Hydrogen is a
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
; it has
symbol
A symbol is a mark, Sign (semiotics), sign, or word that indicates, signifies, or is understood as representing an idea, physical object, object, or wikt:relationship, relationship. Symbols allow people to go beyond what is known or seen by cr ...
H and
atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
1. It is the lightest and
most abundant chemical element in the
universe
The universe is all of space and time and their contents. It comprises all of existence, any fundamental interaction, physical process and physical constant, and therefore all forms of matter and energy, and the structures they form, from s ...
, constituting about 75% of all
normal matter. Under
standard conditions, hydrogen is a
gas of
diatomic molecules with the
formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwe ...
, called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly
combustible.
Stars
A star is a luminous spheroid of plasma held together by self-gravity. The nearest star to Earth is the Sun. Many other stars are visible to the naked eye at night; their immense distances from Earth make them appear as fixed points of ...
, including the
Sun, mainly consist of hydrogen in a
plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in
molecular forms, such as in
water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
and
organic compounds
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
. The most common
isotope of hydrogen (H) consists of one
proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
, one
electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
, and no
neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s.
Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals.
Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovered its property of producing water when burned; hence its name means 'water-former' in Greek. Understanding the colors of light absorbed and emitted by hydrogen was a crucial part of developing
quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
.
Hydrogen, typically
nonmetallic except under
extreme pressure, readily forms
covalent bonds with most nonmetals, contributing to the formation of compounds like water and various organic substances. Its role is crucial in
acid-base reactions, which mainly involve proton exchange among soluble molecules. In
ionic compounds, hydrogen can take the form of either a negatively charged
anion, where it is known as
hydride, or as a positively charged
cation, , called a proton. Although tightly bonded to water molecules, protons strongly affect the behavior of
aqueous solutions, as reflected in the importance of pH. Hydride, on the other hand, is rarely observed because it tends to deprotonate solvents, yielding .
In the
early universe, neutral hydrogen atoms formed about 370,000 years after the
Big Bang as the universe expanded and plasma had cooled enough for electrons to remain bound to protons. Once stars formed most of the atoms in the
intergalactic medium re-ionized.
Nearly all
hydrogen production
Hydrogen gas is produced by several industrial methods. Nearly all of the world's current supply of hydrogen is created from fossil fuels. Article in press. Most hydrogen is ''gray hydrogen'' made through steam methane reforming. In this process, ...
is done by transforming fossil fuels, particularly
steam reforming of
natural gas
Natural gas (also fossil gas, methane gas, and gas) is a naturally occurring compound of gaseous hydrocarbons, primarily methane (95%), small amounts of higher alkanes, and traces of carbon dioxide and nitrogen, hydrogen sulfide and helium ...
. It can also be produced from water or saline by
electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of c ...
, but this process is more expensive. Its main industrial uses include
fossil fuel processing and
ammonia production for fertilizer. Emerging uses for hydrogen include the use of
fuel cell
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen fuel, hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most bat ...
s to generate electricity.
Properties
Atomic hydrogen
Electron energy levels
The
ground state energy level
A quantum mechanics, quantum mechanical system or particle that is bound state, bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical mechanics, classical pa ...
of the electron in a hydrogen atom is −13.6
eV, equivalent to an
ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
of roughly 91 nm wavelength. The energy levels of hydrogen are referred to by consecutive
quantum number
In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system.
To fully specify the state of the electron in a hydrogen atom, four quantum numbers are needed. The traditional set of quantu ...
s, with
being the ground state. The
hydrogen spectral series corresponds to emission of light due to transitions from higher to lower energy levels. Each energy level is further split by
spin interactions between the electron and proton into 4
hyperfine levels.
High precision values for the hydrogen atom energy levels are required for definitions of physical constants. Quantum calculations have identified 9 contributions to the energy levels. The eigenvalue from the
Dirac equation is the largest contribution. Other terms include
relativistic recoil, the
self-energy, and the
vacuum polarization terms.
Isotopes

Hydrogen has three naturally occurring isotopes, denoted , and . Other, highly unstable nuclei ( to ) have been synthesized in the laboratory but not observed in nature.
is the most common hydrogen isotope, with an abundance of >99.98%. Because the
nucleus of this isotope consists of only a single proton, it is given the descriptive but rarely used formal name ''protium''. It is the only stable isotope with no neutrons; see
diproton for a discussion of why others do not exist.
, the other stable hydrogen isotope, is known as
deuterium and contains one proton and one
neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
in the nucleus. Nearly all deuterium nuclei in the universe is thought to have been produced at the time of the
Big Bang, and has endured since then. Deuterium is not radioactive, and is not a significant toxicity hazard. Water enriched in molecules that include deuterium instead of normal hydrogen is called
heavy water
Heavy water (deuterium oxide, , ) is a form of water (molecule), water in which hydrogen atoms are all deuterium ( or D, also known as ''heavy hydrogen'') rather than the common hydrogen-1 isotope (, also called ''protium'') that makes up most o ...
. Deuterium and its compounds are used as a non-radioactive label in chemical experiments and in solvents for -
NMR spectroscopy. Heavy water is used as a
neutron moderator and coolant for nuclear reactors. Deuterium is also a potential fuel for commercial
nuclear fusion
Nuclear fusion is a nuclear reaction, reaction in which two or more atomic nuclei combine to form a larger nuclei, nuclei/neutrons, neutron by-products. The difference in mass between the reactants and products is manifested as either the rele ...
.
is known as
tritium and contains one proton and two neutrons in its nucleus. It is radioactive, decaying into
helium-3 through
beta decay with a
half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 12.32 years.
It is radioactive enough to be used in
luminous paint to enhance the visibility of data displays, such as for painting the hands and dial-markers of watches. The watch glass prevents the small amount of radiation from escaping the case.
Small amounts of tritium are produced naturally by cosmic rays striking atmospheric gases; tritium has also been released in
nuclear weapons tests. It is used in nuclear fusion, as a tracer in
isotope geochemistry, and in specialized
self-powered lighting
Tritium radioluminescence is the use of gaseous tritium, a radioactive isotope of hydrogen, to create visible light. Tritium emits electrons through beta decay and, when they interact with a phosphor material, light is emitted through the proc ...
devices. Tritium has also been used in chemical and biological labeling experiments as a
radiolabel.
Unique among the elements, distinct names are assigned to its isotopes in common use. During the early study of radioactivity, heavy radioisotopes were given their own names, but these are mostly no longer used. The symbols D and T (instead of and ) are sometimes used for deuterium and tritium, but the symbol P was already used for
phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
and thus was not available for protium. In its
nomenclatural guidelines, the
International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
(IUPAC) allows any of D, T, , and to be used, though and are preferred.
