|
Carboxylic Acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parent Chain
In chemistry, a parent structure is the structure of an unadorned ion or molecule from which derivatives can be visualized. Parent structures underpin systematic nomenclature and facilitate classification. Fundamental parent structures have one or no functional groups and often have various types of symmetry. Benzene () is a chemical itself consisting of a hexagonal ring of carbon atoms with a hydrogen atom attached to each, and is the parent of many derivatives that have substituent atoms or groups replacing one or more of the hydrogens. Some parents are rare or nonexistent themselves, as in the case of porphine, though many simple and complex derivatives are known. File:Benzene circle.png, Benzene is the parent. File:Toluene-vec.svg, Toluene is a derivative of benzene. File:Porphyrin.svg, Porphine is the parent of porphyrins. File:PPIXtransH.png, Protoporphyrin IX is a natural derivative of the parent porphine. File:H2octaethylporphyrin.png , Octaethylporphyrin is a syntheti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinegar
Vinegar is an aqueous solution of acetic acid and trace compounds that may include flavorings. Vinegar typically contains 5–8% acetic acid by volume. Usually, the acetic acid is produced by a double fermentation, converting simple sugars to ethanol using yeast, and ethanol to acetic acid by acetic acid bacteria. Many types of vinegar are available, depending on source materials. It is now mainly used in the culinary arts as a flavorful, acidic cooking ingredient, or in pickling. Various types are used as condiments or garnishes, including balsamic vinegar and malt vinegar. As the most easily manufactured mild acid, it has a wide variety of industrial and domestic uses, including use as a household cleaner. Etymology The word "vinegar" arrived in Middle English from Old French (''vyn egre''; sour wine), which in turn derives from Latin: ''vinum'' (wine) + ''acer'' (sour). Chemistry The conversion of ethanol (CH3CH2OH) and oxygen (O2) to acetic acid (CH3COOH) takes plac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats. The global ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insect Stings
Insect bites and stings occur when an insect is agitated and seeks to defend itself through its natural defense mechanisms, or when an insect seeks to feed off the bitten person. Some insects inject formic acid, which can cause an immediate skin reaction often resulting in redness and swelling in the injured area. Stings from fire ants, bees, wasps and hornets are usually painful, and may stimulate a dangerous allergic reaction called anaphylaxis for at-risk patients, and some wasps can also have a powerful bite along with a sting. Bites from mosquitoes and fleas are more likely to cause itching than pain. The skin reaction to insect bites and stings usually lasts for up to a few days. However, in some cases, the local reaction can last for up to two years. These bites are sometimes misdiagnosed as other types of benign or cancerous lesions. Signs and symptoms The reaction to a sting is of three types. The normal reaction involves the area around the bite with redness, itchin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formic Acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure . It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Esters, salts and the anion derived from formic acid are called formates. Industrially, formic acid is produced from methanol. Natural occurrence In nature, formic acid is found in most ants and in stingless bees of the genus ''Oxytrigona''. Wood ants from the genus ''Formica'' can spray formic acid on their prey or to defend the nest. The puss moth caterpillar (''Cerura vinula'') will spray it as well when threatened by predators. It is also found in the trichomes of stinging nettle (''Urtica dioica''). Apart from that, this acid is incorporated in many fruits such as pineapple (0.21mg per 100g), apple (2mg per 100g) and kiwi (1mg per 100g), as well as in many vegetables, namely onion (45mg per 100g), eggplant (1.34 mg per 100g) and, in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Moiety (chemistry)
In organic chemistry, a moiety ( ) is a part of a molecule that is given a name because it is identified as a part of other molecules as well. Typically, the term is used to describe the larger and characteristic parts of organic molecules, and it should not be used to describe or name smaller functional groups of atoms that chemically react in similar ways in most molecules that contain them. Occasionally, a moiety may contain smaller moieties and functional groups. A moiety that acts as a branch extending from the backbone of a hydrocarbon molecule is called a substituent or side chain, which typically can be removed from the molecule and substituted with others. Active moiety In pharmacology, an active moiety is the part of a molecule or ion – excluding appended inactive portions – that is responsible for the physiological or pharmacological action of a drug substance. Inactive appended portions of the drug substance may include either the alcohol or acid moiety of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
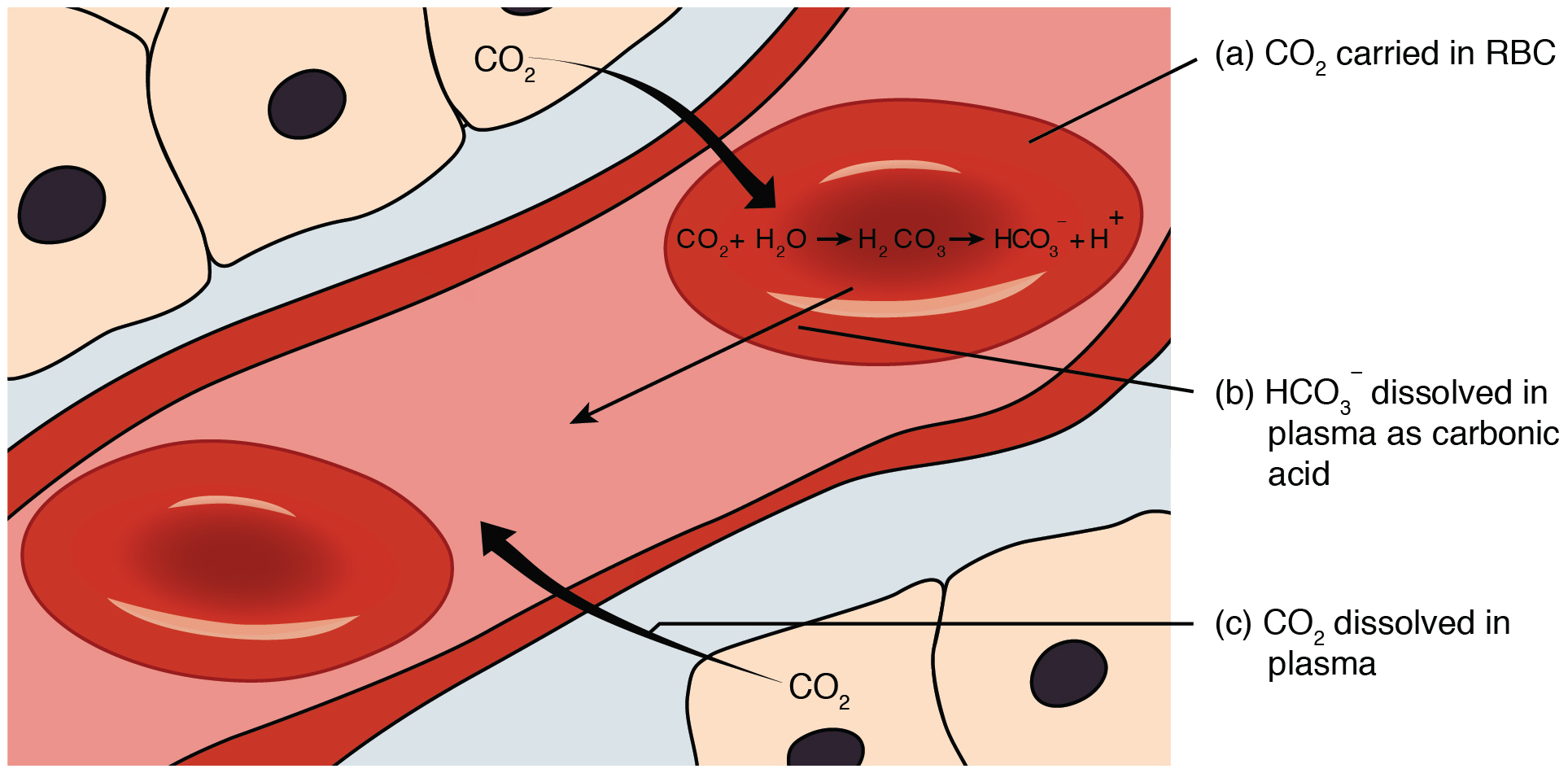
Bicarbonate Buffer System
The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance of carbonic acid (H2CO3), bicarbonate ion (HCO), and carbon dioxide (CO2) in order to maintain pH in the blood and duodenum, among other tissues, to support proper metabolic function. Catalyzed by carbonic anhydrase, carbon dioxide (CO2) reacts with water (H2O) to form carbonic acid (H2CO3), which in turn rapidly dissociates to form a bicarbonate ion (HCO ) and a hydrogen ion (H+) as shown in the following reaction: \rm CO_2 + H_2O \rightleftarrows H_2CO_3 \rightleftarrows HCO_3^- + H^+ As with any buffer system, the pH is balanced by the presence of both a weak acid (for example, H2CO3) and its conjugate base (for example, HCO) so that any excess acid or base introduced to the system is neutralized. Failure of this system to function properly results in acid-base imbalance, such as acidemia (pH 7.45) in the blood. In systemic acid–base balance In tissue, cellular respiration produc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetate
An acetate is a salt (chemistry), salt formed by the combination of acetic acid with a base (e.g. Alkali metal, alkaline, Alkaline earth metal, earthy, Transition metal, metallic, nonmetallic or radical Radical (chemistry), base). "Acetate" also describes the conjugate acid, conjugate base or ion (specifically, the negatively charged ion called an anion) typically found in aqueous solution and written with the chemical formula . The neutral molecules formed by the combination of the acetate ion and a ''positive'' ion (called a cation) are also commonly called "acetates" (hence, ''acetate of lead'', ''acetate of aluminum'', etc.). The simplest of these is hydrogen acetate (called acetic acid) with corresponding salts, esters, and the polyatomic ion, polyatomic anion , or . Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In nature, acetate is the most common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats. The global ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |