not combine with perfectly dry
hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride ga ...
gas; moisture is necessary to bring about the reaction.
As a demonstration experiment under air with ambient moisture, opened bottles of concentrated ammonia and
hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
solutions produce a cloud of
ammonium chloride, which seems to appear "out of nothing" as the salt
aerosol
An aerosol is a suspension (chemistry), suspension of fine solid particles or liquid Drop (liquid), droplets in air or another gas. Aerosols can be natural or Human impact on the environment, anthropogenic. Examples of natural aerosols are fog o ...
forms where the two
diffusing
Molecular diffusion, often simply called diffusion, is the thermal motion of all (liquid or gas) particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size (mass) of ...
clouds of reagents meet between the two bottles.
:
The salts produced by the action of ammonia on acids are known as the
ammonium salts and all contain the
ammonium ion
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
().
Although ammonia is well known as a weak base, it can also act as an extremely weak acid. It is a
protic substance and is capable of formation of
amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
s (which contain the ion). For example,
lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid el ...
dissolves in
liquid ammonia to give a blue solution (
solvated electron
A solvated electron is a free electron in (solvated in) a solution, and is the smallest possible anion. Solvated electrons occur widely. Often, discussions of solvated electrons focus on their solutions in ammonia, which are stable for days, but s ...
) of
lithium amide
Lithium amide or lithium azanide is an inorganic compound with the chemical formula . It is a white solid with a tetragonal crystal structure. Lithium amide can be made by treating lithium metal with liquid ammonia:
:
Other lithium amides
The co ...
:
:
Self-dissociation
Like water, liquid ammonia undergoes
molecular autoionisation to form its
acid and base conjugates:
:
Ammonia often functions as a
weak base
A weak base is a base that, upon dissolution in water, does not dissociate completely, so that the resulting aqueous solution contains only a small proportion of hydroxide ions and the concerned basic radical, and a large proportion of undissociat ...
, so it has some
buffering ability. Shifts in pH will cause more or fewer
ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
cations () and
amide anions () to be present in
solution
Solution may refer to:
* Solution (chemistry), a mixture where one substance is dissolved in another
* Solution (equation), in mathematics
** Numerical solution, in numerical analysis, approximate solutions within specified error bounds
* Soluti ...
. At standard pressure and temperature,
:K = = 10
−30.
Combustion
The
combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
of ammonia to form nitrogen and water is
exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
:
:,
Δ''H''°r = −1267.20 kJ (or −316.8 kJ/mol if expressed per mol of )
The
standard enthalpy change of combustion, Δ''H''°
c, expressed per
mole
Mole (or Molé) may refer to:
Animals
* Mole (animal) or "true mole", mammals in the family Talpidae, found in Eurasia and North America
* Golden moles, southern African mammals in the family Chrysochloridae, similar to but unrelated to Talpida ...
of ammonia and with condensation of the water formed, is −382.81 kJ/mol. Dinitrogen is the thermodynamic product of
combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
: all
nitrogen oxide Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:
Charge-neutral
*Nitric oxide (NO), nitrogen(II) oxide, or nitrogen monoxide
*Nitrogen dioxide (), nitrogen(IV) oxide
* Nitrogen trioxide (), or n ...
s are unstable with respect to and , which is the principle behind the
catalytic converter
A catalytic converter is an exhaust emission control device that converts toxic gases and pollutants in exhaust gas from an internal combustion engine into less-toxic pollutants by catalyzing a redox reaction. Catalytic converters are usually ...
. Nitrogen oxides can be formed as
kinetic products in the presence of appropriate
catalysts
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
, a reaction of great industrial importance in the production of
nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
:
:
A subsequent reaction leads to :
:
The combustion of ammonia in air is very difficult in the absence of a
catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
(such as
platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
gauze or warm
chromium(III) oxide
Chromium(III) oxide (or chromia) is an inorganic compound with the formula . It is one of the principal oxides of chromium and is used as a pigment. In nature, it occurs as the rare mineral eskolaite.
Structure and properties
has the corundum ...
), due to the relatively low
heat of combustion, a lower laminar burning velocity, high
auto-ignition temperature
The autoignition temperature or kindling point of a substance is the lowest temperature in which it spontaneously ignites in a normal atmosphere without an external source of ignition, such as a flame or spark. This temperature is required to s ...
, high
heat of vaporization
The enthalpy of vaporization (symbol ), also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy (enthalpy) that must be added to a liquid substance to transform a quantity of that substance into a gas. T ...
, and a narrow
flammability range. However, recent studies have shown that efficient and stable combustion of ammonia can be achieved using swirl combustors, thereby rekindling research interest in ammonia as a fuel for thermal power production.
The flammable range of ammonia in dry air is 15.15–27.35% and in 100% relative humidity air is 15.95–26.55%. For studying the
kinetics
Kinetics ( grc, κίνησις, , kinesis, ''movement'' or ''to move'') may refer to:
Science and medicine
* Kinetics (physics), the study of motion and its causes
** Rigid body kinetics, the study of the motion of rigid bodies
* Chemical ki ...
of ammonia combustion, knowledge of a detailed reliable reaction mechanism is required, but this has been challenging to obtain.
Formation of other compounds
Ammonia is a direct or indirect precursor to most
manufactured nitrogen-containing compounds.
In
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
, ammonia can act as a
nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
in
substitution reactions.
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
s can be formed by the reaction of ammonia with
alkyl halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely ...
s or with
alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s. The resulting − group is also nucleophilic so
secondary and tertiary amines are often formed. When such multiple substitution is not desired, an excess of ammonia helps minimise it. For example,
methylamine
Methylamine is an organic compound with a formula of . This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine.
Methylamine is sold as a solution in methanol, ...
is prepared by the reaction of ammonia with
chloromethane
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula . One of the haloalkanes, it is a colorless, odorless, flammable gas. Methyl chloride is a crucial reagent in industrial ...
or with
methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
. In both cases,
dimethylamine
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to aroun ...
and
trimethylamine
Trimethylamine (TMA) is an organic compound with the formula N(CH3)3. It is a colorless, hygroscopic, and flammable tertiary amine. It is a gas at room temperature but is usually sold as a 40% solution in water. (It is also sold in pressurized ...
are co-produced.
Ethanolamine
Ethanolamine (2-aminoethanol, monoethanolamine, ETA, or MEA) is an organic chemical compound with the formula or . The molecule is bifunctional, containing both a primary amine and a primary alcohol. Ethanolamine is a colorless, viscous liquid wit ...
is prepared by a ring-opening reaction with
ethylene oxide
Ethylene oxide is an organic compound with the chemical formula, formula . It is a cyclic ether and the simplest epoxide: a three-membered Ring (chemistry), ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless a ...
, and when the reaction is allowed to go further it produces
diethanolamine
Diethanolamine, often abbreviated as DEA or DEOA, is an organic compound with the formula HN(CH2CH2OH)2. Pure diethanolamine is a white solid at room temperature, but its tendencies to absorb water and to supercool meaning that it is often encou ...
and
triethanolamine
Triethanolamine, or TEA is a viscous organic compound that is both a tertiary amine and a triol. A triol is a molecule with three alcohol groups. Approximately 150,000 tonnes were produced in 1999. It is a colourless compound although samples m ...
. The reaction of ammonia with 2-bromopropanoic acid has been used to prepare
racemic
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side c ...
in 70% yield.
Amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
s can be prepared by the reaction of ammonia with
carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
derivatives. For example, ammonia reacts with
formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure . It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Es ...
(HCOOH) to yield
formamide
Formamide is an amide derived from formic acid. It is a colorless liquid which is miscible with water and has an ammonia-like odor. It is chemical feedstock for the manufacture of sulfa drugs and other pharmaceuticals, herbicides and pesticide ...
() when heated.
Acyl chlorides are the most reactive, but the ammonia must be present in at least a twofold excess to neutralise the
hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride ga ...
formed.
Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
s and
anhydride
An organic acid anhydride is an acid anhydride that is an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the pa ...
s also react with ammonia to form amides. Ammonium salts of carboxylic acids can be
dehydrated
In physiology, dehydration is a lack of total body water, with an accompanying disruption of metabolic processes. It occurs when free water loss exceeds free water intake, usually due to exercise, disease, or high environmental temperature. Mil ...
to amides by heating to 150–200 °C as long as no thermally sensitive groups are present.
The hydrogen in ammonia is susceptible to replacement by a myriad of substituents. When dry ammonia gas is heated with metallic
sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
it converts to
sodamide, . With chlorine,
monochloramine
Monochloramine, often called chloramine, is the chemical compound with the formula NH2Cl. Together with dichloramine (NHCl2) and nitrogen trichloride (NCl3), it is one of the three chloramines of ammonia. It is a colorless liquid at its melting p ...
is formed.
Pentavalent ammonia is known as λ
5-amine or, more commonly, ammonium hydride . This crystalline solid is only stable under high pressure and decomposes back into trivalent ammonia (λ
3-amine) and hydrogen gas at normal conditions. This substance was once investigated as a possible solid rocket fuel in 1966.
Ammonia as a ligand
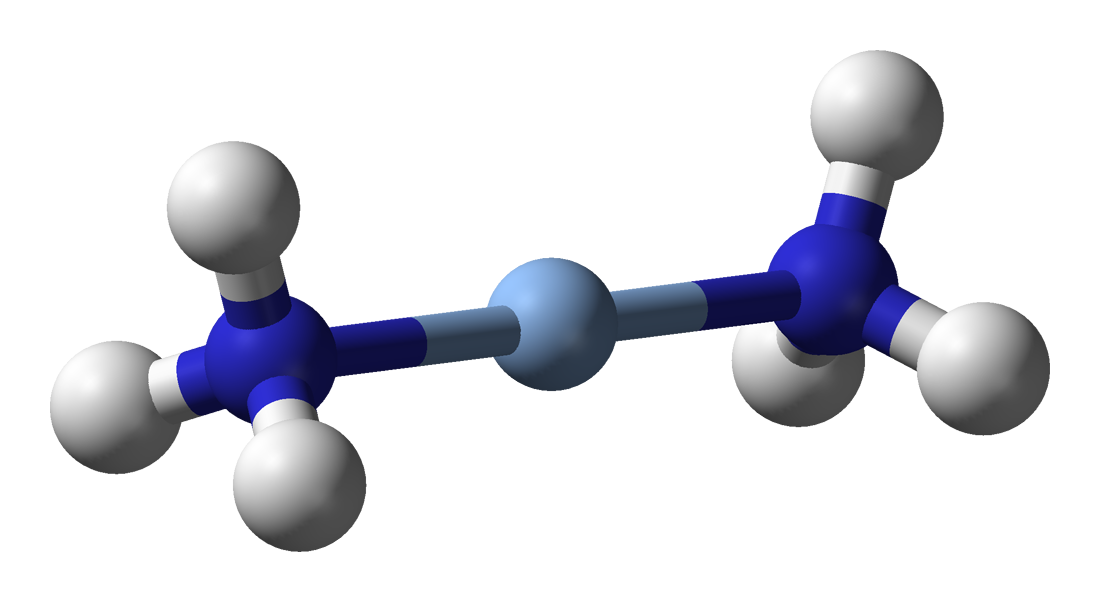

Ammonia can act as a
ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
in
transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
complexes. It is a pure σ-donor, in the middle of the
spectrochemical series
A spectrochemical series is a list of ligands ordered by ligand "strength", and a list of metal ions based on oxidation number, group and element. For a metal ion, the ligands modify the difference in energy Δ between the d orbitals, called the l ...
, and shows intermediate
hard–soft behaviour (see also
ECW model). Its relative donor strength toward a series of acids, versus other Lewis bases, can be illustrated by
C-B plots. For historical reasons, ammonia is named ammine in the nomenclature of
coordination compound
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
s. Some notable ammine complexes include tetraamminediaquacopper(II) (), a dark blue complex formed by adding ammonia to a solution of copper(II) salts. Tetraamminediaquacopper(II) hydroxide is known as
Schweizer's reagent
Schweizer's reagent is the metal ammine complex with the formula u(NH3)4(H2O)2OH)2. This deep-blue compound is used in purifying cellulose.
It is prepared by precipitating copper(II) hydroxide from an aqueous solution of copper sulfate using sod ...
, and has the remarkable ability to dissolve
cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell wall ...
