|
Triple Point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at which the sublimation curve, fusion curve and the vaporisation curve meet. For example, the triple point of mercury occurs at a temperature of and a pressure of 0.165 m Pa. In addition to the triple point for solid, liquid, and gas phases, a triple point may involve more than one solid phase, for substances with multiple polymorphs. Helium-4 is a special case that presents a triple point involving two different fluid phases (lambda point). The triple point of water was used to define the kelvin, the base unit of thermodynamic temperature in the International System of Units (SI). The value of the triple point of water was fixed by definition, rather than measured, but that changed with the 2019 redefinition of SI base units. The triple points of s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to formulate a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kelvin
The kelvin, symbol K, is the primary unit of temperature in the International System of Units (SI), used alongside its prefixed forms and the degree Celsius. It is named after the Belfast-born and University of Glasgow-based engineer and physicist William Thomson, 1st Baron Kelvin (1824–1907). The Kelvin scale is an absolute thermodynamic temperature scale, meaning it uses absolute zero as its null (zero) point. Historically, the Kelvin scale was developed by shifting the starting point of the much-older Celsius scale down from the melting point of water to absolute zero, and its increments still closely approximate the historic definition of a degree Celsius, but since 2019 the scale has been defined by fixing the Boltzmann constant to be exactly . Hence, one kelvin is equal to a change in the thermodynamic temperature that results in a change of thermal energy by . The temperature in degree Celsius is now defined as the temperature in kelvins minus 273.15, meaning t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquefaction
In materials science, liquefaction is a process that generates a liquid from a solid or a gas or that generates a non-liquid phase which behaves in accordance with fluid dynamics. It occurs both naturally and artificially. As an example of the latter, a "major commercial application of liquefaction is the liquefaction of air to allow separation of the constituents, such as oxygen, nitrogen, and the noble gases." Another is the conversion of solid coal into a liquid form usable as a substitute for liquid fuels. Geology In geology, soil liquefaction refers to the process by which water-saturated, unconsolidated sediments are transformed into a substance that acts like a liquid, often in an earthquake. Soil liquefaction was blamed for building collapses in the city of Palu, Indonesia in October 2018. In a related phenomenon, liquefaction of bulk materials in cargo ships may cause a dangerous shift in the load. Physics and chemistry In physics and chemistry, the phase transiti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium. Overview Common components of a phase diagram are ''lines of equilibrium'' or ''phase boundaries'', which refer to lines that mark conditions under which multiple phases can coexist at equilibrium. Phase transitions occur along lines of equilibrium. Metastable phases are not shown in phase diagrams as, despite their common occurrence, they are not equilibrium phases. Triple points are points on phase diagrams where lines of equilibrium intersect. Triple points mark conditions at which three different phases can coexist. For example, the water phase diagram has a triple point corresponding to the single temperature and pressure at which solid, liquid, and gaseous water can coexist in a stabl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sublimation (phase Transition)
Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point in its phase diagram, which corresponds to the lowest pressure at which the substance can exist as a liquid. The reverse process of sublimation is deposition or desublimation, in which a substance passes directly from a gas to a solid phase. Sublimation has also been used as a generic term to describe a solid-to-gas transition (sublimation) followed by a gas-to-solid transition ( deposition). While vaporization from liquid to gas occurs as evaporation from the surface if it occurs below the boiling point of the liquid, and as boiling with formation of bubbles in the interior of the liquid if it occurs at the boiling point, there is no such distinction for the solid-to-gas transition which always occurs as sublimation from the surface. At ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Outer Space
Outer space, commonly shortened to space, is the expanse that exists beyond Earth and its atmosphere and between celestial bodies. Outer space is not completely empty—it is a near-perfect vacuum containing a low density of particles, predominantly a plasma of hydrogen and helium, as well as electromagnetic radiation, magnetic fields, neutrinos, dust, and cosmic rays. The baseline temperature of outer space, as set by the background radiation from the Big Bang, is . The plasma between galaxies is thought to account for about half of the baryonic (ordinary) matter in the universe, having a number density of less than one hydrogen atom per cubic metre and a kinetic temperature of millions of kelvins. Local concentrations of matter have condensed into stars and galaxies. Studies indicate that 90% of the mass in most galaxies is in an unknown form, called dark matter, which interacts with other matter through gravitational but not electromagnetic forces. Observations s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
