|
Edward Divers
Edward Divers Royal Society, FRS (27 November 1837 – 8 April 1912) was a British experimental chemist who rose to prominence despite being visually impaired from young age. Between 1873 and 1899, Divers lived and worked in Japan and significantly contributed to the science and education of that country. Biography Divers was born in London and was of Kentish ancestry. He had one brother, who was connected with the Thames Ironworks and Shipbuilding Company, and a sister. Inflammation in the eyes during infancy seriously impaired his vision, which could not be properly corrected by glasses. This deficiency was further aggravated by an explosion during an experiment in 1884 making him blind in the right eye. In 1850, Divers entered the City of London School where he became inspired by chemistry lectures given by Thomas Hall. In 1853–1854 he became an assistant in John Stenhouse's laboratory at the medical school of St Bartholomew's Hospital. Stenhouse regarded defective vision o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Society
The Chemical Society was a scientific society formed in 1841 (then named the Chemical Society of London) by 77 scientists as a result of increased interest in scientific matters. Chemist Robert Warington was the driving force behind its creation. History One of the aims of the Chemical Society was to hold meetings for "the communication and discussion of discoveries and observations, an account of which shall be published by the Society". In 1847, its importance was recognised by a Royal Charter, which added to its role in the advancement of science, the development of chemical applications in industry. Its members included eminent chemists from overseas including August Wilhelm von Hofmann, who became its president in 1861. Membership was open to all those interested in chemistry, but fellowship was for long restricted to men. In 1904, Edith Humphrey, thought to be the first British woman to gain a doctorate in chemistry (at the University of Zurich), was one of nineteen women ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Institute Of Chemistry
The Royal Institute of Chemistry was a British scientific organisation. Founded in 1877 as the Institute of Chemistry of Great Britain and Ireland (ICGBI), its role was to focus on qualifications and the professional status of chemists, and its aim was to ensure that consulting and analytical chemists were properly trained and qualified. The society received its first Royal Charter on 13 June 1885, and King George VI awarded the society royal patronage with effect from 14 May 1943, from which date it became the Royal Institute of Chemistry of Great Britain and Ireland (RICGBI). This re-designation was formally confirmed by the grant of a Supplemental Charter on 29 March 1944. As well as insisting on thorough professional qualifications, it also laid down strict ethical standards. Its main qualifications were Licentiate (LRIC) (professional training following a course of practical study to a standard lower than an honours degree), Graduate (GRIC) (completion of study equivalent to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
British Association
The British Science Association (BSA) is a charity and learned society founded in 1831 to aid in the promotion and development of science. Until 2009 it was known as the British Association for the Advancement of Science (BA). The current Chief Executive is Katherine Mathieson. The BSA's mission is to get more people engaged in the field of science by coordinating, delivering, and overseeing different projects that are suited to achieve these goals. The BSA "envisions a society in which a diverse group of people can learn and apply the sciences in which they learn." and is managed by a professional staff located at their Head Office in the Wellcome Wolfson Building. The BSA offers a wide variety of activities and events that both recognize and encourage people to be involved in science. These include the British Science Festival, British Science Week, the CREST Awards, Huxley Summit, Media Fellowships Scheme, along with regional and local events. History Foundation The Asso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Divers' Solution
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both causti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Nitrate
Ammonium nitrate is a chemical compound with the chemical formula . It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer.Karl-Heinz Zapp "Ammonium Compounds" in ''Ullmann's Encyclopedia of Industrial Chemistry'' 2012, Wiley-VCH, Weinheim. Global production was estimated at 21.6 million tonnes in 2017. Its other major use is as a component of explosive mixtures used in mining, quarrying, and civil construction. It is the major constituent of ANFO, a popular industrial explosive which accounts for 80% of explosives used in North America; similar formulations have been used in improvised explosive devices. Many countries are phasing out its use in consumer applications due to concerns over its potential for misuse. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyponitrite
In chemistry, hyponitrite may refer to the anion ( N=NOsup>2−), or to any ionic compound that contains it. In organic chemistry, it may also refer to the group −O−N=N−O−, or any organic compound with the generic formula R1−O−N=N−O−R2, where R1 and R2 are organic groups.M. N. Hughes (1968), "Hyponitrites". Quarterly Reviews of the Chemical Society, volume 22, issue 1, pages 1–13. . Such compounds can be viewed as salts and esters of respectively hyponitrous acid or HON=NOH. An acid hyponitrite is an ionic compound with the anion ( ON=NOsup>−). Hyponitrite ion Hyponitrite exhibits cis–trans isomerism.Egon Wiberg, Arnold Frederick Holleman (2001) ''Inorganic Chemistry'', Elsevier The ''trans'' (''E'') form is generally found in hyponitrite salts such as sodium hyponitrite () and silver(I) hyponitrite (). The ''cis'' (''Z'') form of sodium hyponitrite can be obtained too, and it is more reactive than the ''trans'' form. The ''cis'' hyponitrite anion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite also refers to organic compounds having the –ONO group, which are esters of nitrous acid. Production Sodium nitrite is made industrially by passing a mixture of nitrogen oxides into aqueous sodium hydroxide or sodium carbonate solution: : The product is purified by recrystallization. Alkali metal nitrites are thermally stable up to and beyond their melting point (441 °C for KNO2). Ammonium nitrite can be made from dinitrogen trioxide, N2O3, which is formally the anhydride of nitrous acid: :2 NH3 + H2O + N2O3 → 2 NH4NO2 Structure The nitrite ion has a symmetrical structure (C2v molecular point group, symmetry), with both N–O bonds having equal length and a bond angle of about 115°. In valence bond theory, it is des ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gelatinous Acids
Gelatin or gelatine (from la, gelatus meaning "stiff" or "frozen") is a translucent, colorless, flavorless food ingredient, commonly derived from collagen taken from animal body parts. It is brittle when dry and rubbery when moist. It may also be referred to as hydrolyzed collagen, collagen hydrolysate, gelatine hydrolysate, hydrolyzed gelatine, and collagen peptides after it has undergone hydrolysis. It is commonly used as a gelling agent in food, beverages, medications, drug or vitamin capsules, photographic films, papers, and cosmetics. Substances containing gelatin or functioning in a similar way are called gelatinous substances. Gelatin is an irreversibly hydrolyzed form of collagen, wherein the hydrolysis reduces protein fibrils into smaller peptides; depending on the physical and chemical methods of denaturation, the molecular weight of the peptides falls within a broad range. Gelatin is present in gelatin desserts, most gummy candy and marshmallows, ice creams, dips, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guncotton
Nitrocellulose (also known as cellulose nitrate, flash paper, flash cotton, guncotton, pyroxylin and flash string, depending on form) is a highly flammable compound formed by nitrating cellulose through exposure to a mixture of nitric acid and sulfuric acid. One of its first major uses was as guncotton, a replacement for gunpowder as propellant in firearms. It was also used to replace gunpowder as a low-order explosive in mining and other applications. In the form of collodion it was also a critical component in an early photographic emulsion, the use of which revolutionized photography in the 1860s. Production The process uses a mixture of nitric acid and sulfuric acid to convert cellulose into nitrocellulose. The quality of the cellulose is important. Hemicellulose, lignin, pentosans, and mineral salts give inferior nitrocelluloses. In precise chemical terms, nitrocellulose is not a nitro compound, but a nitrate ester. The glucose repeat unit (anhydroglucose) within the ce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations (), where one or more hydrogen atoms are replaced by organic groups (indicated by R). Acid–base properties The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted acids (proton donors): :H+ + NH3 -> H4 The ammonium ion is mildly acidic, reacting with Brønsted bases to return to the uncharged ammonia molecule: : H4 + B- -> HB + NH3 Thus, treatment of concentrated solutions of ammonium salts with strong base gives ammonia. When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions: :H2O + NH3 OH- + H4 The degree to which ammonia forms the ammonium ion depends on the pH of the solution. If the pH is low, the equilibrium shifts to the right: more ammonia molecules are co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
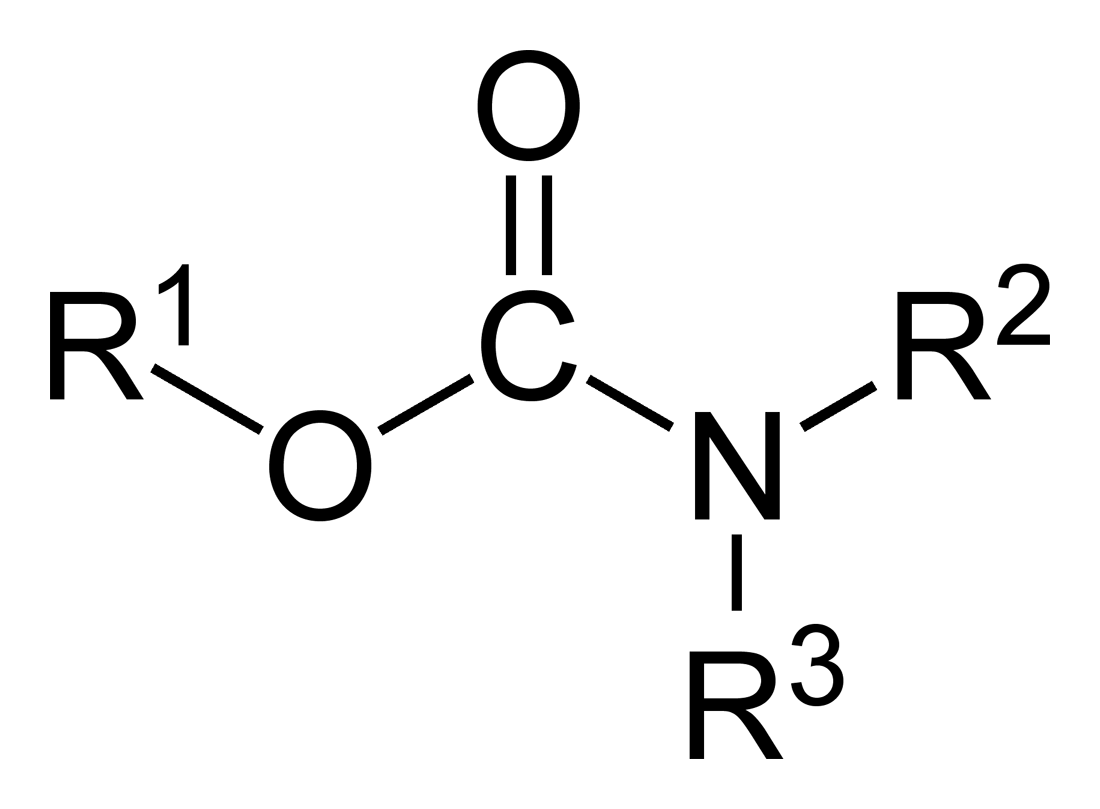
Carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion (e.g. ammonium carbamate). Polymers whose units are joined by carbamate groups are an important family of plastics, the polyurethanes. Properties While carbamic acids are unstable, many carbamate esters or ionic) are stable and well known. Equilibrium with carbonate and bicarbonate In water solutions, the carbamate anion slowly equilibrates with the ammonium cation and the carbonate or bicarbonate anions: : : Calcium carbamate is soluble in water, whereas calcium carbonate is not. Adding a calcium salt to an ammonium carbamate/carbonate solution will precipitate some calcium carbonate immediatel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


-3D-balls.png)