. Diamminesilver(I) () is the active species in
Tollens' reagent
Tollens' reagent (chemical formula Ag(NH3)2OH) is a chemical reagent used to distinguish between aldehydes and ketones along with some alpha-hydroxy ketones which can tautomerize into aldehydes. The reagent consists of a solution of silver nit ...
. Formation of this complex can also help to distinguish between precipitates of the different silver halides:
silver chloride
Silver chloride is a chemical compound with the chemical formula Ag Cl. This white crystalline solid is well known for its low solubility in water (this behavior being reminiscent of the chlorides of Tl+ and Pb2+). Upon illumination or heating, ...
(AgCl) is soluble in dilute (2 M) ammonia solution,
silver bromide
Silver bromide (AgBr) is a soft, pale-yellow, water-insoluble salt well known (along with other silver halides) for its unusual sensitivity to light. This property has allowed silver halides to become the basis of modern photographic materials. A ...
(AgBr) is only soluble in concentrated ammonia solution, whereas
silver iodide
Silver iodide is an inorganic compound with the formula Ag I. The compound is a bright yellow solid, but samples almost always contain impurities of metallic silver that give a gray coloration. The silver contamination arises because AgI is hig ...
(AgI) is insoluble in aqueous ammonia.
Ammine complexes of
chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hardne ...
(III) were known in the late 19th century, and formed the basis of
Alfred Werner
Alfred Werner (12 December 1866 – 15 November 1919) was a Swiss chemist who was a student at ETH Zurich and a professor at the University of Zurich. He won the Nobel Prize in Chemistry in 1913 for proposing the octahedral configuration of ...
's revolutionary theory on the structure of coordination compounds. Werner noted only two
isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
s (''fac''- and ''mer''-) of the complex could be formed, and concluded the ligands must be arranged around the metal ion at the
vertices of an
octahedron
In geometry, an octahedron (plural: octahedra, octahedrons) is a polyhedron with eight faces. The term is most commonly used to refer to the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at ea ...
. This proposal has since been confirmed by
X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
.
An ammine ligand bound to a metal ion is markedly more acidic than a free ammonia molecule, although
deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
in
aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be re ...
is still rare. One example is the
Calomel reaction, where the resulting amidomercury(II) compound is highly insoluble.
:
Ammonia forms 1:1
adduct
An adduct (from the Latin ''adductus'', "drawn toward" alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all co ...
s with a variety of
Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
s such as
,
phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it req ...
, and
. Ammonia is a
hard base (HSAB theory) and its
E & C parameters are E
B = 2.31 and C
B = 2.04. Its relative donor strength toward a series of acids, versus other Lewis bases, can be illustrated by
C-B plots.
Detection and determination
Ammonia in solution
Ammonia and ammonium salts can be readily detected, in very minute traces, by the addition of
Nessler's solution
Potassium tetraiodomercurate(II) is an inorganic compound consisting of potassium cations and the tetraiodomercurate(II) anion. It is mainly used as Nessler's reagent, a 0.09 mol/L solution of potassium tetraiodomercurate(II) (K2 gI4 in 2.5&n ...
, which gives a distinct yellow colouration in the presence of the slightest trace of ammonia or ammonium salts. The amount of ammonia in ammonium salts can be estimated quantitatively by distillation of the salts with
sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
(NaOH) or
potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which exp ...
(KOH), the ammonia evolved being absorbed in a known volume of standard
sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
and the excess of acid then determined
volumetrically; or the ammonia may be absorbed in
hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
and the ammonium chloride so formed precipitated as
ammonium hexachloroplatinate
Ammonium hexachloroplatinate, also known as ammonium chloroplatinate, is the inorganic compound with the formula (NH4)2 tCl6 It is a rare example of a soluble platinum(IV) salt that is not hygroscopic. It forms intensely yellow solutions in water. ...
, .
Gaseous ammonia
Sulfur sticks are burnt to detect small leaks in industrial ammonia refrigeration systems. Larger quantities can be detected by warming the salts with a caustic alkali or with
quicklime
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "''lime''" connotes calcium-containing inorganic ma ...
, when the characteristic smell of ammonia will be at once apparent. Ammonia is an irritant and irritation increases with concentration; the
permissible exposure limit
The permissible exposure limit (PEL or OSHA PEL) is a legal limit in the United States for exposure of an employee to a chemical substance or physical agent such as high level noise. Permissible exposure limits are established by the Occupational ...
is 25
ppm, and lethal above 500 ppm. Higher concentrations are hardly detected by conventional detectors, the type of detector is chosen according to the sensitivity required (e.g. semiconductor, catalytic, electrochemical). Holographic sensors have been proposed for detecting concentrations up to 12.5% in volume.
Ammoniacal nitrogen (NH3-N)
Ammoniacal nitrogen Ammoniacal nitrogen (NH3-N) is a measure for the amount of ammonia, a toxic pollutant often found in landfill leachate and in waste products, such as sewage, liquid manure and other liquid organic waste products. It can also be used as a measure ...
(NH
3-N) is a measure commonly used for testing the quantity of
ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
ions, derived naturally from ammonia, and returned to ammonia via organic processes, in water or waste liquids. It is a measure used mainly for quantifying values in waste treatment and water purification systems, as well as a measure of the health of natural and man-made water reserves. It is measured in units of mg/L (
milligram
The kilogram (also kilogramme) is the unit of mass in the International System of Units (SI), having the unit symbol kg. It is a widely used measure in science, engineering and commerce worldwide, and is often simply called a kilo colloquially. ...
per
litre
The litre (international spelling) or liter (American English spelling) (SI symbols L and l, other symbol used: ℓ) is a metric unit of volume. It is equal to 1 cubic decimetre (dm3), 1000 cubic centimetres (cm3) or 0.001 cubic metre (m3). ...
).
History


The ancient Greek historian
Herodotus
Herodotus ( ; grc, , }; BC) was an ancient Greek historian and geographer from the Greek city of Halicarnassus, part of the Persian Empire (now Bodrum, Turkey) and a later citizen of Thurii in modern Calabria ( Italy). He is known f ...
mentioned that there were
outcrop
An outcrop or rocky outcrop is a visible exposure of bedrock or ancient superficial deposits on the surface of the Earth.
Features
Outcrops do not cover the majority of the Earth's land surface because in most places the bedrock or superficial ...
s of salt in an area of Libya that was inhabited by a people called the "Ammonians" (now: the
Siwa oasis
The Siwa Oasis ( ar, واحة سيوة, ''Wāḥat Sīwah,'' ) is an urban oasis in Egypt; between the Qattara Depression and the Great Sand Sea in the Western Desert (Egypt), Western Desert, 50 km (30 mi) east of the Libyan Egypt–Li ...
in northwestern Egypt, where salt lakes still exist). The Greek geographer
Strabo
Strabo''Strabo'' (meaning "squinty", as in strabismus) was a term employed by the Romans for anyone whose eyes were distorted or deformed. The father of Pompey was called "Pompeius Strabo". A native of Sicily so clear-sighted that he could see ...
also mentioned the salt from this region. However, the ancient authors
Dioscorides
Pedanius Dioscorides ( grc-gre, Πεδάνιος Διοσκουρίδης, ; 40–90 AD), “the father of pharmacognosy”, was a Greek physician, pharmacologist, botanist, and author of ''De materia medica'' (, On Medical Material) —a 5-vol ...
,
Apicius
''Apicius'', also known as ''De re culinaria'' or ''De re coquinaria'' (''On the Subject of Cooking'') is a collection of Roman cookery recipes. It is thought to have been compiled in the fifth century AD. Its language is in many ways closer ...
,
Arrian
Arrian of Nicomedia (; Greek: ''Arrianos''; la, Lucius Flavius Arrianus; )
was a Greek historian, public servant, military commander and philosopher of the Roman period.
''The Anabasis of Alexander'' by Arrian is considered the best ...
,
Synesius
Synesius (; el, Συνέσιος; c. 373 – c. 414), was a Greek bishop of Ptolemais in ancient Libya, a part of the Western Pentapolis of Cyrenaica after 410. He was born of wealthy parents at Balagrae (now Bayda, Libya) near Cyrene between ...
, and
Aëtius of Amida
Aëtius of Amida (; grc-gre, Ἀέτιος Ἀμιδηνός; Latin: ''Aëtius Amidenus''; fl. mid-5th century to mid-6th century) was a Byzantine Greek physician and medical writer, particularly distinguished by the extent of his erudition. His ...
described this salt as forming clear crystals that could be used for cooking and that were essentially
rock salt
Halite (), commonly known as rock salt, is a type of salt, the mineral (natural) form of sodium chloride ( Na Cl). Halite forms isometric crystals. The mineral is typically colorless or white, but may also be light blue, dark blue, purple, pi ...
. ''Hammoniacus sal'' appears in the writings of
Pliny
Pliny may refer to:
People
* Pliny the Elder (23–79 CE), ancient Roman nobleman, scientist, historian, and author of ''Naturalis Historia'' (''Pliny's Natural History'')
* Pliny the Younger (died 113), ancient Roman statesman, orator, w ...
, although it is not known whether the term is identical with the more modern sal ammoniac (ammonium chloride).
The fermentation of urine by bacteria produces a
solution of ammonia; hence fermented urine was used in
Classical Antiquity
Classical antiquity (also the classical era, classical period or classical age) is the period of cultural history between the 8th century BC and the 5th century AD centred on the Mediterranean Sea, comprising the interlocking civilizations of ...
to wash cloth and clothing, to remove hair from hides in preparation for tanning, to serve as a
mordant
A mordant or dye fixative is a substance used to set (i.e. bind) dyes on fabrics by forming a coordination complex with the dye, which then attaches to the fabric (or tissue). It may be used for dyeing fabrics or for intensifying stains in ...
in dying cloth, and to remove rust from iron. It was also used by
ancient dentists to wash teeth.
In the form of sal ammoniac ''(نشادر, nushadir)'', ammonia was important to the
Muslim alchemists. It was mentioned in the ''Book of Stones'', likely written in the 9th century and attributed to
Jābir ibn Hayyān
Abū Mūsā Jābir ibn Ḥayyān (Arabic: , variously called al-Ṣūfī, al-Azdī, al-Kūfī, or al-Ṭūsī), died 806−816, is the purported author of an enormous number and variety of works in Arabic, often called the Jabirian corpus. The ...
.
It was also important to the European
alchemists
Alchemy (from Arabic: ''al-kīmiyā''; from Ancient Greek: χυμεία, ''khumeía'') is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practiced in China, India, the Muslim world, ...
of the 13th century, being mentioned by
Albertus Magnus
Albertus Magnus (c. 1200 – 15 November 1280), also known as Saint Albert the Great or Albert of Cologne, was a German Dominican friar, philosopher, scientist, and bishop. Later canonised as a Catholic saint, he was known during his life ...
. It was also used by
dye
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution an ...
rs in the
Middle Ages
In the history of Europe, the Middle Ages or medieval period lasted approximately from the late 5th to the late 15th centuries, similar to the post-classical period of global history. It began with the fall of the Western Roman Empire a ...
in the form of fermented
urine
Urine is a liquid by-product of metabolism in humans and in many other animals. Urine flows from the kidneys through the ureters to the urinary bladder. Urination results in urine being excretion, excreted from the body through the urethra.
Cel ...
to alter the colour of vegetable dyes. In the 15th century,
Basilius Valentinus
Basil Valentine is the Anglicised version of the name Basilius Valentinus, ostensibly a 15th-century alchemist, possibly Canon of the Benedictine Priory of Saint Peter in Erfurt, Germany but more likely a pseudonym used by one or several 16th-ce ...
showed that ammonia could be obtained by the action of alkalis on sal ammoniac. At a later period, when sal ammoniac was obtained by distilling the hooves and horns of oxen and neutralizing the resulting carbonate with
hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
, the name "spirit of hartshorn" was applied to ammonia.
Gaseous ammonia was first isolated by
Joseph Black
Joseph Black (16 April 1728 – 6 December 1799) was a Scottish physicist and chemist, known for his discoveries of magnesium, latent heat, specific heat, and carbon dioxide. He was Professor of Anatomy and Chemistry at the University of Glas ...
in 1756 by reacting ''sal ammoniac'' (
ammonium chloride) with ''calcined magnesia'' (
magnesium oxide
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions ...