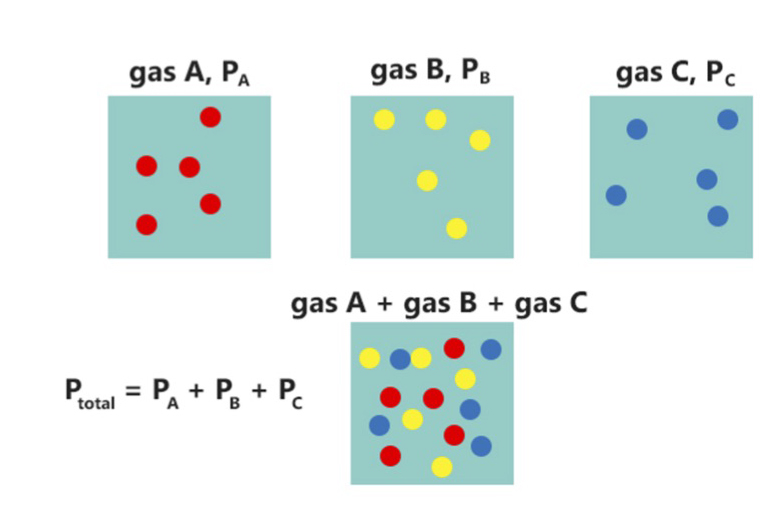
Partial Pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture (Dalton's Law). The partial pressure of a gas is a measure of thermodynamic activity of the gas's molecules. Gases dissolve, diffuse, and react according to their partial pressures but not according to their concentrations in gas mixtures or liquids. This general property of gases is also true in chemical reactions of gases in biology. For example, the necessary amount of oxygen for human respiration, and the amount that is toxic, is set by the partial pressure of oxygen alone. This is true across a very wide range of different concentrations of oxygen present in various inhaled breathing gases or dissolved in blood; consequently, mixture ratios, like that of breathab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Vapor
(99.9839 °C) , - , Boiling point , , - , specific gas constant , 461.5 J/( kg·K) , - , Heat of vaporization , 2.27 MJ/kg , - , Heat capacity , 1.864 kJ/(kg·K) Water vapor, water vapour or aqueous vapor is the gaseous phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Water vapor is transparent, like most constituents of the atmosphere. Under typical atmospheric conditions, water vapor is continuously generated by evaporation and removed by condensation. It is less dense than most of the other constituents of air and triggers convection currents that can lead to clouds. Being a component of Earth's hydrosphere and hydrologic cycle, it is particularly abundant in Earth's atmosphere, where it acts as a greenhouse gas and warming feedback, contributing more to total greenhouse effect than non-condensable gases such as carbon dioxide an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
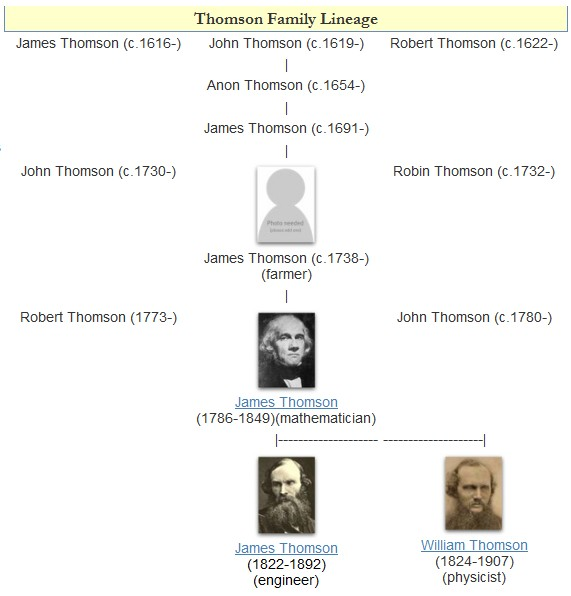
William Thomson, 1st Baron Kelvin
William Thomson, 1st Baron Kelvin, (26 June 182417 December 1907) was a British mathematician, mathematical physicist and engineer born in Belfast. Professor of Natural Philosophy at the University of Glasgow for 53 years, he did important work in the mathematical analysis of electricity and formulation of the first and second laws of thermodynamics, and did much to unify the emerging discipline of physics in its contemporary form. He received the Royal Society's Copley Medal in 1883, was its president 1890–1895, and in 1892 was the first British scientist to be elevated to the House of Lords. Absolute temperatures are stated in units of kelvin in his honour. While the existence of a coldest possible temperature ( absolute zero) was known prior to his work, Kelvin is known for determining its correct value as approximately −273.15 degrees Celsius or −459.67 degrees Fahrenheit. The Joule–Thomson effect is also named in his honour. He worked closely with mathematics ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
James Thomson (engineer)
James Thomson FRS FRSE LLD (16 February 1822 – 8 May 1892) was a British engineer and physicist, born in Belfast, and older brother of William Thomson (Lord Kelvin). Biography Born in Belfast, much of his youth was spent in Glasgow. His father James was professor of mathematics at the University of Glasgow from 1832 onward and his younger brother William was to become Baron Kelvin. James attended Glasgow University from a young age and graduated (1839) with high honours in his late teens. After graduation, he served brief apprenticeships with practical engineers in several domains; and then gave a considerable amount of his time to theoretical and mathematical engineering studies, often in collaboration with his brother, during his twenties in Glasgow. In his late twenties he entered into private practice as a professional engineer with special expertise in water transport. In his early thirties, in 1855, he was appointed professor of civil engineering at Queen's Universi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ITS-90
The International Temperature Scale of 1990 (ITS-90) is an equipment calibration standard specified by the International Committee of Weights and Measures (CIPM) for making measurements on the Kelvin and Celsius temperature scales. It is an approximation of thermodynamic temperature that facilitates the comparability and compatibility of temperature measurements internationally. It defines fourteen calibration points ranging from to ( to ) and is subdivided into multiple temperature ranges which overlap in some instances. ITS-90 is the most recent of a series of International Temperature Scales adopted by the CIPM since 1927. Adopted at the 1989 General Conference on Weights and Measures, it supersedes the International Practical Temperature Scale of 1968 (amended edition of 1975) and the 1976 "Provisional 0.5 K to 30 K Temperature Scale". The CCT has also published several online guidebooks to aid realisations of the ITS-90. The lowest temperature covered by the ITS-90 is 0.65 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |