engineering
Engineering is the use of scientific method, scientific principles to design and build machines, structures, and other items, including bridges, tunnels, roads, vehicles, and buildings. The discipline of engineering encompasses a broad rang ...
, mineralogy
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical) properties of minerals and mineralized artifacts. Specific studies within mineralogy include the proces ...
, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium.
Overview
Common components of a phase diagram are ''lines of equilibrium'' or ''phase boundaries'', which refer to lines that mark conditions under which multiple phases can coexist at equilibrium. Phase transitions occur along lines of equilibrium. Metastable phases are not shown in phase diagrams as, despite their common occurrence, they are not equilibrium phases. Triple points are points on phase diagrams where lines of equilibrium intersect. Triple points mark conditions at which three different phases can coexist. For example, the water phase diagram has a triple point corresponding to the single temperature and pressure at which solid, liquid, and gaseous water can coexist in a stable equilibrium ( and a partial vapor pressure of ). The solidus is the temperature below which the substance is stable in the solid state. Theliquidus
The liquidus temperature, TL or Tliq, specifies the temperature above which a material is completely liquid, and the maximum temperature at which crystals can co-exist with the melt in thermodynamic equilibrium. It is mostly used for impure subst ...
is the temperature above which the substance is stable in a liquid state. There may be a gap between the solidus and liquidus; within the gap, the substance consists of a mixture of crystals and liquid (like a "slurry
A slurry is a mixture of denser solids suspended in liquid, usually water. The most common use of slurry is as a means of transporting solids or separating minerals, the liquid being a carrier that is pumped on a device such as a centrifugal pu ...
").
Working fluids are often categorized on the basis of the shape of their phase diagram.
Types
2-dimensional diagrams
Pressure vs temperature
The simplest phase diagrams are pressure–temperature diagrams of a single simple substance, such as water. The axes correspond to thepressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country a ...
and temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied on ...
. The phase diagram shows, in pressure–temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, a ...
, and gas.
The curves on the phase diagram show the points where the free energy (and other derived properties) becomes non-analytic: their derivatives with respect to the coordinates (temperature and pressure in this example) change discontinuously (abruptly). For example, the heat capacity of a container filled with ice will change abruptly as the container is heated past the melting point. The open spaces, where the free energy is analytic, correspond to single phase regions. Single phase regions are separated by lines of non-analytical behavior, where phase transitions occur, which are called phase boundaries.
In the diagram on the right, the phase boundary between liquid and gas does not continue indefinitely. Instead, it terminates at a point on the phase diagram called the critical point. This reflects the fact that, at extremely high temperatures and pressures, the liquid and gaseous phases become indistinguishable, in what is known as a supercritical fluid
A supercritical fluid (SCF) is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist, but below the pressure required to compress it into a solid. It can effuse through porous so ...
. In water, the critical point occurs at around ''T''c = , ''p''c = and ''ρ''c = 356 kg/m3.
The existence of the liquid–gas critical point reveals a slight ambiguity in labelling the single phase regions. When going from the liquid to the gaseous phase, one usually crosses the phase boundary, but it is possible to choose a path that never crosses the boundary by going to the right of the critical point. Thus, the liquid and gaseous phases can blend continuously into each other. The solid–liquid phase boundary can only end in a critical point if the solid and liquid phases have the same symmetry group
In group theory, the symmetry group of a geometric object is the group of all transformations under which the object is invariant, endowed with the group operation of composition. Such a transformation is an invertible mapping of the ambient ...
.
For most substances, the solid–liquid phase boundary (or fusion curve) in the phase diagram has a positive slope
In mathematics, the slope or gradient of a line is a number that describes both the ''direction'' and the ''steepness'' of the line. Slope is often denoted by the letter ''m''; there is no clear answer to the question why the letter ''m'' is used ...
so that the melting point increases with pressure. This is true whenever the solid phase is denser than the liquid phase. The greater the pressure on a given substance, the closer together the molecules of the substance are brought to each other, which increases the effect of the substance's intermolecular forces. Thus, the substance requires a higher temperature for its molecules to have enough energy to break out of the fixed pattern of the solid phase and enter the liquid phase. A similar concept applies to liquid–gas phase changes.
Water is an exception which has a solid-liquid boundary with negative slope so that the melting point decreases with pressure. This occurs because ice (solid water) is less dense than liquid water, as shown by the fact that ice floats on water. At a molecular level, ice is less dense because it has a more extensive network of hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
ing which requires a greater separation of water molecules. Other exceptions include antimony and bismuth.
At very high pressures above 50 GPa (500 000 atm), liquid nitrogen
Liquid nitrogen—LN2—is nitrogen in a liquid state at low temperature. Liquid nitrogen has a boiling point of about . It is produced industrially by fractional distillation of liquid air. It is a colorless, low viscosity liquid that is wide ...
undergoes a liquid-liquid phase transition to a polymeric form and becomes denser than solid nitrogen at the same pressure. Under these conditions therefore, solid nitrogen also floats in its liquid.
The value of the slope d''P''/d''T'' is given by the Clausius–Clapeyron equation for fusion (melting)
:
where Δ''H''fus is the heat of fusion which is always positive, and Δ''V''fus is the volume change for fusion. For most substances Δ''V''fus is positive so that the slope is positive. However for water and other exceptions, Δ''V''fus is negative so that the slope is negative.
Other thermodynamic properties
In addition to temperature and pressure, other thermodynamic properties may be graphed in phase diagrams. Examples of such thermodynamic properties include specific volume, specific enthalpy, or specific entropy. For example, single-component graphs of temperature vs. specific entropy (''T'' vs. ''s'') for water/steam
Steam is a substance containing water in the gas phase, and sometimes also an aerosol of liquid water droplets, or air. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization ...
or for a refrigerant
A refrigerant is a working fluid used in the heat pump and refrigeration cycle, refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Ref ...
are commonly used to illustrate thermodynamic cycles such as a Carnot cycle, Rankine cycle, or vapor-compression refrigeration cycle.
Any two thermodynamic quantities may be shown on the horizontal and vertical axes of a two-dimensional diagram. Additional thermodynamic quantities may each be illustrated in increments as a series of lines – curved, straight, or a combination of curved and straight. Each of these iso-lines represents the thermodynamic quantity at a certain constant value.
3-dimensional diagrams
 An
An orthographic projection
Orthographic projection (also orthogonal projection and analemma) is a means of representing Three-dimensional space, three-dimensional objects in Two-dimensional space, two dimensions. Orthographic projection is a form of parallel projection in ...
of the 3D ''p''–''v''–''T'' graph showing pressure and temperature as the vertical and horizontal axes collapses the 3D plot into the standard 2D pressure–temperature diagram. When this is done, the solid–vapor, solid–liquid, and liquid–vapor surfaces collapse into three corresponding curved lines meeting at the triple point, which is the collapsed orthographic projection of the triple line.
Binary mixtures
Other much more complex types of phase diagrams can be constructed, particularly when more than one pure component is present. In that case, concentration becomes an important variable. Phase diagrams with more than two dimensions can be constructed that show the effect of more than two variables on the phase of a substance. Phase diagrams can use other variables in addition to or in place of temperature, pressure and composition, for example the strength of an applied electrical or magnetic field, and they can also involve substances that take on more than just three states of matter. One type of phase diagram plots temperature against the relative concentrations of two substances in a binarymixture
In chemistry, a mixture is a material made up of two or more different chemical substances which are not chemically bonded. A mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the ...
called a ''binary phase diagram'', as shown at right. Such a mixture can be either a solid solution, eutectic or peritectic, among others. These two types of mixtures result in very different graphs. Another type of binary phase diagram is a ''boiling-point diagram'' for a mixture of two components, i. e. chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one ele ...
s. For two particular volatile components at a certain pressure such as atmospheric pressure
Atmospheric pressure, also known as barometric pressure (after the barometer), is the pressure within the atmosphere of Earth. The standard atmosphere (symbol: atm) is a unit of pressure defined as , which is equivalent to 1013.25 millibar ...
, a boiling-point diagram shows what vapor (gas) compositions are in equilibrium with given liquid compositions depending on temperature. In a typical binary boiling-point diagram, temperature is plotted on a vertical axis and mixture composition on a horizontal axis.
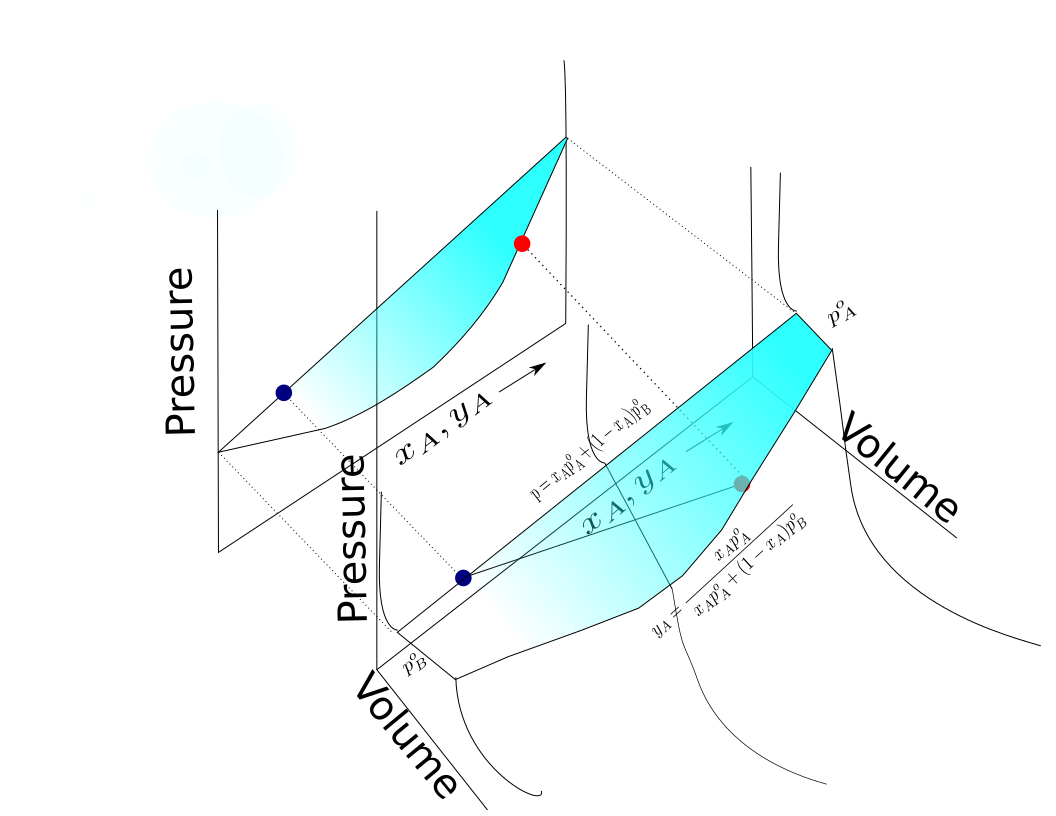 A two component diagram with components A and B in an "ideal" solution is shown. The construction of a liquid vapor phase diagram assumes an ideal liquid solution obeying Raoult's law and an ideal gas mixture obeying Dalton's law of partial pressure. A tie line from the liquid to the gas at constant pressure would indicate the two compositions of the liquid and gas respectively.
A simple example diagram with hypothetical components 1 and 2 in a non- azeotropic mixture is shown at right. The fact that there are two separate curved lines joining the boiling points of the pure components means that the vapor composition is usually not the same as the liquid composition the vapor is in equilibrium with. See Vapor–liquid equilibrium for more information.
In addition to the above-mentioned types of phase diagrams, there are many other possible combinations. Some of the major features of phase diagrams include congruent points, where a solid phase transforms directly into a liquid. There is also the
A two component diagram with components A and B in an "ideal" solution is shown. The construction of a liquid vapor phase diagram assumes an ideal liquid solution obeying Raoult's law and an ideal gas mixture obeying Dalton's law of partial pressure. A tie line from the liquid to the gas at constant pressure would indicate the two compositions of the liquid and gas respectively.
A simple example diagram with hypothetical components 1 and 2 in a non- azeotropic mixture is shown at right. The fact that there are two separate curved lines joining the boiling points of the pure components means that the vapor composition is usually not the same as the liquid composition the vapor is in equilibrium with. See Vapor–liquid equilibrium for more information.
In addition to the above-mentioned types of phase diagrams, there are many other possible combinations. Some of the major features of phase diagrams include congruent points, where a solid phase transforms directly into a liquid. There is also the peritectoid
A eutectic system or eutectic mixture ( ) is a homogeneous mixture that has a melting point lower than those of the constituents. The lowest possible melting point over all of the mixing ratios of the constituents is called the ''eutectic tempe ...
, a point where two solid phases combine into one solid phase during cooling. The inverse of this, when one solid phase transforms into two solid phases during cooling, is called the eutectoid.
A complex phase diagram of great technological importance is that of the iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
–carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes ...
system for less than 7% carbon (see steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant ...
).
The x-axis of such a diagram represents the concentration variable of the mixture. As the mixtures are typically far from dilute and their density as a function of temperature is usually unknown, the preferred concentration measure is mole fraction. A volume-based measure like molarity would be inadvisable.
Ternary phase diagrams
A system with three components is called a ternary system. At constant pressure the maximum number of independent variables is three – the temperature and two concentration values. For a representation of ternary equilibria a three-dimensional phase diagram is required. Often such a diagram is drawn with the composition as a horizontal plane and the temperature on an axis perpendicular to this plane. To represent composition in a ternary system an equilateral triangle is used, called Gibbs triangle (see also Ternary plot).Crystals
Polymorphic and polyamorphic substances have multiplecrystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macr ...
or amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal.
Etymology
The term comes from the Greek language ...
phases, which can be graphed in a similar fashion to solid, liquid, and gas phases.
Mesophases
Some organic materials pass through intermediate states between solid and liquid; these states are called mesophases. Attention has been directed to mesophases because they enable display devices and have become commercially important through the so-called liquid-crystal technology. Phase diagrams are used to describe the occurrence of mesophases.See also
* CALPHAD (method) * Computational thermodynamics *Congruent melting
Congruent melting occurs during melting of a compound when the composition of the liquid that forms is the same as the composition of the solid. It can be contrasted with incongruent melting. This generally happens in two- component systems. To tak ...
and incongruent melting
Incongruent melting occurs when a solid substance does not melt uniformly, so that the chemical composition of the resulting liquid is not the same as that of the original solid. During incongruent melting a new solid of different composition forms ...
* Gibbs phase rule
* Glass databases Glass databases are a collection of glass compositions, glass properties, glass models, associated trademark names, patents etc. These data were collected from publications in scientific papers and patents, from personal communication with scien ...
* Hamiltonian mechanics
* Phase separation
* Saturation dome A saturation dome is a graphical representation of the combination of vapor and gas that is used in thermodynamics. It can be used to find either the pressure or the specific volume as long as one already has at least one of these properties.
Descr ...
* Schreinemaker's analysis Schreinemaker's Analysis is the use of Schreinemaker's Rules to create a phase diagram.
After applying Schreinemaker's Rules and creating a phase diagram, the resulting geometric figure will be thermodynamically accurate, although the axes will b ...
References
External links
Iron-Iron Carbide Phase Diagram Example
DoITPoMS Phase Diagram Library
DoITPoMS Teaching and Learning Package – "Phase Diagrams and Solidification"
Phase Diagrams: The Beginning of Wisdom – Open Access Journal Article
Binodal curves, tie-lines, lever rule and invariant points – How to read phase diagrams
(Video by SciFox on TIB AV-Portal)
The Alloy Phase Diagram International Commission (APDIC)
{{Authority control Diagram Equilibrium chemistry Materials science Metallurgy Charts Diagrams Gases Chemical engineering thermodynamics