). It was isolated again by
Peter Woulfe
Peter Woulfe (1727–1803) was an Anglo-Irish chemist and mineralogist. He first had the idea that wolframite might contain a previously undiscovered element (tungsten).
In 1771, Woulfe reported the formation of a yellow dye when indigo was tr ...
in 1767, by
Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hydrog ...
in 1770 and by
Joseph Priestley
Joseph Priestley (; 24 March 1733 – 6 February 1804) was an English chemist, natural philosopher, separatist theologian, grammarian, multi-subject educator, and liberal political theorist. He published over 150 works, and conducted exp ...
in 1773 and was termed by him "alkaline air". Eleven years later in 1785,
Claude Louis Berthollet
Claude Louis Berthollet (, 9 December 1748 – 6 November 1822) was a Savoyard-French chemist who became vice president of the French Senate in 1804. He is known for his scientific contributions to theory of chemical equilibria via the mecha ...
ascertained its composition.
The
Haber–Bosch process
The Haber process, also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. It is named after its inventors, the German chemists Fritz Haber and C ...
to produce ammonia from the nitrogen in the air was developed by
Fritz Haber
Fritz Haber (; 9 December 186829 January 1934) was a German chemist who received the Nobel Prize in Chemistry in 1918 for his invention of the Haber–Bosch process, a method used in industry to synthesize ammonia from nitrogen gas and hydrogen ...
and
Carl Bosch
Carl Bosch (; 27 August 1874 – 26 April 1940) was a German chemist and engineer and Nobel Laureate in Chemistry. He was a pioneer in the field of high-pressure industrial chemistry and founder of IG Farben, at one point the world's largest ...
in 1909 and patented in 1910. It was first used on an industrial scale in Germany during
World War I
World War I (28 July 1914 11 November 1918), often abbreviated as WWI, was one of the deadliest global conflicts in history. Belligerents included much of Europe, the Russian Empire, the United States, and the Ottoman Empire, with fightin ...
,
following the allied blockade that cut off the supply of nitrates from
Chile
Chile, officially the Republic of Chile, is a country in the western part of South America. It is the southernmost country in the world, and the closest to Antarctica, occupying a long and narrow strip of land between the Andes to the east a ...
. The ammonia was used to produce explosives to sustain war efforts.
Before the availability of natural gas, hydrogen as a precursor to
ammonia production was produced via the
electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
of water or using the
chloralkali process
The chloralkali process (also chlor-alkali and chlor alkali) is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide (caustic soda), which are comm ...
.
With the advent of the steel industry in the 20th century, ammonia became a byproduct of the production of
coking
Coking is the heating of coal in the absence of oxygen to a temperature above 600 °C to drive off the volatile components of the raw coal, leaving a hard, strong, porous material of high carbon content called coke. Coke consists almost ent ...
coal.
Applications
Solvent
Liquid ammonia is the best-known and most widely studied nonaqueous ionising solvent. Its most conspicuous property is its ability to dissolve alkali metals to form highly coloured, electrically conductive solutions containing
solvated electron
A solvated electron is a free electron in (solvated in) a solution, and is the smallest possible anion. Solvated electrons occur widely. Often, discussions of solvated electrons focus on their solutions in ammonia, which are stable for days, but s ...
s. Apart from these remarkable solutions, much of the chemistry in liquid ammonia can be classified by analogy with related reactions in aqueous solutions. Comparison of the physical properties of with those of water shows has the lower melting point, boiling point, density,
viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inte ...
,
dielectric constant
The relative permittivity (in older texts, dielectric constant) is the permittivity of a material expressed as a ratio with the electric permittivity of a vacuum. A dielectric is an insulating material, and the dielectric constant of an insulat ...
and
electrical conductivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allow ...
; this is due at least in part to the weaker hydrogen bonding in and because such bonding cannot form cross-linked networks, since each molecule has only one lone pair of electrons compared with two for each molecule. The ionic self-
dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (K_D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex fa ...
of liquid at −50 °C is about 10
−33.

Solubility of salts
Liquid ammonia is an ionising solvent, although less so than water, and dissolves a range of ionic compounds, including many
nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
s,
nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
s,
cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of ...
s,
thiocyanate
Thiocyanate (also known as rhodanide) is the anion . It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) thiocyanate was formerly used in pyrot ...
s,
metal cyclopentadienyl complexes and
metal bis(trimethylsilyl)amides
Metal bis(trimethylsilyl)amides (often abbreviated as metal silylamides) are coordination complexes composed of a cationic metal with anionic bis(trimethylsilyl)amide ligands and are part of a broader category of metal amides.
Due to the bulky hy ...
.
Most ammonium salts are soluble and act as acids in liquid ammonia solutions. The solubility of
halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
salts increases from
fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typ ...
to
iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine defic ...
. A saturated solution of
ammonium nitrate
Ammonium nitrate is a chemical compound with the chemical formula . It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is ...
(Divers' solution, named after
Edward Divers
Edward Divers FRS (27 November 1837 – 8 April 1912) was a British experimental chemist who rose to prominence despite being visually impaired from young age. Between 1873 and 1899, Divers lived and worked in Japan and significantly contribute ...
) contains 0.83 mol solute per mole of ammonia and has a
vapour pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases ...
of less than 1 bar even at .
Solutions of metals
Liquid ammonia will dissolve all of the
alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
s and other
electropositive
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
metals such as
Ca,
Sr,
Ba,
Eu, and
Yb (also
Mg using an electrolytic process
). At low concentrations (<0.06 mol/L), deep blue solutions are formed: these contain metal cations and
solvated electron
A solvated electron is a free electron in (solvated in) a solution, and is the smallest possible anion. Solvated electrons occur widely. Often, discussions of solvated electrons focus on their solutions in ammonia, which are stable for days, but s ...
s, free electrons that are surrounded by a cage of ammonia molecules.
These solutions are very useful as strong reducing agents. At higher concentrations, the solutions are metallic in appearance and in electrical conductivity. At low temperatures, the two types of solution can coexist as
immiscible phases.
Redox properties of liquid ammonia
The range of thermodynamic stability of liquid ammonia solutions is very narrow, as the potential for oxidation to dinitrogen,
''E''° (), is only +0.04 V. In practice, both oxidation to dinitrogen and reduction to dihydrogen are slow. This is particularly true of reducing solutions: the solutions of the alkali metals mentioned above are stable for several days, slowly decomposing to the
metal amide Metal amides (systematic name metal azanides) are a class of coordination compounds composed of a metal center with amide ligands of the form NR2−. Amide ligands have two electron pairs available for bonding. In principle, they can be terminal or ...
and dihydrogen. Most studies involving liquid ammonia solutions are done in reducing conditions; although oxidation of liquid ammonia is usually slow, there is still a risk of explosion, particularly if transition metal ions are present as possible catalysts.

Fertilizer
In the US as of 2019, approximately 88% of ammonia was used as fertilizers either as its salts, solutions or anhydrously.
When applied to soil, it helps provide increased yields of crops such as maize and wheat. 30% of agricultural nitrogen applied in the US is in the form of anhydrous ammonia and worldwide 110 million tonnes are applied each year.
Precursor to nitrogenous compounds
Ammonia is directly or indirectly the precursor to most nitrogen-containing compounds. Virtually all synthetic nitrogen compounds are derived from ammonia. An important derivative is
nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
. This key material is generated via the
Ostwald process The Ostwald process is a chemical process used for making nitric acid (HNO3). Wilhelm Ostwald developed the process, and he patented it in 1902. The Ostwald process is a mainstay of the modern chemical industry, and it provides the main raw materi ...
by
oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
of ammonia with air over a
platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
catalyst at , ≈9 atm.
Nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its che ...
is an intermediate in this conversion:
:
Nitric acid is used for the production of
fertilizer
A fertilizer (American English) or fertiliser (British English; see spelling differences) is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from ...
s,
explosive
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An expl ...
s, and many organonitrogen compounds.
Ammonia is also used to make the following compounds:
*
Hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine ...
, in the
Olin Raschig process The Olin Raschig process is a chemical process for the production of hydrazine. The main steps in this process, patented by German chemist Friedrich Raschig in 1906 and one of three reactions named after him, are the formation of monochloramine from ...
and the
peroxide process The peroxide process is a method for the industrial production of hydrazine.
In this process hydrogen peroxide is used as an oxidant instead of sodium hypochlorite, which is traditionally used to generate hydrazine. The main advantage of the perox ...
*
Hydrogen cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on an ...
, in the
BMA process The BMA process or Degussa process is a chemical process developed by the German chemical company Degussa for the production of hydrogen cyanide from methane and ammonia in presence of a platinum catalyst. Hydrogen cyanide is used in the chemical i ...
and the
Andrussow process
The Andrussow process is an industrial process for the production of hydrogen cyanide from methane and ammonia in the presence of oxygen and a platinum catalyst.
:2 CH4 + 2 NH3 + 3 O2 → 2 HCN + 6 H2O
The process is based on a reaction ...
*
Hydroxylamine
Hydroxylamine is an inorganic compound with the formula . The material is a white crystalline, hygroscopic compound.Greenwood and Earnshaw. ''Chemistry of the Elements.'' 2nd Edition. Reed Educational and Professional Publishing Ltd. pp. 431–43 ...
and
ammonium carbonate
Ammonium carbonate is a salt with the chemical formula (NH4)2CO3. Since it readily degrades to gaseous ammonia and carbon dioxide upon heating, it is used as a leavening agent and also as smelling salt. It is also known as baker's ammonia and is ...
, in the
Raschig process
*
Phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it req ...
, in the
Raschig–Hooker process
The Raschig–Hooker process is a chemical process for the production of chlorobenzene and phenol.
The Raschig–Hooker process was patented by Friedrich Raschig, a German chemist and politician also known for the Raschig process, the Olin Rasch ...
*
Urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important r ...
, in the
Bosch–Meiser urea process and in
Wöhler synthesis
The Wöhler synthesis is the conversion of ammonium cyanate into urea. This chemical reaction was described in 1828 by Friedrich Wöhler. It is often cited as the starting point of modern organic chemistry. Although the Wöhler reaction concerns ...
*
Amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
s, using
Strecker amino-acid synthesis
*
Acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions. In terms of its molecular ...
, in the
Sohio process
Ammonia can also be used to make compounds in reactions which are not specifically named. Examples of such compounds include:
ammonium perchlorate
Ammonium perchlorate ("AP") is an inorganic compound with the formula NH4 ClO4. It is a colorless or white solid that is soluble in water. It is a powerful oxidizer. Combined with a fuel, it can be used as a rocket propellant called ammonium pe ...
,
ammonium nitrate
Ammonium nitrate is a chemical compound with the chemical formula . It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is ...
,
formamide
Formamide is an amide derived from formic acid. It is a colorless liquid which is miscible with water and has an ammonia-like odor. It is chemical feedstock for the manufacture of sulfa drugs and other pharmaceuticals, herbicides and pesticide ...
,
dinitrogen tetroxide
Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide (NTO), and occasionally (usually among ex-USSR/Russia rocket engineers) as amyl, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium ...
,
alprazolam
Alprazolam, sold under the brand name Xanax, among others, is a fast-acting, potent tranquilizer of medium duration in the triazolobenzodiazepine (TBZD) class, which are benzodiazepines (BZDs) fused with a triazole ring. It is most commonly u ...
,
ethanolamine
Ethanolamine (2-aminoethanol, monoethanolamine, ETA, or MEA) is an organic chemical compound with the formula or . The molecule is bifunctional, containing both a primary amine and a primary alcohol. Ethanolamine is a colorless, viscous liquid wit ...
,
ethyl carbamate
Ethyl carbamate (also called urethane) is an organic compound with the formula CH3CH2OC(O)NH2. It is an ester of carbamic acid and a white solid. Despite its name, it is not a component of polyurethanes. Because it is a carcinogen, it is rarely us ...
,
hexamethylenetetramine
Hexamethylenetetramine, also known as methenamine, hexamine, or urotropin, is a heterocyclic organic compound with the formula (CH2)6N4. This white crystalline compound is highly soluble in water and polar organic solvents. It has a cage-like s ...
, and
ammonium bicarbonate
Ammonium bicarbonate is an inorganic compound with formula (NH4)HCO3. The compound has many names, reflecting its long history. Chemically speaking, it is the bicarbonate salt of the ammonium ion. It is a colourless solid that degrades readily to ...
.
Cleansing agent

Household "ammonia" (more correctly called ammonium hydroxide) is a
solution of in water, and is used as a general purpose cleaner for many surfaces. Because ammonia results in a relatively streak-free shine, one of its most common uses is to clean glass, porcelain and stainless steel. It is also frequently used for cleaning ovens and soaking items to loosen baked-on grime. Household ammonia ranges in concentration by weight from 5 to 10% ammonia. United States manufacturers of cleaning products are required to provide the product's
material safety data sheet
A safety data sheet (SDS), material safety data sheet (MSDS), or product safety data sheet (PSDS) is a document that lists information relating to occupational safety and health for the use of various substances and products. SDSs are a widely ...
which lists the concentration used.
Solutions of ammonia (5–10% by weight) are used as household cleaners, particularly for glass. These solutions are irritating to the eyes and
mucous membrane
A mucous membrane or mucosa is a membrane that lines various cavities in the body of an organism and covers the surface of internal organs. It consists of one or more layers of epithelial cells overlying a layer of loose connective tissue. It is ...
s (respiratory and digestive tracts), and to a lesser extent the skin. Experts advise that caution be used to ensure the substance is not mixed into any liquid containing bleach, due to the danger of toxic gas. Mixing with
chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
-containing products or strong oxidants, such as household
bleach
Bleach is the generic name for any chemical product that is used industrially or domestically to remove color (whitening) from a fabric or fiber or to clean or to remove stains in a process called bleaching. It often refers specifically, to ...
, can generate
chloramines
Chloramines refer to derivatives of ammonia and organic amines wherein one or more N-H bonds have been replaced by N-Cl bonds. Two classes of compounds are considered: inorganic chloramines and organic chloramines.
Inorganic chloramines
Inorgan ...
.
Experts also warn not to use ammonia-based cleaners (such as glass or window cleaners) on car touchscreens, due to the risk of damage to the screen's anti-glare and anti-fingerprint coatings.
Fermentation
Solutions of ammonia ranging from 16% to 25% are used in the
fermentation
Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food ...
industry as a source of nitrogen for microorganisms and to adjust pH during fermentation.
Antimicrobial agent for food products
As early as in 1895, it was known that ammonia was "strongly
antiseptic
An antiseptic (from Greek ἀντί ''anti'', "against" and σηπτικός ''sēptikos'', "putrefactive") is an antimicrobial substance or compound that is applied to living tissue/skin to reduce the possibility of infection, sepsis, or putre ...
... it requires 1.4 grams per litre to preserve
beef tea (broth)." In one study, anhydrous ammonia destroyed 99.999% of
zoonotic bacteria
A zoonosis (; plural zoonoses) or zoonotic disease is an infectious disease of humans caused by a pathogen (an infectious agent, such as a bacterium, virus, parasite or prion) that has jumped from a non-human (usually a vertebrate) to a human. ...
in 3 types of
animal feed
Animal feed is food given to domestic animals, especially livestock, in the course of animal husbandry. There are two basic types: fodder and forage. Used alone, the word ''feed'' more often refers to fodder. Animal feed is an important input to ...
, but not
silage
Silage () is a type of fodder made from green foliage crops which have been preserved by fermentation to the point of acidification. It can be fed to cattle, sheep and other such ruminants (cud-chewing animals). The fermentation and storage p ...
. Anhydrous ammonia is currently used commercially to reduce or eliminate
microbial
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
contamination of
beef
Beef is the culinary name for meat from cattle (''Bos taurus'').
In prehistoric times, humankind hunted aurochs and later domesticated them. Since that time, numerous breeds of cattle have been bred specifically for the quality or quantity ...
.
Lean finely textured beef (popularly known as "
pink slime
Pink slime (also known as lean finely textured beef or LFTB, finely textured beef, or boneless lean beef trimmings or BLBT) is a meat by-product used as a food additive to ground beef and beef-based processed meats, as a filler, or to red ...
") in the beef industry is made from fatty
beef trimmings (c. 50–70% fat) by removing the fat using heat and
centrifugation
Centrifugation is a mechanical process which involves the use of the centrifugal force to separate particles from a solution according to their size, shape, density, medium viscosity and rotor speed. The denser components of the mixture migrate ...
, then treating it with ammonia to kill ''
E. coli''. The process was deemed effective and safe by the
US Department of Agriculture
The United States Department of Agriculture (USDA) is the United States federal executive departments, federal executive department responsible for developing and executing federal laws related to farming, forestry, rural economic development, ...
based on a study that found that the treatment reduces ''E. coli'' to undetectable levels. There have been safety concerns about the process as well as consumer complaints about the taste and smell of ammonia-treated beef.
Fuel


The raw
energy density
In physics, energy density is the amount of energy stored in a given system or region of space per unit volume. It is sometimes confused with energy per unit mass which is properly called specific energy or .
Often only the ''useful'' or extract ...
of liquid ammonia is 11.5 MJ/L,
which is about a third that of
diesel
Diesel may refer to:
* Diesel engine, an internal combustion engine where ignition is caused by compression
* Diesel fuel, a liquid fuel used in diesel engines
* Diesel locomotive, a railway locomotive in which the prime mover is a diesel engin ...
. There is the opportunity to convert ammonia back to hydrogen, where it can be used to power hydrogen fuel cells, or it may be used directly within high-temperature
solid oxide direct ammonia fuel cells to provide efficient power sources that do not emit
greenhouse gas
A greenhouse gas (GHG or GhG) is a gas that Absorption (electromagnetic radiation), absorbs and Emission (electromagnetic radiation), emits radiant energy within the thermal infrared range, causing the greenhouse effect. The primary greenhouse ...
es.
The conversion of ammonia to hydrogen via the
sodium amide
Sodium amide, commonly called sodamide (systematic name sodium azanide), is the inorganic compound with the formula . It is a salt composed of the sodium cation and the azanide anion. This solid, which is dangerously reactive toward water, is white ...
process,
either for combustion or as fuel for a
proton exchange membrane fuel cell
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
,
[ is possible. Another method is the catalytic decomposition of ammonia using solid catalysts. Conversion to hydrogen would allow the storage of hydrogen at nearly 18 wt% compared to ≈5% for gaseous hydrogen under pressure.
Ammonia engines or ammonia motors, using ammonia as a ]working fluid
For fluid power, a working fluid is a gas or liquid that primarily transfers force, motion, or mechanical energy. In hydraulics, water or hydraulic fluid transfers force between hydraulic components such as hydraulic pumps, hydraulic cylinders, a ...
, have been proposed and occasionally used. The principle is similar to that used in a fireless locomotive
A fireless locomotive is a type of locomotive which uses reciprocating engines powered from a reservoir of compressed air or steam, which is filled at intervals from an external source. They offer advantages over conventional steam locomotives of ...
, but with ammonia as the working fluid, instead of steam or compressed air. Ammonia engines were used experimentally in the 19th century by Goldsworthy Gurney
Sir Goldsworthy Gurney (14 February 1793 – 28 February 1875) was an English surgeon, chemist, architect, builder, lecturer and consultant. He was a prototypical British gentleman scientist and inventor of the Victorian era.
Amongst many acc ...
in the UK and the St. Charles Avenue Streetcar line in New Orleans in the 1870s and 1880s,World War II
World War II or the Second World War, often abbreviated as WWII or WW2, was a world war that lasted from 1939 to 1945. It involved the vast majority of the world's countries—including all of the great powers—forming two opposin ...
ammonia was used to power buses in Belgium.[
Ammonia is sometimes proposed as a practical alternative to ]fossil fuel
A fossil fuel is a hydrocarbon-containing material formed naturally in the Earth's crust from the remains of dead plants and animals that is extracted and burned as a fuel. The main fossil fuels are coal, oil, and natural gas. Fossil fuels m ...
for internal combustion engine
An internal combustion engine (ICE or IC engine) is a heat engine in which the combustion of a fuel occurs with an oxidizer (usually air) in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal combus ...
s.octane rating
An octane rating, or octane number, is a standard measure of a fuel's ability to withstand compression in an internal combustion engine without detonating. The higher the octane number, the more compression the fuel can withstand before detonating ...
of 120carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
, carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
, hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ex ...
, or soot
Soot ( ) is a mass of impure carbon particles resulting from the incomplete combustion of hydrocarbons. It is more properly restricted to the product of the gas-phase combustion process but is commonly extended to include the residual pyrolysed ...
.
Ammonia production currently creates 1.8% of global emissions. "Green ammonia" is ammonia produced by using green hydrogen (hydrogen produced by electrolysis), whereas "blue ammonia" is ammonia produced using blue hydrogen (hydrogen produced by steam methane reforming where the carbon dioxide has been captured and stored).
However, ammonia cannot be easily used in existing Otto cycle
An Otto cycle is an idealized thermodynamic cycle that describes the functioning of a typical spark ignition piston engine. It is the thermodynamic cycle most commonly found in automobile engines.
The Otto cycle is a description of what happ ...
engines because of its very narrow flammability range. The 60 MW Rjukan dam in Telemark
Telemark is a traditional region, a former county, and a current electoral district in southern Norway. In 2020, Telemark merged with the former county of Vestfold to form the county of Vestfold og Telemark. Telemark borders the traditional ...
, Norway, produced ammonia for many years from 1913, providing fertilizer for much of Europe. Despite this, several tests have been run.
Compared to hydrogen as a fuel, ammonia is much more energy efficient, and could be produced, stored, and delivered at a much lower cost than hydrogen, which must be kept compressed or as a cryogenic liquid.[
Rocket engines have also been fueled by ammonia. The Reaction Motors XLR99 rocket engine that powered the hypersonic research aircraft used liquid ammonia. Although not as powerful as other fuels, it left no soot in the reusable rocket engine, and its density approximately matches the density of the oxidizer, liquid oxygen, which simplified the aircraft's design.
In early August 2018, scientists from ]Australia
Australia, officially the Commonwealth of Australia, is a Sovereign state, sovereign country comprising the mainland of the Australia (continent), Australian continent, the island of Tasmania, and numerous List of islands of Australia, sma ...
's Commonwealth Scientific and Industrial Research Organisation
The Commonwealth Scientific and Industrial Research Organisation (CSIRO) is an Australian Government agency responsible for scientific research.
CSIRO works with leading organisations around the world. From its headquarters in Canberra, CSIRO ...
(CSIRO) announced the success of developing a process to release hydrogen from ammonia and harvest that at ultra-high purity as a fuel for cars. This uses a special membrane. Two demonstration fuel cell vehicle
A fuel cell vehicle (FCV) or fuel cell electric vehicle (FCEV) is an electric vehicle that uses a fuel cell, sometimes in combination with a small battery or supercapacitor, to power its onboard electric motor. Fuel cells in vehicles generate e ...
s have the technology, a Hyundai Nexo
The Hyundai Nexo ( ko, 현대 넥쏘, translit=Hyeondae Negso) is a hydrogen fuel cell powered crossover SUV that was revealed at the 2018 Consumer Electronics Show on January 8, 2018. Replacing the Hyundai Tucson FCEV, the Nexo is the flagship ...
and Toyota Mirai
The (from , Japanese for 'future') is a mid-size hydrogen fuel cell vehicle (FCV) manufactured by Toyota, and is one of the first FCV automobiles to be mass-produced and sold commercially. The Mirai was unveiled at the November 2014 Los Angel ...
.['Carbon-free fuel': Australian hydrogen car breakthrough could go global](_blank)
Lexy Hamilton-Smith, ABC News Online
ABC News, or ABC News and Current Affairs, is a public news service produced by the Australian Broadcasting Corporation. Broadcasting within Australia and the rest of the world, the service covers both local and world affairs.
The division of ...
, 2018-08-08
In 2020, Saudi Arabia
Saudi Arabia, officially the Kingdom of Saudi Arabia (KSA), is a country in Western Asia. It covers the bulk of the Arabian Peninsula, and has a land area of about , making it the fifth-largest country in Asia, the second-largest in the A ...
shipped 40 metric tons
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United States ...
of liquid "blue ammonia" to Japan for use as a fuel. It was produced as a by-product by petrochemical industries, and can be burned without giving off greenhouse gas
A greenhouse gas (GHG or GhG) is a gas that Absorption (electromagnetic radiation), absorbs and Emission (electromagnetic radiation), emits radiant energy within the thermal infrared range, causing the greenhouse effect. The primary greenhouse ...
es. Its energy density by volume is nearly double that of liquid hydrogen. If the process of creating it can be scaled up via purely renewable resources, producing green ammonia, it could make a major difference in avoiding climate change. The company ACWA Power
ACWA Power is a developer, investor, co-owner and operator of a portfolio of power generation and desalinated water production plants currently with presence in 10 countries including in the Middle East and North Africa, Southern Africa and Sout ...
and the city of Neom
Neom (styled NEOM; ''Neom,'' ) is a city being built in Tabuk Province in northwestern Saudi Arabia. It is planned to incorporate smart city technologies and function as a tourist destination. The site is north of the Red Sea, east of Egypt acr ...
have announced the construction of a green hydrogen and ammonia plant in 2020.
Green ammonia is considered as a potential fuel for future container ships. In 2020, the companies DSME
Daewoo Shipbuilding & Marine Engineering Co., Ltd ( ko, 대우조선해양; abbreviated DSME) is one of the "Big Three" shipbuilders of South Korea, along with Hyundai and Samsung.
History
On 21 February 2011, the A. P. Moller-Maersk Group (M ...
and MAN Energy Solutions
MAN Energy Solutions SE is a German multinational company based in Augsburg that produces large-bore gas and diesel engines and also turbomachinery for marine, rail and stationary applications, as locomotive and marine propulsion systems, power p ...
announced the construction of an ammonia-based ship, DSME plans to commercialize it by 2025. The use of ammonia as a potential alternative fuel for aircraft
An aircraft is a vehicle that is able to fly by gaining support from the air. It counters the force of gravity by using either static lift or by using the dynamic lift of an airfoil, or in a few cases the downward thrust from jet engines ...
jet engine
A jet engine is a type of reaction engine discharging a fast-moving jet of heated gas (usually air) that generates thrust by jet propulsion. While this broad definition can include rocket, Pump-jet, water jet, and hybrid propulsion, the term ...
s is also being explored.
Japan intends to implement a plan to develop ammonia co-firing technology that can increase the use of ammonia in power generation, as part of efforts to assist domestic and other Asian utilities to accelerate their transition to carbon neutrality
Carbon neutrality is a state of net-zero carbon dioxide emissions. This can be achieved by balancing emissions of carbon dioxide with its removal (often through carbon offsetting) or by eliminating emissions from society (the transition to the "p ...
.
In October 2021, the first International Conference on Fuel Ammonia (ICFA2021) was held.
In June 2022, IHI Corporation
, formerly known as , is a Japanese engineering corporation headquartered in Tokyo, Japan that produces and offers ships, space launch vehicles, aircraft engines, marine diesel engines, gas turbines, gas engines, railway systems, turbochargers ...
succeeded in reducing greenhouse gases by over 99% during combustion of liquid ammonia in a 2,000-kilowatt-class gas turbine achieving truly CO₂-free power generation.
In July 2022, Quad nations of Japan, the U.S., Australia and India agreed to promote technological development for clean-burning hydrogen and ammonia as fuels at the security grouping's first energy meeting. , however, significant amounts of NOx are produced. Nitrous oxide may also be a problem.
Other
Remediation of gaseous emissions
Ammonia is used to scrub from the burning of fossil fuels, and the resulting product is converted to ammonium sulfate for use as fertilizer. Ammonia neutralises the nitrogen oxide () pollutants emitted by diesel engines. This technology, called SCR (selective catalytic reduction
Selective catalytic reduction (SCR) is a means of converting nitrogen oxides, also referred to as with the aid of a catalyst into diatomic nitrogen (), and water (). A reductant, typically anhydrous ammonia (), aqueous ammonia (), or a urea () s ...
), relies on a vanadia-based catalyst.
Ammonia may be used to mitigate gaseous spills of phosgene
Phosgene is the organic chemical compound with the formula COCl2. It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. Phosgene is a valued and important industrial building block, espe ...
.
As a hydrogen carrier
Due to its attributes, being liquid at ambient temperature under its own vapour pressure and having high volumetric and gravimetric energy density, ammonia is considered a suitable carrier for hydrogen, and may be cheaper than direct transport of liquid hydrogen.
Refrigeration – R717
Because of ammonia's vaporization properties, it is a useful refrigerant
A refrigerant is a working fluid used in the heat pump and refrigeration cycle, refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Ref ...
.[ It was commonly used before the popularisation of ]chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and prop ...
s (Freons). Anhydrous ammonia is widely used in industrial refrigeration applications and hockey rinks because of its high energy efficiency
Energy efficiency may refer to:
* Energy efficiency (physics), the ratio between the useful output and input of an energy conversion process
** Electrical efficiency, useful power output per electrical power consumed
** Mechanical efficiency, a ra ...
and low cost. It suffers from the disadvantage of toxicity, and requiring corrosion resistant components, which restricts its domestic and small-scale use. Along with its use in modern vapor-compression refrigeration
Vapour-compression refrigeration or vapor-compression refrigeration system (VCRS), in which the refrigerant undergoes phase changes, is one of the many refrigeration cycles and is the most widely used method for air conditioning of buildings ...
it is used in a mixture along with hydrogen and water in absorption refrigerator
An absorption refrigerator is a refrigerator that uses a heat source (e.g., solar energy, a fossil-fueled flame, waste heat from factories, or district heating systems) to provide the energy needed to drive the cooling process. The system uses tw ...
s. The Kalina cycle
The Kalina cycle, developed by Alexander Kalina, is a thermodynamic process for converting thermal energy into usable mechanical power.
It uses a solution of 2 fluids with different boiling points for its working fluid. Since the solution boil ...
, which is of growing importance to geothermal power plants, depends on the wide boiling range of the ammonia–water mixture. Ammonia coolant is also used in the S1 radiator aboard the International Space Station
The International Space Station (ISS) is the largest modular space station currently in low Earth orbit. It is a multinational collaborative project involving five participating space agencies: NASA (United States), Roscosmos (Russia), JAXA ...
in two loops which are used to regulate the internal temperature and enable temperature-dependent experiments.
The potential importance of ammonia as a refrigerant has increased with the discovery that vented CFCs and HFCs are extremely potent and stable greenhouse gases.
Stimulant
 Ammonia, as the vapor released by
Ammonia, as the vapor released by smelling salts
Smelling salts, also known as ammonia inhalants, spirit of hartshorn or sal volatile, are chemical compounds used as stimulants to restore consciousness after fainting.
Usage
The usual active compound is ammonium carbonate—a colorless-to-w ...
, has found significant use as a respiratory stimulant. Ammonia is commonly used in the illegal manufacture of methamphetamine
Methamphetamine (contracted from ) is a potent central nervous system (CNS) stimulant that is mainly used as a recreational drug and less commonly as a second-line treatment for attention deficit hyperactivity disorder and obesity. Methamph ...
through a Birch reduction
The Birch reduction is an organic reaction that is used to convert arenes to cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch and involves the organic reduction of aromatic rings in an amine solvent (traditionally ...
. The Birch method of making methamphetamine is dangerous because the alkali metal and liquid ammonia are both extremely reactive, and the temperature of liquid ammonia makes it susceptible to explosive boiling when reactants are added.
Textile
Liquid ammonia is used for treatment of cotton materials, giving properties like mercerisation
Mercerisation is a textile finishing treatment for cellulose fabric and yarn, mainly cotton and flax, which improves dye uptake and tear strength, reduces fabric shrinkage, and imparts a silk-like luster.
Development
The process was devise ...
, using alkalis. In particular, it is used for prewashing of wool.
Lifting gas
At standard temperature and pressure, ammonia is less dense than atmosphere and has approximately 45–48% of the lifting power of hydrogen or helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
. Ammonia has sometimes been used to fill balloons as a lifting gas
A lifting gas or lighter-than-air gas is a gas that has a density lower than normal atmospheric gases and rises above them as a result. It is required for aerostats to create buoyancy, particularly in lighter-than-air aircraft, which include ballo ...
. Because of its relatively high boiling point (compared to helium and hydrogen), ammonia could potentially be refrigerated and liquefied aboard an airship
An airship or dirigible balloon is a type of aerostat or lighter-than-air aircraft that can navigate through the air under its own power. Aerostats gain their lift from a lifting gas that is less dense than the surrounding air.
In early ...
to reduce lift and add ballast (and returned to a gas to add lift and reduce ballast).
Fuming
Ammonia has been used to darken quartersawn white oak in Arts & Crafts and Mission-style furniture. Ammonia fumes react with the natural tannin
Tannins (or tannoids) are a class of astringent, polyphenolic biomolecules that bind to and precipitate proteins and various other organic compounds including amino acids and alkaloids.
The term ''tannin'' (from Anglo-Norman ''tanner'', ...
s in the wood
Wood is a porous and fibrous structural tissue found in the stems and roots of trees and other woody plants. It is an organic materiala natural composite of cellulose fibers that are strong in tension and embedded in a matrix of lignin th ...
and cause it to change colours.
Safety
 The U.S. Occupational Safety and Health Administration (OSHA) has set a 15-minute exposure limit for gaseous ammonia of 35 ppm by volume in the environmental air and an 8-hour exposure limit of 25 ppm by volume.
The U.S. Occupational Safety and Health Administration (OSHA) has set a 15-minute exposure limit for gaseous ammonia of 35 ppm by volume in the environmental air and an 8-hour exposure limit of 25 ppm by volume.National Institute for Occupational Safety and Health
The National Institute for Occupational Safety and Health (NIOSH, ) is the United States federal agency responsible for conducting research and making recommendations for the prevention of work-related injury and illness. NIOSH is part of the C ...
(NIOSH) recently reduced the IDLH (Immediately Dangerous to Life and Health, the level to which a healthy worker can be exposed for 30 minutes without suffering irreversible health effects) from 500 to 300 based on recent more conservative interpretations of original research in 1943. Other organizations have varying exposure levels. U.S. Navy Standards .S. Bureau of Ships 1962maximum allowable concentrations (MACs): for continuous exposure (60 days) is 25 ppm; for exposure of 1 hour is 400 ppm. Ammonia vapour has a sharp, irritating, pungent odour that acts as a warning of potentially dangerous exposure. The average odour threshold is 5 ppm, well below any danger or damage. Exposure to very high concentrations of gaseous ammonia can result in lung damage and death.[ Ammonia is regulated in the United States as a non-flammable gas, but it meets the definition of a material that is toxic by inhalation and requires a hazardous safety permit when transported in quantities greater than 13,248 L (3,500 gallons).
Liquid ammonia is dangerous because it is ]hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance ...
and because it can cause caustic burns. See for more information.
Toxicity
The toxicity of ammonia solutions does not usually cause problems for humans and other mammals, as a specific mechanism exists to prevent its build-up in the bloodstream. Ammonia is converted to carbamoyl phosphate
Carbamoyl phosphate is an anion of biochemical significance. In land-dwelling animals, it is an intermediary metabolite in nitrogen disposal through the urea cycle and the synthesis of pyrimidines. Its enzymatic counterpart, carbamoyl phosphate syn ...
by the enzyme carbamoyl phosphate synthetase
Carbamoyl phosphate synthetase catalyzes the ATP-dependent synthesis
of carbamoyl phosphate from glutamine () or ammonia () and bicarbonate. This enzyme catalyzes the reaction of ATP and bicarbonate to produce carboxy phosphate and ADP. Carb ...
, and then enters the urea cycle
The urea cycle (also known as the ornithine cycle) is a cycle of biochemical reactions that produces urea (NH2)2CO from ammonia (NH3). Animals that use this cycle, mainly amphibians and mammals, are called ureotelic.
The urea cycle converts highl ...
to be either incorporated into amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
s or excreted in the urine. Fish
Fish are aquatic, craniate, gill-bearing animals that lack limbs with digits. Included in this definition are the living hagfish, lampreys, and cartilaginous and bony fish as well as various extinct related groups. Approximately 95% of li ...
and amphibian
Amphibians are tetrapod, four-limbed and ectothermic vertebrates of the Class (biology), class Amphibia. All living amphibians belong to the group Lissamphibia. They inhabit a wide variety of habitats, with most species living within terres ...
s lack this mechanism, as they can usually eliminate ammonia from their bodies by direct excretion. Ammonia even at dilute concentrations is highly toxic to aquatic animals, and for this reason it is classified as ''dangerous for the environment''. Atmospheric ammonia plays a key role in the formation of fine particulate matter.
Ammonia is a constituent of tobacco smoke
Tobacco smoke is a sooty aerosol produced by the incomplete combustion of tobacco during the tobacco smoking, smoking of cigarettes and other tobacco products. Temperatures in burning cigarettes range from about 400 °C between puffs to abo ...
.
Coking wastewater
Ammonia is present in coking wastewater streams, as a liquid by-product of the production of coke from coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal is formed when dea ...
. In some cases, the ammonia is discharged to the marine environment
Marine habitats are habitats that support marine life. Marine life depends in some way on the saltwater that is in the sea (the term ''marine'' comes from the Latin ''mare'', meaning sea or ocean). A habitat is an ecological or environmental ...
where it acts as a pollutant. The Whyalla steelworks
The Whyalla Steelworks is a fully integrated steelworks and the only manufacturer of rail in Australia. Iron ore is mined in the Middleback Range to feed the steelworks, resulting in the distribution of finished steel products of over 90 different ...
in South Australia
South Australia (commonly abbreviated as SA) is a state in the southern central part of Australia. It covers some of the most arid parts of the country. With a total land area of , it is the fourth-largest of Australia's states and territories ...
is one example of a coke-producing facility which discharges ammonia into marine waters.
Aquaculture
Ammonia toxicity is believed to be a cause of otherwise unexplained losses in fish hatcheries. Excess ammonia may accumulate and cause alteration of metabolism or increases in the body pH of the exposed organism. Tolerance varies among fish species.
Storage information
Similar to propane
Propane () is a three-carbon alkane with the molecular formula . It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used a ...
, anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achie ...
ammonia boils below room temperature when at atmospheric pressure. A storage vessel capable of is suitable to contain the liquid. Ammonia is used in numerous different industrial application requiring carbon or stainless steel storage vessels. Ammonia with at least 0.2% by weight water content is not corrosive to carbon steel. carbon steel construction storage tanks with 0.2% by weight or more of water could last more than 50 years in service. Experts warn that ammonium compounds not be allowed to come in contact with bases (unless in an intended and contained reaction), as dangerous quantities of ammonia gas could be released.
Laboratory
 The hazards of ammonia solutions depend on the concentration: "dilute" ammonia solutions are usually 5–10% by weight (<5.62 mol/L); "concentrated" solutions are usually prepared at >25% by weight. A 25% (by weight) solution has a density of 0.907 g/cm3, and a solution that has a lower density will be more concentrated. The European Union classification of ammonia solutions is given in the table.
The ammonia vapour from concentrated ammonia solutions is severely irritating to the eyes and the respiratory tract, and experts warn that these solutions only be handled in a fume hood. Saturated ("0.880" – see #Properties) solutions can develop a significant pressure inside a closed bottle in warm weather, and experts also warn that the bottle be opened with care. This is not usually a problem for 25% ("0.900") solutions.
Experts warn that ammonia solutions not be mixed with
The hazards of ammonia solutions depend on the concentration: "dilute" ammonia solutions are usually 5–10% by weight (<5.62 mol/L); "concentrated" solutions are usually prepared at >25% by weight. A 25% (by weight) solution has a density of 0.907 g/cm3, and a solution that has a lower density will be more concentrated. The European Union classification of ammonia solutions is given in the table.
The ammonia vapour from concentrated ammonia solutions is severely irritating to the eyes and the respiratory tract, and experts warn that these solutions only be handled in a fume hood. Saturated ("0.880" – see #Properties) solutions can develop a significant pressure inside a closed bottle in warm weather, and experts also warn that the bottle be opened with care. This is not usually a problem for 25% ("0.900") solutions.
Experts warn that ammonia solutions not be mixed with halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
s, as toxic and/or explosive products are formed. Experts also warn that prolonged contact of ammonia solutions with silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
, mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
or iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine defic ...
salts can also lead to explosive products: such mixtures are often formed in qualitative inorganic analysis
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may ...
, and that it needs to be lightly acidified but not concentrated (<6% w/v) before disposal once the test is completed.
Laboratory use of anhydrous ammonia (gas or liquid)
Anhydrous ammonia is classified as toxic (T) and dangerous for the environment (N). The gas is flammable (autoignition temperature
The autoignition temperature or kindling point of a substance is the lowest temperature in which it spontaneously ignites in a normal atmosphere without an external source of ignition, such as a flame or spark. This temperature is required to su ...
: 651 °C) and can form explosive mixtures with air (16–25%). The permissible exposure limit
The permissible exposure limit (PEL or OSHA PEL) is a legal limit in the United States for exposure of an employee to a chemical substance or physical agent such as high level noise. Permissible exposure limits are established by the Occupational ...
(PEL) in the United States is 50 ppm (35 mg/m3), while the IDLH
The term immediately dangerous to life or health (IDLH) is defined by the US National Institute for Occupational Safety and Health (NIOSH) as exposure to airborne contaminants that is "likely to cause death or immediate or delayed permanent advers ...
concentration is estimated at 300 ppm. Repeated exposure to ammonia lowers the sensitivity to the smell of the gas: normally the odour is detectable at concentrations of less than 50 ppm, but desensitised individuals may not detect it even at concentrations of 100 ppm. Anhydrous ammonia corrodes copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
- and zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
-containing alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductility, ...
s which makes brass
Brass is an alloy of copper (Cu) and zinc (Zn), in proportions which can be varied to achieve different mechanical, electrical, and chemical properties. It is a substitutional alloy: atoms of the two constituents may replace each other with ...
fittings not appropriate for handling the gas. Liquid ammonia can also attack rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, and ...
and certain plastics.
Ammonia reacts violently with the halogens. Nitrogen triiodide
Nitrogen triiodide is an inorganic compound with the formula N I3. It is an extremely sensitive contact explosive: small quantities explode with a loud, sharp snap when touched even lightly, releasing a purple cloud of iodine vapor; it can even b ...
, a primary
Primary or primaries may refer to:
Arts, entertainment, and media Music Groups and labels
* Primary (band), from Australia
* Primary (musician), hip hop musician and record producer from South Korea
* Primary Music, Israeli record label
Works
* ...
high explosive
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An exp ...
, is formed when ammonia comes in contact with iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
. Ammonia causes the explosive polymerisation
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many for ...
of ethylene oxide
Ethylene oxide is an organic compound with the chemical formula, formula . It is a cyclic ether and the simplest epoxide: a three-membered Ring (chemistry), ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless a ...
. It also forms explosive fulminating compounds with compounds of gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile met ...
, silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
, mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
, germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors s ...
or tellurium
Tellurium is a chemical element with the symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally fou ...
, and with stibine. Violent reactions have also been reported with acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the mos ...
, hypochlorite
In chemistry, hypochlorite is an anion with the chemical formula ClO−. It combines with a number of cations to form hypochlorite salts. Common examples include sodium hypochlorite (household bleach) and calcium hypochlorite (a component of ble ...
solutions, potassium ferricyanide
Potassium ferricyanide is the chemical compound with the formula K3 e(CN)6 This bright red salt contains the octahedrally coordinated 3−.html" ;"title="e(CN)6sup>3−">e(CN)6sup>3− ion. It is soluble in water and its solution shows some g ...
and peroxide
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable.
The most common peroxide is hydrogen p ...
s.
Ammonia adsorption followed by FTIR as well as temperature programmed desorption of ammonia (NH3-TPD) are very valuable methods to characterize acid-base properties of heterogeneous catalysts.
Production
 Ammonia is one of the most produced inorganic chemicals, with global production reported at 175 million tonnes in 2018.
Ammonia is one of the most produced inorganic chemicals, with global production reported at 175 million tonnes in 2018.World War I
World War I (28 July 1914 11 November 1918), often abbreviated as WWI, was one of the deadliest global conflicts in history. Belligerents included much of Europe, the Russian Empire, the United States, and the Ottoman Empire, with fightin ...
, most ammonia was obtained by the dry distillation
Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids). The method may involve pyrolysis or thermolysis, or it may not (for instance, a simple mixture of ice and glass could be se ...
of nitrogenous vegetable and animal waste products, including camel
A camel (from: la, camelus and grc-gre, κάμηλος (''kamēlos'') from Hebrew or Phoenician: גָמָל ''gāmāl''.) is an even-toed ungulate in the genus ''Camelus'' that bears distinctive fatty deposits known as "humps" on its back. C ...
dung, where it was distilled by the reduction of nitrous acid
Nitrous acid (molecular formula ) is a weak and monoprotic acid known only in Solution (chemistry), solution, in the gas phase and in the form of nitrite () salts. Nitrous acid is used to make diazonium salts from amines. The resulting diazoni ...
and nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
s with hydrogen; in addition, it was produced by the distillation of coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal is formed when dea ...
, and also by the decomposition of ammonium salts by alkaline hydroxides such as quicklime
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "''lime''" connotes calcium-containing inorganic ma ...
:
:
For small scale laboratory synthesis, one can heat urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important r ...
and calcium hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed or slaked with water. It has m ...
:
:
Haber–Bosch
Mass production uses the Haber–Bosch process
The Haber process, also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. It is named after its inventors, the German chemists Fritz Haber and C ...
, a gas phase
In the outline of physical science, physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, ref ...
reaction between hydrogen () and nitrogen () at a moderately-elevated temperature (450 °C) and high pressure ():
:, Δ''H''° = −91.8 kJ/mol
This reaction is exothermic and results in decreased entropy, meaning that the reaction is favoured at lower temperatures and higher pressures. It is difficult and expensive to achieve, as lower temperatures result in slower reaction kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in w ...
(hence a slower reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit ...
) and high pressure requires high-strength pressure vessels that are not weakened by hydrogen embrittlement
Hydrogen embrittlement (HE), also known as hydrogen-assisted cracking or hydrogen-induced cracking (HIC), is a reduction in the ductility of a metal due to absorbed hydrogen. Hydrogen atoms are small and can permeate solid metals. Once absorbed ...
. Diatomic
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear. Ot ...
nitrogen is bound together by a triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
, which makes it rather inert. Yield and efficiency are low, meaning that the output must be continuously separated and extracted for the reaction to proceed at an acceptable pace. Combined with the energy needed to produce hydrogen and purified atmospheric nitrogen, ammonia production is energy-intensive, accounting for 1% to 2% of global energy consumption, 3% of global carbon emissions
Greenhouse gas emissions from human activities strengthen the greenhouse effect, contributing to climate change. Most is carbon dioxide from burning fossil fuels: coal, oil, and natural gas. The largest emitters include coal in China and larg ...
, and 3 to 5% of natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbo ...
consumption.
The choice of catalyst is important for synthesizing ammonia. In 2012, Hideo Hosono
, ForMemRS, is a Japanese material scientist most known for the discovery of iron-based superconductors.
Career and research
Hosono is also a pioneer in developing transparent oxide semiconductors: he proposed a material design concept for a t ...
's group found that Ru-loaded calcium-aluminum oxide C12A7: electride
An electride is an ionic compound in which an electron is the anion. Solutions of alkali metals in ammonia are electride salts. In the case of sodium, these blue solutions consist of a(NH3)6sup>+ and solvated electrons:
:Na + 6 NH3 → ...
works well as a catalyst and pursued more efficient formation. This method is implemented in a small plant for ammonia synthesis in Japan. In 2019, Hosono's group found another catalyst, a novel perovskite
Perovskite (pronunciation: ) is a calcium titanium oxide mineral composed of calcium titanate (chemical formula ). Its name is also applied to the class of compounds which have the same type of crystal structure as (XIIA2+VIB4+X2−3), known as ...
oxynitride-hydride , that works at lower temperature and without costly ruthenium.
Electrochemical
Ammonia can be synthesized electrochemically. The only required inputs are sources of nitrogen (potentially atmospheric) and hydrogen (water), allowing generation at the point of use. The availability of renewable energy creates the possibility of zero emission production.
Another electrochemical
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outco ...
synthesis mode involves the reductive formation of lithium nitride
Lithium nitride is a compound with the formula Li3N. It is the only stable alkali metal nitride. The solid has a reddish-pink color and high melting point.
Preparation and handling
Lithium nitride is prepared by direct combination of elemental ...
, which can be protonated
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid, ...
to ammonia, given a proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
source. Ethanol has been used as such a source, although it may degrade. The first use of this chemistry was reported in 1930, where lithium solutions in ethanol were used to produce ammonia at pressures of up to 1000 bar. In 1994, Tsuneto et al. used lithium electrodeposition in tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is ma ...
to synthesize ammonia at more moderate pressures with reasonable Faradaic efficiency Faraday efficiency (also called ''faradaic efficiency'', ''faradaic yield'', ''coulombic efficiency'' or ''current efficiency'') describes the efficiency with which charge (electrons) is transferred in a system facilitating an electrochemical reacti ...
. Other studies have since used the ethanol-tetrahydrofuran system for electrochemical ammonia synthesis.gas diffusion electrode
Gas diffusion electrodes (GDE) are electrodes with a conjunction of a solid, liquid and gaseous interface, and an electrical conducting catalyst supporting an electrochemical reaction between the liquid and the gaseous phase.
Principle
GDEs are us ...
to improve nitrogen transport to the reactive lithium. The study observed production rates of up to 30 ± 5 nanomoles/s/cm2 and Faradaic efficiencies of up to 47.5 ± 4% at ambient temperature and 1 bar pressure.
In 2021, Suryanto et al. replaced ethanol with a tetraalkyl phosphonium salt
In polyatomic cations with the chemical formula (where R is a hydrogen or an alkyl, aryl, or halide group). These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.
Types of phosphonium c ...
. This cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
can stably undergo deprotonation–reprotonation cycles, while it enhances the medium's ionic conductivity.faradaic efficiency Faraday efficiency (also called ''faradaic efficiency'', ''faradaic yield'', ''coulombic efficiency'' or ''current efficiency'') describes the efficiency with which charge (electrons) is transferred in a system facilitating an electrochemical reacti ...
experiments under 0.5-bar
Bar or BAR may refer to:
Food and drink
* Bar (establishment), selling alcoholic beverages
* Candy bar
* Chocolate bar
Science and technology
* Bar (river morphology), a deposit of sediment
* Bar (tropical cyclone), a layer of cloud
* Bar (u ...
hydrogen and 19.5-bar nitrogen partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas ...
at ambient temperature.
Role in biological systems and human disease
 Ammonia is both a
Ammonia is both a metabolic waste
Metabolic wastes or excrements are substances left over from metabolic processes (such as cellular respiration) which cannot be used by the organism (they are surplus or toxic), and must therefore be excreted. This includes nitrogen compounds, ...
and a metabolic input throughout the biosphere
The biosphere (from Greek βίος ''bíos'' "life" and σφαῖρα ''sphaira'' "sphere"), also known as the ecosphere (from Greek οἶκος ''oîkos'' "environment" and σφαῖρα), is the worldwide sum of all ecosystems. It can also be ...
. It is an important source of nitrogen for living systems. Although atmospheric nitrogen abounds (more than 75%), few living creatures are capable of using atmospheric nitrogen in its diatomic form, gas. Therefore, nitrogen fixation
Nitrogen fixation is a chemical process by which molecular nitrogen (), with a strong triple covalent bond, in the air is converted into ammonia () or related nitrogenous compounds, typically in soil or aquatic systems but also in industry. Atmo ...
is required for the synthesis of amino acids, which are the building blocks of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
. Some plants rely on ammonia and other nitrogenous wastes incorporated into the soil by decaying matter. Others, such as nitrogen-fixing legume
A legume () is a plant in the family Fabaceae (or Leguminosae), or the fruit or seed of such a plant. When used as a dry grain, the seed is also called a pulse. Legumes are grown agriculturally, primarily for human consumption, for livestock f ...
s, benefit from symbiotic
Symbiosis (from Greek , , "living together", from , , "together", and , bíōsis, "living") is any type of a close and long-term biological interaction between two different biological organisms, be it mutualistic, commensalistic, or parasit ...
relationships with rhizobia
Rhizobia are diazotrophic bacteria that fix nitrogen after becoming established inside the root nodules of legumes (Fabaceae). To express genes for nitrogen fixation, rhizobia require a plant host; they cannot independently fix nitrogen. In gene ...
bacteria that create ammonia from atmospheric nitrogen.
In humans, inhaling ammonia in high concentrations can be fatal. Exposure to ammonia can cause headaches
Headache is the symptom of pain in the face, head, or neck. It can occur as a migraine, tension-type headache, or cluster headache. There is an increased risk of depression in those with severe headaches.
Headaches can occur as a result of m ...
, edema
Edema, also spelled oedema, and also known as fluid retention, dropsy, hydropsy and swelling, is the build-up of fluid in the body's Tissue (biology), tissue. Most commonly, the legs or arms are affected. Symptoms may include skin which feels t ...
, impaired memory, seizures
An epileptic seizure, informally known as a seizure, is a period of symptoms due to abnormally excessive or neural oscillation, synchronous neuronal activity in the brain. Outward effects vary from uncontrolled shaking movements involving much o ...
and coma
A coma is a deep state of prolonged unconsciousness in which a person cannot be awakened, fails to respond normally to painful stimuli, light, or sound, lacks a normal wake-sleep cycle and does not initiate voluntary actions. Coma patients exhi ...
as it is neurotoxic
Neurotoxicity is a form of toxicity in which a biological, chemical, or physical agent produces an adverse effect on the structure or function of the central and/or peripheral nervous system. It occurs when exposure to a substance – specificall ...
in nature.
Biosynthesis
In certain organisms, ammonia is produced from atmospheric nitrogen by enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
s called nitrogenase
Nitrogenases are enzymes () that are produced by certain bacteria, such as cyanobacteria (blue-green bacteria) and rhizobacteria. These enzymes are responsible for the Organic redox reaction, reduction of nitrogen (N2) to ammonia (NH3). Nitrog ...
s. The overall process is called nitrogen fixation
Nitrogen fixation is a chemical process by which molecular nitrogen (), with a strong triple covalent bond, in the air is converted into ammonia () or related nitrogenous compounds, typically in soil or aquatic systems but also in industry. Atmo ...
. Intense effort has been directed toward understanding the mechanism of biological nitrogen fixation. The scientific interest in this problem is motivated by the unusual structure of the active site of the enzyme, which consists of an ensemble.
Ammonia is also a metabolic product of amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
deamination
Deamination is the removal of an amino group from a molecule. Enzymes that catalyse this reaction are called deaminases.
In the human body, deamination takes place primarily in the liver, however it can also occur in the kidney. In situations of e ...
catalyzed by enzymes such as glutamate dehydrogenase 1
GLUD1 (glutamate dehydrogenase 1) is a mitochondrial matrix enzyme, one of the family of glutamate dehydrogenases that are ubiquitous in life, with a key role in nitrogen and glutamate (Glu) metabolism and energy homeostasis. This dehydrogenase is ...
. Ammonia excretion is common in aquatic animals. In humans, it is quickly converted to urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important r ...
, which is much less toxic, particularly less basic
BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Thomas E. Kurtz at Dartmouth College ...
. This urea is a major component of the dry weight of urine
Urine is a liquid by-product of metabolism in humans and in many other animals. Urine flows from the kidneys through the ureters to the urinary bladder. Urination results in urine being excretion, excreted from the body through the urethra.
Cel ...
. Most reptiles, birds, insects, and snails excrete uric acid
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates, such as ammonium acid urate. Uric acid is a product of the metabolic breakdown of ...
solely as nitrogenous waste.
Physiology
Ammonia plays a role in both normal and abnormal animal physiology
Physiology (; ) is the scientific study of functions and mechanisms in a living system. As a sub-discipline of biology, physiology focuses on how organisms, organ systems, individual organs, cells, and biomolecules carry out the chemical ...
. It is biosynthesised through normal amino acid metabolism and is toxic in high concentrations. The liver
The liver is a major Organ (anatomy), organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of proteins and biochemicals necessary for ...
converts ammonia to urea through a series of reactions known as the urea cycle
The urea cycle (also known as the ornithine cycle) is a cycle of biochemical reactions that produces urea (NH2)2CO from ammonia (NH3). Animals that use this cycle, mainly amphibians and mammals, are called ureotelic.
The urea cycle converts highl ...
. Liver dysfunction, such as that seen in cirrhosis
Cirrhosis, also known as liver cirrhosis or hepatic cirrhosis, and end-stage liver disease, is the impaired liver function caused by the formation of scar tissue known as fibrosis due to damage caused by liver disease. Damage causes tissue repai ...
, may lead to elevated amounts of ammonia in the blood (hyperammonemia
Hyperammonemia is a metabolic disturbance characterised by an excess of ammonia in the blood. It is a dangerous condition that may lead to brain injury and death. It may be primary or secondary.
Ammonia is a substance that contains nitrogen. It i ...
). Likewise, defects in the enzymes responsible for the urea cycle, such as ornithine transcarbamylase
Ornithine transcarbamylase (OTC) (also called ornithine carbamoyltransferase) is an enzyme () that catalyzes the reaction between carbamoyl phosphate (CP) and ornithine (Orn) to form citrulline (Cit) and phosphate (Pi). There are two classes of OT ...
, lead to hyperammonemia. Hyperammonemia contributes to the confusion and coma
A coma is a deep state of prolonged unconsciousness in which a person cannot be awakened, fails to respond normally to painful stimuli, light, or sound, lacks a normal wake-sleep cycle and does not initiate voluntary actions. Coma patients exhi ...
of hepatic encephalopathy
Hepatic encephalopathy (HE) is an altered level of consciousness as a result of liver failure. Its onset may be gradual or sudden. Other symptoms may include movement problems, changes in mood, or changes in personality. In the advanced stages ...
, as well as the neurologic disease common in people with urea cycle defects and organic aciduria
Organic acidemia, is a term used to classify a group of metabolic disorders which disrupt normal amino acid metabolism, particularly branched-chain amino acids, causing a buildup of acids which are usually not present.
The branched-chain amino a ...
s.
Ammonia is important for normal animal acid/base balance. After formation of ammonium from glutamine
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral, ...
, α-ketoglutarate may be degraded to produce two bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochemic ...
ions, which are then available as buffers for dietary acids. Ammonium is excreted in the urine, resulting in net acid loss. Ammonia may itself diffuse across the renal tubules, combine with a hydrogen ion, and thus allow for further acid excretion.
Excretion
Ammonium ions are a toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subst ...
waste product of metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cell ...
in animal
Animals are multicellular, eukaryotic organisms in the Kingdom (biology), biological kingdom Animalia. With few exceptions, animals Heterotroph, consume organic material, Cellular respiration#Aerobic respiration, breathe oxygen, are Motilit ...
s. In fish and aquatic invertebrates, it is excreted directly into the water. In mammals, sharks, and amphibians, it is converted in the urea cycle
The urea cycle (also known as the ornithine cycle) is a cycle of biochemical reactions that produces urea (NH2)2CO from ammonia (NH3). Animals that use this cycle, mainly amphibians and mammals, are called ureotelic.
The urea cycle converts highl ...
to urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important r ...
, which is less toxic and can be stored more efficiently. In birds, reptiles, and terrestrial snails, metabolic ammonium is converted into uric acid
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates, such as ammonium acid urate. Uric acid is a product of the metabolic breakdown of ...
, which is solid and can therefore be excreted with minimal water loss.
Beyond Earth
 Ammonia has been detected in the atmospheres of the
Ammonia has been detected in the atmospheres of the giant planet
The giant planets constitute a diverse type of planet much larger than Earth. They are usually primarily composed of low-boiling-point materials (volatiles), rather than rock or other solid matter, but massive solid planets can also exist. Ther ...
s, including Jupiter
Jupiter is the fifth planet from the Sun and the List of Solar System objects by size, largest in the Solar System. It is a gas giant with a mass more than two and a half times that of all the other planets in the Solar System combined, but ...
, along with other gases such as methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Eart ...
, hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
, and helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
. The interior of Saturn
Saturn is the sixth planet from the Sun and the second-largest in the Solar System, after Jupiter. It is a gas giant with an average radius of about nine and a half times that of Earth. It has only one-eighth the average density of Earth; h ...
may include frozen ammonia crystals. It is found on Deimos and Phobos – the two moons of Mars
The two moons of Mars are Phobos (moon), Phobos and Deimos (moon), Deimos. They are irregular in shape. Both were discovered by American astronomer Asaph Hall in August 1877 and are named after the Greek mythology, Greek mythological twin charac ...
.