Antihydrogen () is the
antimatter counterpart to hydrogen. It consists of an
antiproton with a
positron. Antihydrogen is the only type of antimatter atom to have been produced .
The
exotic atom muonium (symbol Mu), composed of an anti
muon and an
electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
, is analogous hydrogen and IUPAC nomenclature incorporates such hypothetical compounds as muonium chloride (MuCl) and sodium muonide (NaMu), analogous to
hydrogen chloride and
sodium hydride respectively.
Dihydrogen
Under
standard conditions, hydrogen is a
gas of
diatomic molecules with the
formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwe ...
, officially called "dihydrogen", but also called "molecular hydrogen",
or simply hydrogen. Dihydrogen is a colorless, odorless, flammable gas.
Combustion
Hydrogen gas is highly flammable, reacting with
oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
in air, to produce liquid water:
:
The
amount of heat released per
mole of hydrogen is −286 kJ or 141.865 MJ for a kilogram mass.
Hydrogen gas forms explosive mixtures with air in concentrations from 4–74% and with chlorine at 5–95%. The hydrogen
autoignition temperature, the temperature of spontaneous ignition in air, is . In a high-pressure hydrogen leak, the shock wave from the leak itself can heat air to the autoignition temperature, leading to flaming and possibly explosion.
Hydrogen flames emit faint blue and
ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
light.
Flame detectors are used to detect hydrogen fires as they are nearly invisible to the naked eye in daylight.
Spin isomers
Molecular exists as two
nuclear isomers that differ in the
spin states of their nuclei.
In the orthohydrogen form, the spins of the two nuclei are parallel, forming a spin
triplet state having a
total molecular spin ; in the parahydrogen form the spins are antiparallel and form a spin
singlet state
In quantum mechanics, a singlet state usually refers to a system in which all electrons are paired. The term 'singlet' originally meant a linked set of particles whose net angular momentum is zero, that is, whose overall spin quantum number s=0. A ...
having spin
. The equilibrium ratio of ortho- to para-hydrogen depends on temperature. At room temperature or warmer, equilibrium hydrogen gas contains about 25% of the para form and 75% of the ortho form.
The ortho form is an
excited state, having higher energy than the para form by 1.455 kJ/mol,
and it converts to the para form over the course of several minutes when cooled to low temperature. The thermal properties of these isomers differ because each has distinct
rotational quantum states.
The ortho-to-para ratio in is an important consideration in the
liquefaction and storage of
liquid hydrogen: the conversion from ortho to para is
exothermic and produces sufficient heat to evaporate most of the liquid if not converted first to parahydrogen during the cooling process.
s for the ortho-para interconversion, such as
ferric oxide and
activated carbon compounds, are used during hydrogen cooling to avoid this loss of liquid.
Phases
 Liquid hydrogen
Liquid hydrogen can exist at temperatures below hydrogen's
critical point of 33
K. However, for it to be in a fully liquid state at
atmospheric pressure
Atmospheric pressure, also known as air pressure or barometric pressure (after the barometer), is the pressure within the atmosphere of Earth. The standard atmosphere (symbol: atm) is a unit of pressure defined as , which is equivalent to 1,013. ...
, H
2 needs to be cooled to . Hydrogen was liquefied by
James Dewar in 1898 by using
regenerative cooling and his invention, the
vacuum flask.
Liquid hydrogen becomes
solid hydrogen at
standard pressure below hydrogen's
melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
of . Distinct solid phases exist, known as Phase I through Phase V, each exhibiting a characteristic molecular arrangement.
Liquid and solid phases can exist in combination at the
triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three Phase (matter), phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at ...
, a substance known as
slush hydrogen.
Metallic hydrogen, a phase obtained at extremely high pressures (in excess of ), is an electrical conductor. It is believed to exist deep within
giant planets like
Jupiter
Jupiter is the fifth planet from the Sun and the List of Solar System objects by size, largest in the Solar System. It is a gas giant with a Jupiter mass, mass more than 2.5 times that of all the other planets in the Solar System combined a ...
.
When
ionized, hydrogen becomes a
plasma. This is the form in which hydrogen exists within
star
A star is a luminous spheroid of plasma (physics), plasma held together by Self-gravitation, self-gravity. The List of nearest stars and brown dwarfs, nearest star to Earth is the Sun. Many other stars are visible to the naked eye at night sk ...
s.
Thermal and physical properties
History
18th century
In 1671, Irish scientist
Robert Boyle discovered and described the reaction between
iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
filings and dilute
acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
s, which results in the production of hydrogen gas.
Boyle did not note that the gas was inflammable, but hydrogen would play a key role in overturning the
phlogiston theory of combustion.
In 1766,
Henry Cavendish was the first to recognize hydrogen gas as a discrete substance, by naming the gas from a
metal-acid reaction "inflammable air". He speculated that "inflammable air" was in fact identical to the hypothetical substance "
phlogiston"
and further finding in 1781 that the gas produces water when burned. He is usually given credit for the discovery of hydrogen as an element.
In 1783,
Antoine Lavoisier identified the element that came to be known as hydrogen when he and
Laplace reproduced Cavendish's finding that water is produced when hydrogen is burned.
Lavoisier produced hydrogen for his experiments on mass conservation by treating metallic
iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
with a stream of H
2O through an incandescent iron tube heated in a fire. Anaerobic oxidation of iron by the protons of water at high temperature can be schematically represented by the set of following reactions:
*
*
*
Many metals react similarly with water leading to the production of hydrogen. In some situations, this H
2-producing process is problematic as is the case of zirconium cladding on nuclear fuel rods.
19th century
By 1806 hydrogen was used to fill balloons.
François Isaac de Rivaz built the first
de Rivaz engine, an internal combustion engine powered by a mixture of hydrogen and oxygen in 1806.
Edward Daniel Clarke invented the hydrogen gas blowpipe in 1819. The
Döbereiner's lamp and
limelight were invented in 1823. Hydrogen was
liquefied for the first time by
James Dewar in 1898 by using
regenerative cooling and his invention, the
vacuum flask. He produced
solid hydrogen the next year.
One of the first
quantum effects to be explicitly noticed (but not understood at the time) was
James Clerk Maxwell
James Clerk Maxwell (13 June 1831 – 5 November 1879) was a Scottish physicist and mathematician who was responsible for the classical theory of electromagnetic radiation, which was the first theory to describe electricity, magnetism an ...
's observation that the
specific heat capacity of unaccountably departs from that of a
diatomic
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear mol ...
gas below room temperature and begins to increasingly resemble that of a monatomic gas at cryogenic temperatures. According to quantum theory, this behavior arises from the spacing of the (quantized) rotational energy levels, which are particularly wide-spaced in because of its low mass. These widely spaced levels inhibit equal partition of heat energy into rotational motion in hydrogen at low temperatures. Diatomic gases composed of heavier atoms do not have such widely spaced levels and do not exhibit the same effect.
20th century
The existence of the
hydride anion was suggested by
Gilbert N. Lewis in 1916 for group 1 and 2 salt-like compounds. In 1920, Moers electrolyzed molten
lithium hydride (LiH), producing a
stoichiometric quantity of hydrogen at the anode.

Because of its simple atomic structure, consisting only of a proton and an electron, the
hydrogen atom, together with the spectrum of light produced from it or absorbed by it, has been central to the
development of the theory of atomic structure. The energy levels of hydrogen can be calculated fairly accurately using the
Bohr model
In atomic physics, the Bohr model or Rutherford–Bohr model was a model of the atom that incorporated some early quantum concepts. Developed from 1911 to 1918 by Niels Bohr and building on Ernest Rutherford's nuclear Rutherford model, model, i ...
of the atom, in which the electron "orbits" the proton, like how Earth orbits the Sun. However, the electron and proton are held together by electrostatic attraction, while planets and celestial objects are held by
gravity
In physics, gravity (), also known as gravitation or a gravitational interaction, is a fundamental interaction, a mutual attraction between all massive particles. On Earth, gravity takes a slightly different meaning: the observed force b ...
. Due to the discretization of
angular momentum
Angular momentum (sometimes called moment of momentum or rotational momentum) is the rotational analog of Momentum, linear momentum. It is an important physical quantity because it is a Conservation law, conserved quantity – the total ang ...
postulated in early
quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
by Bohr, the electron in the Bohr model can only occupy certain allowed distances from the proton, and therefore only certain allowed energies.
Hydrogen's unique position as the only neutral atom for which the
Schrödinger equation can be directly solved, has significantly contributed to the understanding of quantum mechanics through the exploration of its energetics.
Furthermore, study of the corresponding simplicity of the hydrogen molecule and the corresponding cation
brought understanding of the nature of the chemical bond, which followed shortly after the quantum mechanical treatment of the hydrogen atom had been developed in the mid-1920s.
Hydrogen-lifted airship
Because is only 7% the density of air, it was once widely used as a
lifting gas in balloons and
airship
An airship, dirigible balloon or dirigible is a type of aerostat (lighter-than-air) aircraft that can navigate through the air flying powered aircraft, under its own power. Aerostats use buoyancy from a lifting gas that is less dense than the ...
s.
The first hydrogen-filled
balloon was invented by
Jacques Charles in 1783. Hydrogen provided the lift for the first reliable form of air-travel following the 1852 invention of the first hydrogen-lifted
airship
An airship, dirigible balloon or dirigible is a type of aerostat (lighter-than-air) aircraft that can navigate through the air flying powered aircraft, under its own power. Aerostats use buoyancy from a lifting gas that is less dense than the ...
by
Henri Giffard. German count
Ferdinand von Zeppelin
Graf, Count Ferdinand von Zeppelin (; 8 July 1838 – 8 March 1917) was a General (Germany), German general and later inventor of the Zeppelin rigid airships. His name became synonymous with airships and dominated long-distance flight until the ...
promoted the idea of rigid airships lifted by hydrogen that later were called
Zeppelin
A Zeppelin is a type of rigid airship named after the German inventor Ferdinand von Zeppelin () who pioneered rigid airship development at the beginning of the 20th century. Zeppelin's notions were first formulated in 1874Eckener 1938, pp. 155� ...
s; the first of which had its maiden flight in 1900.
Regularly scheduled flights started in 1910 and by the outbreak of World War I in August 1914, they had carried 35,000 passengers without a serious incident. Hydrogen-lifted airships in the form of
blimps were used as observation platforms and bombers during the War II, especially on the US Eastern seaboard.
The first non-stop transatlantic crossing was made by the British airship ''
R34'' in 1919 and regular passenger service resumed in the 1920s. Hydrogen was used in the
''Hindenburg'' airship, which caught fire over
New Jersey
New Jersey is a U.S. state, state located in both the Mid-Atlantic States, Mid-Atlantic and Northeastern United States, Northeastern regions of the United States. Located at the geographic hub of the urban area, heavily urbanized Northeas ...
on 6 May 1937.
The hydrogen that filled the airship was ignited, possibly by static electricity, and burst into flames. Following this
Hindenburg disaster, commercial hydrogen airship travel
ceased. Hydrogen is still used, in preference to non-flammable but more expensive
helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
, as a lifting gas for
weather balloons.
Deuterium and tritium
Deuterium was discovered in December 1931 by
Harold Urey, and
tritium was prepared in 1934 by
Ernest Rutherford,
Mark Oliphant, and
Paul Harteck.
Heavy water
Heavy water (deuterium oxide, , ) is a form of water (molecule), water in which hydrogen atoms are all deuterium ( or D, also known as ''heavy hydrogen'') rather than the common hydrogen-1 isotope (, also called ''protium'') that makes up most o ...
, which consists of deuterium in the place of regular hydrogen, was discovered by Urey's group in 1932.
Chemistry
Reactions of H2

is relatively unreactive. The thermodynamic basis of this low reactivity is the very strong H–H bond, with a
bond dissociation energy of 435.7 kJ/mol. It does form coordination complexes called
dihydrogen complexes. These species provide insights into the early steps in the interactions of hydrogen with metal catalysts. According to
neutron diffraction, the metal and two H atoms form a triangle in these complexes. The H-H bond remains intact but is elongated. They are acidic.
Although exotic on Earth, the ion is common in the universe. It is a triangular species, like the aforementioned dihydrogen complexes. It is known as
protonated molecular hydrogen or the trihydrogen cation.
Hydrogen reacts with
chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
to produce
HCl and with
bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
to produce
HBr by a
chain reaction. The reaction requires initiation. For example in the case of Br
2, the diatomic molecule is broken into atoms, . Propagating reactions consume hydrogen molecules and produce HBr, as well as Br and H atoms:
:
:
Finally the terminating reaction:
:
:.
consumes the remaining atoms.
The addition of H
2 to unsaturated organic compounds, such as
alkenes and
alkynes, is called
hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
. Even if the reaction is
energetically favorable, it does not take place even at higher temperatures. In the presence of a
catalyst like finely divided
platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
or
nickel, the reaction proceeds at room temperature.
Hydrogen-containing compounds
Hydrogen can exist in both +1 and −1
oxidation states, forming compounds through
ionic and
covalent bonding. It is a part of a wide range of substances, including water,
hydrocarbons, and numerous other
organic compounds
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
.
The H
+ ion—commonly referred to as a proton due to its single proton and absence of electrons—is central to
acid–base chemistry, although the proton does not move freely. In the
Brønsted–Lowry framework, acids are defined by their ability to donate H
+ ions to bases.
Hydrogen forms a vast variety of compounds with
carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
known as hydrocarbons, and an even greater diversity with other elements (
heteroatoms), giving rise to the broad class of organic compounds often associated with living organisms.

Hydrogen compounds with hydrogen in the oxidation state −1 are known as hydrides, which are usually formed between hydrogen and metals. The hydrides can be ionic (aka saline), covalent, nor metallic. With heating, H
2 reacts efficiently with the alkali and alkaline earth metals to give the
ionic hydrides of the formula MH and MH
2, respectively. These salt-like crystalline compounds have high melting points and all react with water to liberate hydrogen. Covalent hydrides are include
boranes and polymeric
aluminium hydride.
Transition metals form
metal hydrides via continuous dissolution of hydrogen into the metal.
[ A well known hydride is lithium aluminium hydride, the anion carries hydridic centers firmly attached to the Al(III). Perhaps the most extensive series of hydrides are the boranes, compounds consisting only of boron and hydrogen.]ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
but also as bridging ligands. In diborane (), four H's are terminal and two bridge between the two B atoms.
Hydrogen bonding
When bonded to a more electronegative element, particularly fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
, oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, or nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
, hydrogen can participate in a form of medium-strength noncovalent bonding with another electronegative element with a lone pair like oxygen or nitrogen, a phenomenon called hydrogen bonding that is critical to the stability of many biological molecules. Hydrogen bonding alters molecule structures, viscosity
Viscosity is a measure of a fluid's rate-dependent drag (physics), resistance to a change in shape or to movement of its neighboring portions relative to one another. For liquids, it corresponds to the informal concept of ''thickness''; for e ...
, solubility
In chemistry, solubility is the ability of a chemical substance, substance, the solute, to form a solution (chemistry), solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form su ...
, as well as melting and boiling points even protein folding dynamics.
Protons and acids
 In water, hydrogen bonding plays an important role in reaction thermodynamics. A hydrogen bond can shift over to proton transfer.
Under the Brønsted–Lowry acid–base theory, acids are proton donors, while bases are proton acceptors.
A bare proton, essentially cannot exist in anything other than a vacuum. Otherwise it attaches to other atoms, ions, or molecules. Even species as inert as methane can be protonated. The term 'proton' is used loosely and metaphorically to refer to refer to solvated " without any implication that any single protons exist freely as a species. To avoid the implication of the naked proton in solution, acidic aqueous solutions are sometimes considered to contain the " hydronium ion" () or still more accurately, .
In water, hydrogen bonding plays an important role in reaction thermodynamics. A hydrogen bond can shift over to proton transfer.
Under the Brønsted–Lowry acid–base theory, acids are proton donors, while bases are proton acceptors.
A bare proton, essentially cannot exist in anything other than a vacuum. Otherwise it attaches to other atoms, ions, or molecules. Even species as inert as methane can be protonated. The term 'proton' is used loosely and metaphorically to refer to refer to solvated " without any implication that any single protons exist freely as a species. To avoid the implication of the naked proton in solution, acidic aqueous solutions are sometimes considered to contain the " hydronium ion" () or still more accurately, .logarithmic scale
A logarithmic scale (or log scale) is a method used to display numerical data that spans a broad range of values, especially when there are significant differences among the magnitudes of the numbers involved.
Unlike a linear Scale (measurement) ...
that reflects its acidity or basicity. Lower pH values indicate higher concentrations of hydronium ions, corresponding to more acidic conditions.
Occurrence
Cosmic
 Hydrogen, as atomic H, is the most abundant
Hydrogen, as atomic H, is the most abundant chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
in the universe, making up 75% of normal matter by mass
Mass is an Intrinsic and extrinsic properties, intrinsic property of a physical body, body. It was traditionally believed to be related to the physical quantity, quantity of matter in a body, until the discovery of the atom and particle physi ...
and >90% by number of atoms. In the early universe, the protons
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' ( elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an electron (the pro ...
formed in the first second after the Big Bang; neutral hydrogen atoms formed about 370,000 years later during the recombination epoch as the universe expanded and plasma had cooled enough for electrons to remain bound to protons.
In astrophysics, neutral hydrogen in the interstellar medium is called ''H I'' and ionized hydrogen is called ''H II''. Radiation from stars ionizes H I to H II, creating spheres of ionized H II around stars. In the chronology of the universe neutral hydrogen dominated until the birth of stars during the era of reionization led to bubbles of ionized hydrogen that grew and merged over 500 million of years.
They are the source of the 21-cm hydrogen line at 1420 MHz that is detected in order to probe primordial hydrogen. The large amount of neutral hydrogen found in the damped Lyman-alpha systems is thought to dominate the cosmological
Cosmology () is a branch of physics and metaphysics dealing with the nature of the universe, the cosmos. The term ''cosmology'' was first used in English in 1656 in Thomas Blount's ''Glossographia'', with the meaning of "a speaking of the wo ...
baryonic density of the universe up to a redshift
In physics, a redshift is an increase in the wavelength, and corresponding decrease in the frequency and photon energy, of electromagnetic radiation (such as light). The opposite change, a decrease in wavelength and increase in frequency and e ...
of ''z'' = 4.
Hydrogen is found in great abundance in stars and gas giant
A gas giant is a giant planet composed mainly of hydrogen and helium. Jupiter and Saturn are the gas giants of the Solar System. The term "gas giant" was originally synonymous with "giant planet". However, in the 1990s, it became known that Uranu ...
planets. Molecular cloud
A molecular cloud—sometimes called a stellar nursery if star formation is occurring within—is a type of interstellar cloud of which the density and size permit absorption nebulae, the formation of molecules (most commonly molecular hydrogen, ...
s of are associated with star formation
Star formation is the process by which dense regions within molecular clouds in interstellar space—sometimes referred to as "stellar nurseries" or "star-forming regions"—Jeans instability, collapse and form stars. As a branch of astronomy, sta ...
. Hydrogen plays a vital role in powering star
A star is a luminous spheroid of plasma (physics), plasma held together by Self-gravitation, self-gravity. The List of nearest stars and brown dwarfs, nearest star to Earth is the Sun. Many other stars are visible to the naked eye at night sk ...
s through the proton-proton reaction in lower-mass stars, and through the CNO cycle of nuclear fusion
Nuclear fusion is a nuclear reaction, reaction in which two or more atomic nuclei combine to form a larger nuclei, nuclei/neutrons, neutron by-products. The difference in mass between the reactants and products is manifested as either the rele ...
in case of stars more massive than the Sun.
A molecular form called protonated molecular hydrogen () is found in the interstellar medium, where it is generated by ionization of molecular hydrogen from cosmic ray
Cosmic rays or astroparticles are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the ...
s. This ion has also been observed in the upper atmosphere of Jupiter. The ion is long-lived in outer space due to the low temperature and density. is one of the most abundant ions in the universe, and it plays a notable role in the chemistry of the interstellar medium. Neutral triatomic hydrogen can exist only in an excited form and is unstable.
Terrestrial
Hydrogen is the third most abundant element on the Earth's surface,chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s such as hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
s and water.Mali
Mali, officially the Republic of Mali, is a landlocked country in West Africa. It is the List of African countries by area, eighth-largest country in Africa, with an area of over . The country is bordered to the north by Algeria, to the east b ...
, France
France, officially the French Republic, is a country located primarily in Western Europe. Overseas France, Its overseas regions and territories include French Guiana in South America, Saint Pierre and Miquelon in the Atlantic Ocean#North Atlan ...
and Australia
Australia, officially the Commonwealth of Australia, is a country comprising mainland Australia, the mainland of the Australia (continent), Australian continent, the island of Tasmania and list of islands of Australia, numerous smaller isl ...
.
Production and storage
Industrial routes
Nearly all of the world's current supply of hydrogen gas () is created from fossil fuels.[ Article in press.] Many methods exist for producing H2, but three dominate commercially: steam reforming often coupled to water-gas shift, partial oxidation of hydrocarbons, and water electrolysis.[
]
Steam reforming
Hydrogen is mainly produced by steam methane reforming (SMR), the reaction of water and methane.methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
to yield carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
and .
:
Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide.methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
, which further contributes to the overall carbon footprint of hydrogen.Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
s other than methane can be used to produce synthesis gas with varying product ratios. One of the many complications to this highly optimized technology is the formation of coke or carbon:
:
Therefore, steam reforming typically employs an excess of . Additional hydrogen can be recovered from the steam by using carbon monoxide through the water gas shift reaction (WGS). This process requires an iron oxide
An iron oxide is a chemical compound composed of iron and oxygen. Several iron oxides are recognized. Often they are non-stoichiometric. Ferric oxyhydroxides are a related class of compounds, perhaps the best known of which is rust.
Iron ...
catalyst:
Partial oxidation of hydrocarbons
Other methods for CO and production include partial oxidation of hydrocarbons:propane
Propane () is a three-carbon chain alkane with the molecular formula . It is a gas at standard temperature and pressure, but becomes liquid when compressed for transportation and storage. A by-product of natural gas processing and petroleum ref ...
.
Water electrolysis
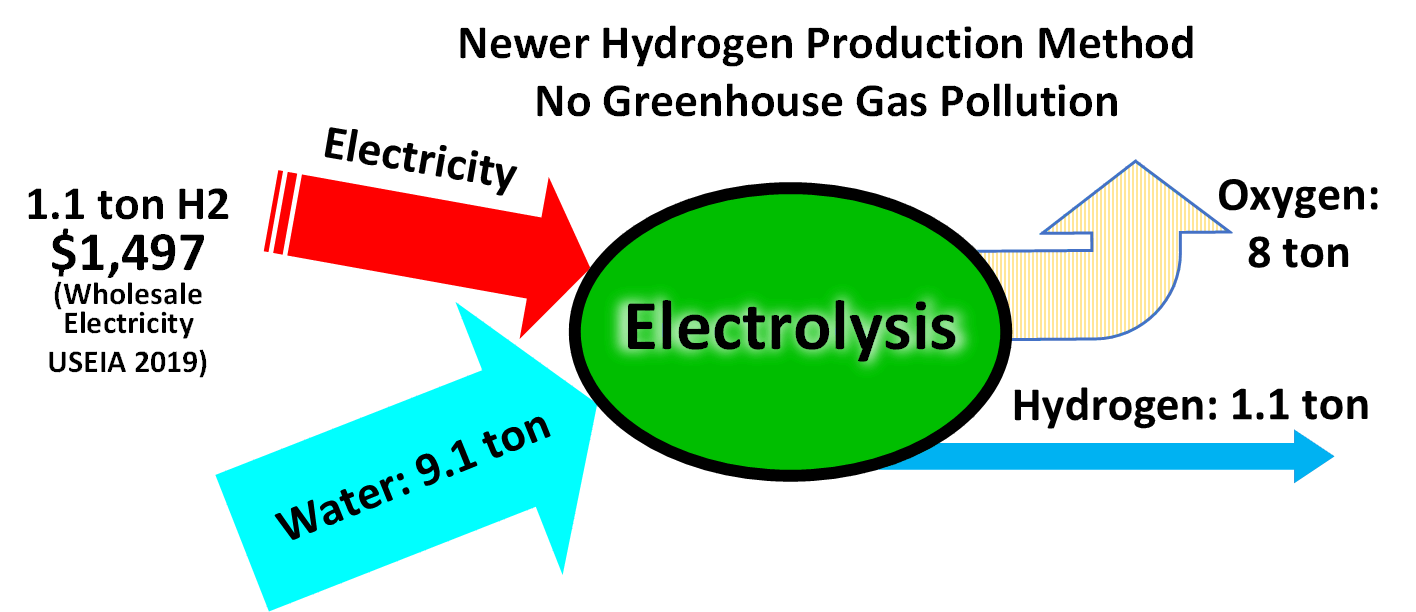 Electrolysis of water is a conceptually simple method of producing hydrogen.
:
Commercial electrolyzers use nickel-based catalysts in strongly alkaline solution. Platinum is a better catalyst but is expensive. The hydrogen created through electrolysis using renewable energy is commonly referred to as " green hydrogen".
Electrolysis of water is a conceptually simple method of producing hydrogen.
:
Commercial electrolyzers use nickel-based catalysts in strongly alkaline solution. Platinum is a better catalyst but is expensive. The hydrogen created through electrolysis using renewable energy is commonly referred to as " green hydrogen".Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of c ...
of brine to yield chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
also produces high purity hydrogen as a co-product, which is used for a variety of transformations such as hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
s.
The electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of c ...
process is more expensive than producing hydrogen from methane without carbon capture and storage.
Methane pyrolysis
Hydrogen can be produced by pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology
The word ''pyrolysis'' is coined from the Gree ...
of natural gas (methane), producing hydrogen gas and solid carbon with the aid a catalyst and 74 kJ/mol input heat:
: (ΔH° = 74 kJ/mol)
The carbon may be sold as a manufacturing feedstock or fuel, or landfilled.
This route could have a lower carbon footprint than existing hydrogen production processes, but mechanisms for removing the carbon and preventing it from reacting with the catalyst remain obstacles for industrial scale use.
Thermochemical
Water splitting is the process by which water is decomposed into its components. Relevant to the biological scenario is this simple equation:
:
The reaction occurs in the light reactions in all photosynthetic
Photosynthesis ( ) is a Biological system, system of biological processes by which Photoautotrophism, photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical ener ...
organisms. A few organisms, including the alga '' Chlamydomonas reinhardtii'' and cyanobacteria
Cyanobacteria ( ) are a group of autotrophic gram-negative bacteria that can obtain biological energy via oxygenic photosynthesis. The name "cyanobacteria" () refers to their bluish green (cyan) color, which forms the basis of cyanobacteri ...
, have evolved a second step in the dark reactions in which protons and electrons are reduced to form gas by specialized hydrogenases in the chloroplast
A chloroplast () is a type of membrane-bound organelle, organelle known as a plastid that conducts photosynthesis mostly in plant cell, plant and algae, algal cells. Chloroplasts have a high concentration of chlorophyll pigments which captur ...
.
Efforts have been undertaken to genetically modify cyanobacterial hydrogenases to more efficiently generate gas even in the presence of oxygen. Efforts have also been undertaken with genetically modified alga in a bioreactor.
Relevant to the thermal water-splitting scenario is this simple equation:
:
More than 200 thermochemical cycles can be used for water splitting. Many of these cycles such as the iron oxide cycle, cerium(IV) oxide–cerium(III) oxide cycle, zinc zinc-oxide cycle, sulfur-iodine cycle, copper-chlorine cycle and hybrid sulfur cycle have been evaluated for their commercial potential to produce hydrogen and oxygen from water and heat without using electricity. A number of labs (including in France
France, officially the French Republic, is a country located primarily in Western Europe. Overseas France, Its overseas regions and territories include French Guiana in South America, Saint Pierre and Miquelon in the Atlantic Ocean#North Atlan ...
, Germany
Germany, officially the Federal Republic of Germany, is a country in Central Europe. It lies between the Baltic Sea and the North Sea to the north and the Alps to the south. Its sixteen States of Germany, constituent states have a total popu ...
, Greece
Greece, officially the Hellenic Republic, is a country in Southeast Europe. Located on the southern tip of the Balkan peninsula, it shares land borders with Albania to the northwest, North Macedonia and Bulgaria to the north, and Turkey to th ...
, Japan
Japan is an island country in East Asia. Located in the Pacific Ocean off the northeast coast of the Asia, Asian mainland, it is bordered on the west by the Sea of Japan and extends from the Sea of Okhotsk in the north to the East China Sea ...
, and the United States
The United States of America (USA), also known as the United States (U.S.) or America, is a country primarily located in North America. It is a federal republic of 50 U.S. state, states and a federal capital district, Washington, D.C. The 48 ...
) are developing thermochemical methods to produce hydrogen from solar energy and water.
Natural routes
Biohydrogen
is produced by enzymes called hydrogenases. This process allows the host organism to use fermentation as a source of energy. These same enzymes also can oxidize H2, such that the host organisms can subsist by reducing oxidized substrates using electrons extracted from H2.
The hydrogenase enzyme feature iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
or nickel-iron centers at their active sites. The natural cycle of hydrogen production and consumption by organisms is called the hydrogen cycle.[
Confirming the existence of hydrogenases in the human gut, occurs in human breath. The concentration in the breath of fasting people at rest is typically less than 5 parts per million (ppm) but can be 50 ppm when people with intestinal disorders consume molecules they cannot absorb during diagnostic hydrogen breath tests.
]
Serpentinization
Serpentinization
Serpentinization is a hydration and Metamorphic rock, metamorphic transformation of ferromagnesian minerals, such as olivine and pyroxene, in mafic and ultramafic rock to produce serpentinite. Minerals formed by serpentinization include the Serp ...
is a geological mechanism that produce highly reducing conditions.iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
and steel
Steel is an alloy of iron and carbon that demonstrates improved mechanical properties compared to the pure form of iron. Due to steel's high Young's modulus, elastic modulus, Yield (engineering), yield strength, Fracture, fracture strength a ...
in oxygen-free groundwater
Groundwater is the water present beneath Earth's surface in rock and Pore space in soil, soil pore spaces and in the fractures of stratum, rock formations. About 30 percent of all readily available fresh water in the world is groundwater. A unit ...
and in reducing soil
Soil, also commonly referred to as earth, is a mixture of organic matter, minerals, gases, water, and organisms that together support the life of plants and soil organisms. Some scientific definitions distinguish dirt from ''soil'' by re ...
s below the water table
The water table is the upper surface of the phreatic zone or zone of saturation. The zone of saturation is where the pores and fractures of the ground are saturated with groundwater, which may be fresh, saline, or brackish, depending on the loc ...
.
Laboratory syntheses
is produced in laboratory settings, such as in the small-scale electrolysis of water using metal electrodes and water containing an electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solven ...
, which liberates hydrogen gas at the cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
:aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
, are slow to react with water because they form passivated oxide coatings of oxides. An alloy of aluminium and gallium
Gallium is a chemical element; it has Chemical symbol, symbol Ga and atomic number 31. Discovered by the French chemist Paul-Émile Lecoq de Boisbaudran in 1875,
elemental gallium is a soft, silvery metal at standard temperature and pressure. ...
, however, does react with water. At high pH, aluminium can produce :
Storage
If H2 is to be used as an energy source, its storage is important. It dissolves only poorly in solvents. For example, at room temperature and 0.1 M pascal, ca. 0.05 moles dissolves in one kilogram of diethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
.critical temperature
Critical or Critically may refer to:
*Critical, or critical but stable, medical states
**Critical, or intensive care medicine
*Critical juncture, a discontinuous change studied in the social sciences.
*Critical Software, a company specializing in ...
. In contrast, ammonia and many hydrocarbons can be liquified at room temperature under pressure. For these reasons, hydrogen ''carriers'' - materials that reversibly bind H2 - have attracted much attention. The key question is then the weight percent of H2-equivalents within the carrier material. For example, hydrogen can be reversibly absorbed into many rare earth and transition metalssolubility
In chemistry, solubility is the ability of a chemical substance, substance, the solute, to form a solution (chemistry), solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form su ...
in metals is influenced by local distortions or impurities in the crystal lattice.hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
s such as tetrahydroquinoline, which reversibly release some H2 when heated in the presence of a catalyst:
:
Applications
Petrochemical industry
Large quantities of are used in the "upgrading" of fossil fuels
A fossil fuel is a flammable carbon compound- or hydrocarbon-containing material formed naturally in the Earth's crust from the buried remains of prehistoric organisms (animals, plants or microplanktons), a process that occurs within geologica ...
. Key consumers of include hydrodesulfurization, and hydrocracking. Many of these reactions can be classified as hydrogenolysis, i.e., the cleavage of bonds by hydrogen. Illustrative is the separation of sulfur from liquid fossil fuels:
Hydrogenation
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
, the addition of to various substrates, is done on a large scale. Hydrogenation of produces ammonia by the Haber process:
Fuel
The potential for using hydrogen (H2) as a fuel has been widely discussed. Hydrogen can be used in fuel cells to produce electricity, or burned to generate heat.ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
and methanol and fuel cell technology.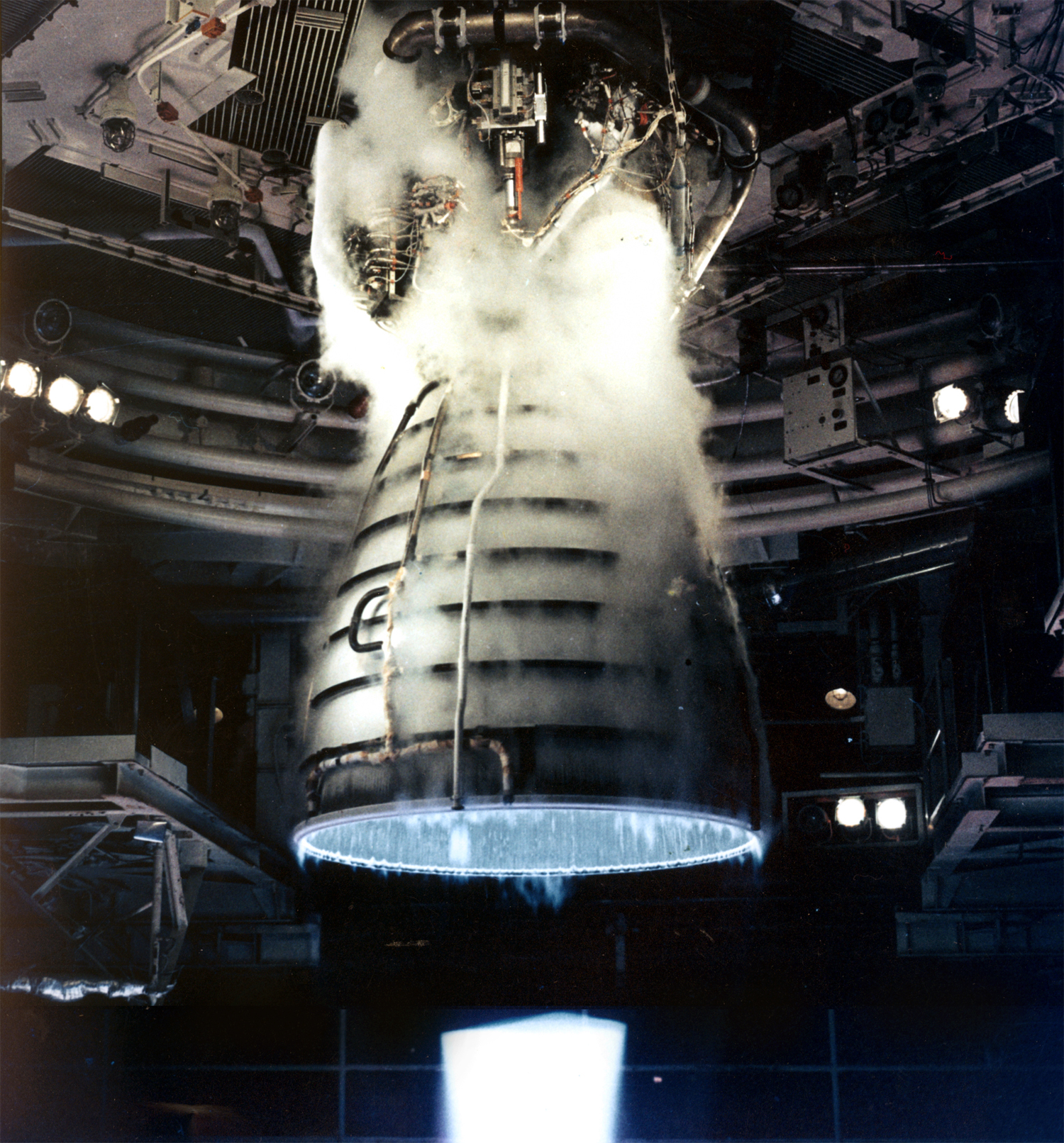 Liquid hydrogen and liquid oxygen together serve as cryogenic propellants in
Liquid hydrogen and liquid oxygen together serve as cryogenic propellants in liquid-propellant rocket
A liquid-propellant rocket or liquid rocket uses a rocket engine burning liquid rocket propellant, liquid propellants. (Alternate approaches use gaseous or Solid-propellant rocket , solid propellants.) Liquids are desirable propellants because th ...
s, as in the Space Shuttle main engines. NASA
The National Aeronautics and Space Administration (NASA ) is an independent agencies of the United States government, independent agency of the federal government of the United States, US federal government responsible for the United States ...
has investigated the use of rocket propellant made from atomic hydrogen, boron or carbon that is frozen into solid molecular hydrogen particles suspended in liquid helium. Upon warming, the mixture vaporizes to allow the atomic species to recombine, heating the mixture to high temperature.
Hydrogen produced when there is a surplus of variable renewable electricity could in principle be stored and later used to generate heat or to re-generate electricity. It can be further transformed into synthetic fuels such as ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
and methanol. Disadvantages of hydrogen fuel include high costs of storage and distribution due to hydrogen's explosivity, its large volume compared to other fuels, and its tendency to make pipes brittle.
Nickel–hydrogen battery
The very long-lived, rechargeable nickel–hydrogen battery developed for satellite power systems uses pressurized gaseous H2. The International Space Station
The International Space Station (ISS) is a large space station that was Assembly of the International Space Station, assembled and is maintained in low Earth orbit by a collaboration of five space agencies and their contractors: NASA (United ...
, Mars Odyssey and the Mars Global Surveyor are equipped with nickel-hydrogen batteries. In the dark part of its orbit, the Hubble Space Telescope
The Hubble Space Telescope (HST or Hubble) is a space telescope that was launched into low Earth orbit in 1990 and remains in operation. It was not the Orbiting Solar Observatory, first space telescope, but it is one of the largest and most ...
is also powered by nickel-hydrogen batteries, which were finally replaced in May 2009, more than 19 years after launch and 13 years beyond their design life.
Semiconductor industry
Hydrogen is employed to saturate broken ("dangling") bonds of amorphous silicon and amorphous carbon that helps stabilizing material properties. Hydrogen, introduced as a unintended side-effect of production, acts as a shallow electron donor leading to n-type conductivity in ZnO, with important uses in transducers and phosphors. Detailed analysis of ZnO and of MgO show evidence of four and six-fold hydrogen multicentre bonds.
The doping behavior of hydrogen varies with the material.
Niche and evolving uses
Other than the uses mentioned above, hydrogen is also used in smaller scales in the following applications:
*Shielding gas: Hydrogen is used as a shielding gas in welding
Welding is a fabrication (metal), fabrication process that joins materials, usually metals or thermoplastics, primarily by using high temperature to melting, melt the parts together and allow them to cool, causing Fusion welding, fusion. Co ...
methods such as atomic hydrogen welding.
*Coolant: Hydrogen is used as a coolant in large power stations generators due to its high thermal conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1.
Heat transfer occurs at a lower rate in materials of low ...
and low density. The first hydrogen-cooled turbogenerator went into service using gaseous hydrogen as a coolant in the rotor and the stator in 1937 at Dayton
Dayton () is a city in Montgomery County, Ohio, United States, and its county seat. It is the List of cities in Ohio, sixth-most populous city in Ohio, with a population of 137,644 at the 2020 United States census, 2020 census. The Dayton metro ...
, Ohio.
*Cryogenic research: Liquid is used in cryogenic research, including superconductivity studies.
*Leak detection: Pure or mixed with nitrogen (sometimes called forming gas), hydrogen is a tracer gas for detection of minute leaks. Applications can be found in the automotive, chemical, power generation, aerospace, and telecommunications industries. Hydrogen is an authorized food additive (E 949) that allows food package leak testing, as well as having anti-oxidizing properties.
*Neutron moderation: Deuterium (hydrogen-2) is used in nuclear fission applications as a moderator to slow neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s.
*Nuclear fusion fuel: Deuterium is used in nuclear fusion
Nuclear fusion is a nuclear reaction, reaction in which two or more atomic nuclei combine to form a larger nuclei, nuclei/neutrons, neutron by-products. The difference in mass between the reactants and products is manifested as either the rele ...
reactions.nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
s, is used in the production of hydrogen bomb
A thermonuclear weapon, fusion weapon or hydrogen bomb (H-bomb) is a second-generation nuclear weapon design. Its greater sophistication affords it vastly greater destructive power than first-generation nuclear bombs, a more compact size, a lo ...
s, as an isotopic label in the biosciences,
Safety and precautions
In hydrogen pipelines and steel storage vessels, hydrogen molecules are prone to react with metals, causing hydrogen embrittlement and leaks in the pipeline or storage vessel.[Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License] Since it is lighter than air, hydrogen does not easily accumulate to form a combustible gas mixture.
See also
* Combined cycle hydrogen power plant
*
*
*
*
*
* (for hydrogen)
*
*
References
Further reading
*
*
*
*
*
External links
Basic Hydrogen Calculations of Quantum Mechanics
at '' The Periodic Table of Videos'' (University of Nottingham)
High temperature hydrogen phase diagram
{{Authority control
Chemical elements
Reactive nonmetals
Diatomic nonmetals
Nuclear fusion fuels
Airship technology
Reducing agents
Refrigerants
Gaseous signaling molecules
E-number additives
 Hydrogen has three naturally occurring isotopes, denoted , and . Other, highly unstable nuclei ( to ) have been synthesized in the laboratory but not observed in nature.
is the most common hydrogen isotope, with an abundance of >99.98%. Because the nucleus of this isotope consists of only a single proton, it is given the descriptive but rarely used formal name ''protium''. It is the only stable isotope with no neutrons; see diproton for a discussion of why others do not exist.
, the other stable hydrogen isotope, is known as deuterium and contains one proton and one
Hydrogen has three naturally occurring isotopes, denoted , and . Other, highly unstable nuclei ( to ) have been synthesized in the laboratory but not observed in nature.
is the most common hydrogen isotope, with an abundance of >99.98%. Because the nucleus of this isotope consists of only a single proton, it is given the descriptive but rarely used formal name ''protium''. It is the only stable isotope with no neutrons; see diproton for a discussion of why others do not exist.
, the other stable hydrogen isotope, is known as deuterium and contains one proton and one  Liquid hydrogen can exist at temperatures below hydrogen's critical point of 33 K. However, for it to be in a fully liquid state at
Liquid hydrogen can exist at temperatures below hydrogen's critical point of 33 K. However, for it to be in a fully liquid state at  Hydrogen compounds with hydrogen in the oxidation state −1 are known as hydrides, which are usually formed between hydrogen and metals. The hydrides can be ionic (aka saline), covalent, nor metallic. With heating, H2 reacts efficiently with the alkali and alkaline earth metals to give the ionic hydrides of the formula MH and MH2, respectively. These salt-like crystalline compounds have high melting points and all react with water to liberate hydrogen. Covalent hydrides are include boranes and polymeric aluminium hydride. Transition metals form metal hydrides via continuous dissolution of hydrogen into the metal. A well known hydride is lithium aluminium hydride, the anion carries hydridic centers firmly attached to the Al(III). Perhaps the most extensive series of hydrides are the boranes, compounds consisting only of boron and hydrogen.
Hydrides can bond to these electropositive elements not only as a terminal
Hydrogen compounds with hydrogen in the oxidation state −1 are known as hydrides, which are usually formed between hydrogen and metals. The hydrides can be ionic (aka saline), covalent, nor metallic. With heating, H2 reacts efficiently with the alkali and alkaline earth metals to give the ionic hydrides of the formula MH and MH2, respectively. These salt-like crystalline compounds have high melting points and all react with water to liberate hydrogen. Covalent hydrides are include boranes and polymeric aluminium hydride. Transition metals form metal hydrides via continuous dissolution of hydrogen into the metal. A well known hydride is lithium aluminium hydride, the anion carries hydridic centers firmly attached to the Al(III). Perhaps the most extensive series of hydrides are the boranes, compounds consisting only of boron and hydrogen.
Hydrides can bond to these electropositive elements not only as a terminal  Hydrogen, as atomic H, is the most abundant
Hydrogen, as atomic H, is the most abundant  Electrolysis of water is a conceptually simple method of producing hydrogen.
:
Commercial electrolyzers use nickel-based catalysts in strongly alkaline solution. Platinum is a better catalyst but is expensive. The hydrogen created through electrolysis using renewable energy is commonly referred to as " green hydrogen".
Electrolysis of water is a conceptually simple method of producing hydrogen.
:
Commercial electrolyzers use nickel-based catalysts in strongly alkaline solution. Platinum is a better catalyst but is expensive. The hydrogen created through electrolysis using renewable energy is commonly referred to as " green hydrogen".
 Liquid hydrogen and liquid oxygen together serve as cryogenic propellants in
Liquid hydrogen and liquid oxygen together serve as cryogenic propellants in