Interstellar space
Ammonia was first detected in interstellar space in 1968, based on microwave
Microwave is a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter corresponding to frequencies between 300 MHz and 300 GHz respectively. Different sources define different frequency ran ...
emissions from the direction of the galactic core
The Galactic Center or Galactic Centre is the rotational center, the barycenter, of the Milky Way galaxy. Its central massive object is a supermassive black hole of about 4 million solar masses, which is called Sagittarius A*, a compact rad ...
. This was the first polyatomic
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. The term molecule may or may no ...
molecule to be so detected.
The sensitivity of the molecule to a broad range of excitations and the ease with which it can be observed in a number of regions has made ammonia one of the most important molecules for studies of molecular cloud
A molecular cloud, sometimes called a stellar nursery (if star formation is occurring within), is a type of interstellar cloud, the density and size of which permit absorption nebulae, the formation of molecules (most commonly molecular hydrogen ...
s.deuterated
Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope (an atom with a detectable variation in neutron count) through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific ...
ammonia was considered a surprise as deuterium is relatively scarce. It is thought that the low-temperature conditions allow this molecule to survive and accumulate.
Since its interstellar discovery, has proved to be an invaluable spectroscopic tool in the study of the interstellar medium. With a large number of transitions sensitive to a wide range of excitation conditions, has been widely astronomically detected – its detection has been reported in hundreds of journal articles. Listed below is a sample of journal articles that highlights the range of detectors that have been used to identify ammonia.
The study of interstellar ammonia has been important to a number of areas of research in the last few decades. Some of these are delineated below and primarily involve using ammonia as an interstellar thermometer.
Interstellar formation mechanisms
The interstellar abundance for ammonia has been measured for a variety of environments. The []/[] ratio has been estimated to range from 10−7 in small dark clouds up to 10−5 in the dense core of the Orion molecular cloud complex. Although a total of 18 total production routes have been proposed, the principal formation mechanism for interstellar is the reaction:
:
The rate constant, ''k'', of this reaction depends on the temperature of the environment, with a value of 5.2×10−6 at 10 K. The rate constant was calculated from the formula . For the primary formation reaction, and . Assuming an abundance of 3×10−7 and an electron abundance of 10−7 typical of molecular clouds, the formation will proceed at a rate of in a molecular cloud of total density .
All other proposed formation reactions have rate constants of between 2 and 13 orders of magnitude smaller, making their contribution to the abundance of ammonia relatively insignificant. As an example of the minor contribution other formation reactions play, the reaction:
:
has a rate constant of 2.2. Assuming densities of 105 and []/[] ratio of 10−7, this reaction proceeds at a rate of 2.2, more than 3 orders of magnitude slower than the primary reaction above.
Some of the other possible formation reactions are:
:
:
Interstellar destruction mechanisms
There are 113 total proposed reactions leading to the destruction of . Of these, 39 were tabulated in extensive tables of the chemistry among C, N, and O compounds. A review of interstellar ammonia cites the following reactions as the principal dissociation mechanisms:[
with rate constants of 4.39×10−9 and 2.2×10−9, respectively. The above equations (, ) run at a rate of 8.8×10−9 and 4.4×10−13, respectively. These calculations assumed the given rate constants and abundances of []/[] = 10−5, []/[] = 2×10−5, []/[] = 2×10−9, and total densities of ''n'' = 105, typical of cold, dense, molecular clouds. Clearly, between these two primary reactions, equation () is the dominant destruction reaction, with a rate ≈10,000 times faster than equation (). This is due to the relatively high abundance of .
]
Single antenna detections
Radio observations of from the Effelsberg 100-m Radio Telescope
The Effelsberg 100-m Radio Telescope is a radio telescope in the Ahr Hills (part of the Eifel) in Bad Münstereifel, Germany. For 29 years the Effelsberg Radio Telescope was the largest fully steerable radio telescope on Earth, surpassing the Lo ...
reveal that the ammonia line is separated into two components – a background ridge and an unresolved core. The background corresponds well with the locations previously detected CO. The 25 m Chilbolton telescope in England detected radio signatures of ammonia in H II region
An H II region or HII region is a region of interstellar atomic hydrogen that is ionized. It is typically in a molecular cloud of partially ionized gas in which star formation has recently taken place, with a size ranging from one to hundreds ...
s, HNH2O maser
A maser (, an acronym for microwave amplification by stimulated emission of radiation) is a device that produces coherent electromagnetic waves through amplification by stimulated emission. The first maser was built by Charles H. Townes, Ja ...
s, H-H objects, and other objects associated with star formation. A comparison of emission line widths indicates that turbulent or systematic velocities do not increase in the central cores of molecular clouds.
Microwave radiation from ammonia was observed in several galactic objects including W3(OH), Orion A, W43, W51, and five sources in the galactic centre. The high detection rate indicates that this is a common molecule in the interstellar medium and that high-density regions are common in the galaxy.
Interferometric studies
VLA VLA or vla may refer to:
Organizations
* Vermont Library Association, professional organization for librarians from Vermont
* Veterinary Laboratories Agency, a UK government agency for researching animal and public health
* Victoria Legal Aid, an ...
observations of in seven regions with high-velocity gaseous outflows revealed condensations of less than 0.1 pc in L1551, S140, and Cepheus A. Three individual condensations were detected in Cepheus A, one of them with a highly elongated shape. They may play an important role in creating the bipolar outflow in the region.
Extragalactic ammonia was imaged using the VLA in IC 342
IC 342 (also known as Caldwell 5) is an intermediate spiral galaxy in the constellation Camelopardalis, located relatively close to the Milky Way. Despite its size and actual brightness, its location behind dusty areas near the galactic equator m ...
. The hot gas has temperatures above 70 K, which was inferred from ammonia line ratios and appears to be closely associated with the innermost portions of the nuclear bar seen in CO. was also monitored by VLA toward a sample of four galactic ultracompact HII regions: G9.62+0.19, G10.47+0.03, G29.96-0.02, and G31.41+0.31. Based upon temperature and density diagnostics, it is concluded that in general such clumps are probably the sites of massive star formation in an early evolutionary phase prior to the development of an ultracompact HII region.
Infrared detections
Absorption at 2.97 micrometres due to solid ammonia was recorded from interstellar grains in the Becklin-Neugebauer Object and probably in NGC 2264-IR as well. This detection helped explain the physical shape of previously poorly understood and related ice absorption lines.
A spectrum of the disk of Jupiter was obtained from the Kuiper Airborne Observatory
The Gerard P. Kuiper Airborne Observatory (KAO) was a national facility operated by NASA to support research in infrared astronomy. The observation platform was a highly modified Lockheed C-141A Starlifter jet transport aircraft (s/n: 6110, reg ...
, covering the 100 to 300 cm−1 spectral range. Analysis of the spectrum provides information on global mean properties of ammonia gas and an ammonia ice haze.
A total of 149 dark cloud positions were surveyed for evidence of 'dense cores' by using the (J,K) = (1,1) rotating inversion line of NH3. In general, the cores are not spherically shaped, with aspect ratios ranging from 1.1 to 4.4. It is also found that cores with stars have broader lines than cores without stars.
Ammonia has been detected in the Draco Nebula and in one or possibly two molecular clouds, which are associated with the high-latitude galactic infrared cirrus
Infrared cirrus or galactic cirrus are galactic filamentary structures seen in space over most of the sky that emit far-infrared light. The name is given because the structures are cloud-like in appearance. These structures were first detected ...
. The finding is significant because they may represent the birthplaces for the Population I metallicity B-type stars in the galactic halo that could have been borne in the galactic disk.
Observations of nearby dark clouds
By balancing and stimulated emission with spontaneous emission, it is possible to construct a relation between excitation temperature
The excitation temperature (T_) is defined for a population of particles via the Boltzmann factor. It satisfies
:
\frac = \frac \exp,
where ''n''u and ''n''l represent the number of particles in an upper (''e.g.'' excited) and lower (''e.g.'' ...
and density. Moreover, since the transitional levels of ammonia can be approximated by a 2-level system at low temperatures, this calculation is fairly simple. This premise can be applied to dark clouds, regions suspected of having extremely low temperatures and possible sites for future star formation. Detections of ammonia in dark clouds show very narrow linesindicative not only of low temperatures, but also of a low level of inner-cloud turbulence. Line ratio calculations provide a measurement of cloud temperature that is independent of previous CO observations. The ammonia observations were consistent with CO measurements of rotation temperatures of ≈10 K. With this, densities can be determined, and have been calculated to range between 104 and 105 cm−3 in dark clouds. Mapping of gives typical clouds sizes of 0.1 pc and masses near 1 solar mass. These cold, dense cores are the sites of future star formation.
UC HII regions
Ultra-compact HII regions are among the best tracers of high-mass star formation. The dense material surrounding UCHII regions is likely primarily molecular. Since a complete study of massive star formation necessarily involves the cloud from which the star formed, ammonia is an invaluable tool in understanding this surrounding molecular material. Since this molecular material can be spatially resolved, it is possible to constrain the heating/ionising sources, temperatures, masses, and sizes of the regions. Doppler-shifted velocity components allow for the separation of distinct regions of molecular gas that can trace outflows and hot cores originating from forming stars.
Extragalactic detection
Ammonia has been detected in external galaxies,Milky Way
The Milky Way is the galaxy that includes our Solar System, with the name describing the galaxy's appearance from Earth: a hazy band of light seen in the night sky formed from stars that cannot be individually distinguished by the naked eye ...
galaxyhot dense molecular cores form around newly forming stars embedded in larger clouds of molecular material on the scale of several hundred pc (giant molecular clouds; GMCs).
See also
*
*
*
*
*
*
*
*
*
Notes
References
Works Cited
*
*
*
Further reading
*
*
*
*
External links
International Chemical Safety Card 0414
(anhydrous ammonia), ilo.org.
(aqueous solutions), ilo.org.
*
*
Emergency Response to Ammonia Fertilizer Releases (Spills)
for the Minnesota Department of Agriculture.ammoniaspills.org
National Institute for Occupational Safety and Health – Ammonia Page
cdc.gov
cdc.gov
Ammonia, video
{{Authority control
Bases (chemistry)
Foul-smelling chemicals
Gaseous signaling molecules
Household chemicals
Industrial gases
Inorganic solvents
Nitrogen cycle
Nitrogen hydrides
Nitrogen(−III) compounds
Refrigerants
Toxicology

 Ammonia can act as a
Ammonia can act as a 
 The ancient Greek historian
The ancient Greek historian 


 The raw
The raw  The U.S. Occupational Safety and Health Administration (OSHA) has set a 15-minute exposure limit for gaseous ammonia of 35 ppm by volume in the environmental air and an 8-hour exposure limit of 25 ppm by volume. The
The U.S. Occupational Safety and Health Administration (OSHA) has set a 15-minute exposure limit for gaseous ammonia of 35 ppm by volume in the environmental air and an 8-hour exposure limit of 25 ppm by volume. The  The hazards of ammonia solutions depend on the concentration: "dilute" ammonia solutions are usually 5–10% by weight (<5.62 mol/L); "concentrated" solutions are usually prepared at >25% by weight. A 25% (by weight) solution has a density of 0.907 g/cm3, and a solution that has a lower density will be more concentrated. The European Union classification of ammonia solutions is given in the table.
The ammonia vapour from concentrated ammonia solutions is severely irritating to the eyes and the respiratory tract, and experts warn that these solutions only be handled in a fume hood. Saturated ("0.880" – see #Properties) solutions can develop a significant pressure inside a closed bottle in warm weather, and experts also warn that the bottle be opened with care. This is not usually a problem for 25% ("0.900") solutions.
Experts warn that ammonia solutions not be mixed with
The hazards of ammonia solutions depend on the concentration: "dilute" ammonia solutions are usually 5–10% by weight (<5.62 mol/L); "concentrated" solutions are usually prepared at >25% by weight. A 25% (by weight) solution has a density of 0.907 g/cm3, and a solution that has a lower density will be more concentrated. The European Union classification of ammonia solutions is given in the table.
The ammonia vapour from concentrated ammonia solutions is severely irritating to the eyes and the respiratory tract, and experts warn that these solutions only be handled in a fume hood. Saturated ("0.880" – see #Properties) solutions can develop a significant pressure inside a closed bottle in warm weather, and experts also warn that the bottle be opened with care. This is not usually a problem for 25% ("0.900") solutions.
Experts warn that ammonia solutions not be mixed with  Ammonia has been detected in the atmospheres of the
Ammonia has been detected in the atmospheres of the