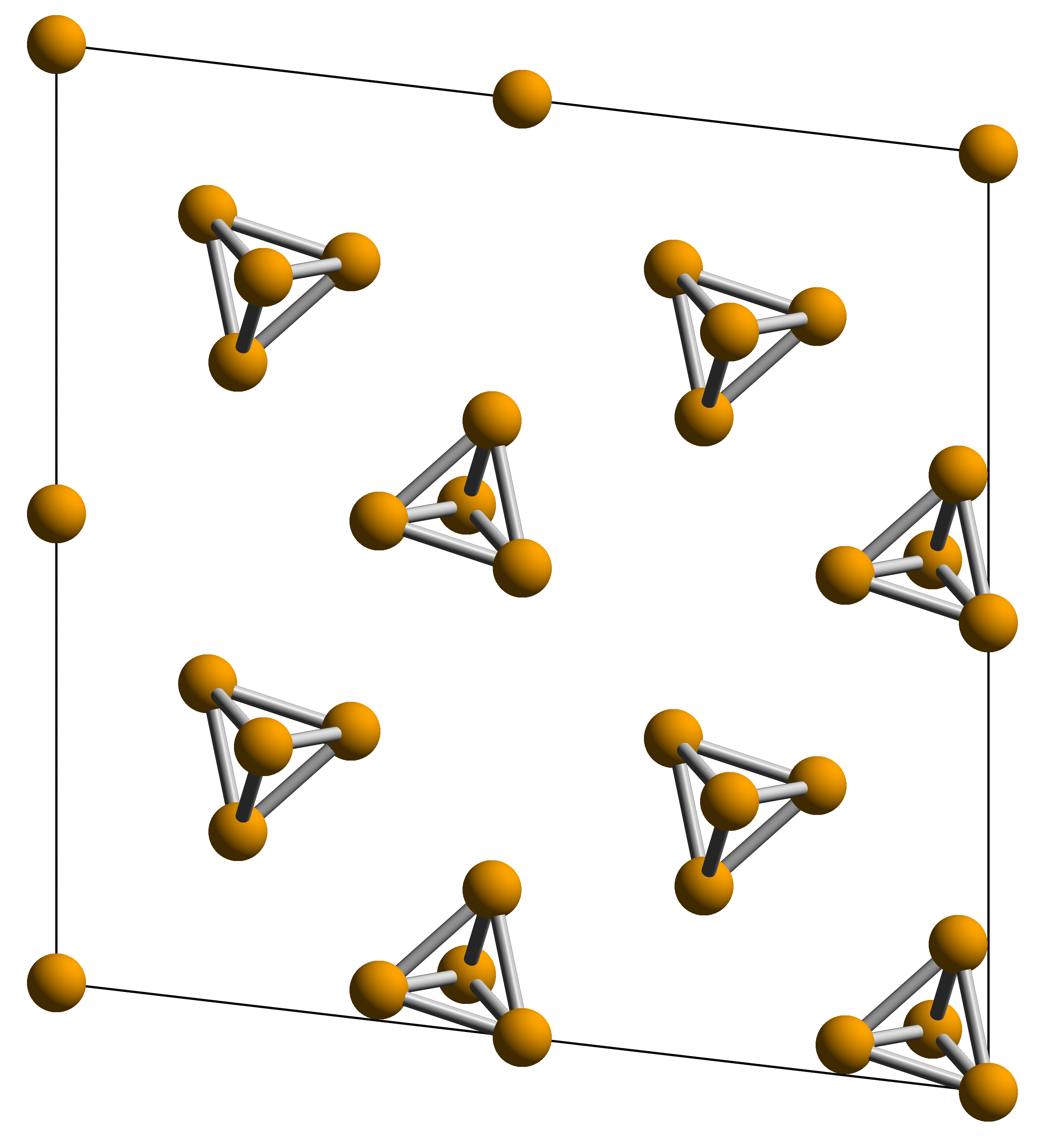|
Phase (matter)
In the physical sciences, a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is a different material, in its own separate phase. (See .) More precisely, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. The term ''phase'' is sometimes used as a synonym for state of matter, but there can be several immiscible phases of the same state of matter (as where oil and water separate into distinct phases, both in the liquid state). Types of phases Distinct phases may be described as different states of matter such as gas, liquid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Outline Of Physical Science
Physical science is a branch of natural science that studies abiotic component, non-living systems, in contrast to life science. It in turn has many branches, each referred to as a "physical science", together is called the "physical sciences". Definition Physical science can be described as all of the following: * A branch of outline of science, science (a systematic enterprise that builds and organizes Outline of knowledge, knowledge in the form of testable explanations and predictions about the universe)."... modern science is a discovery as well as an invention. It was a discovery that nature generally acts regularly enough to be described by laws and even by mathematics; and required invention to devise the techniques, abstractions, apparatus, and organization for exhibiting the regularities and securing their law-like descriptions." —p.vii, J. L. Heilbron, (2003, editor-in-chief). ''The Oxford Companion to the History of Modern Science''. New York: Oxford ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Multiphasic Liquid
A multiphasic liquid is a mixture consisting of more than two immiscible liquid phases. Biphasic mixtures consisting of two immiscible phases are very common and usually consist of an organic solvent and an aqueous phase ("oil and water"). Multiphasic liquids can be used for selective liquid–liquid extractions or for decorative purposes, e.g. in cosmetics. While it is possible to get multilayered phases by layering nonpolar and aqueous phases of decreasing densities on top of each other, these phases will not separate after mixing like true multiphasic liquids. Compositions The following types of multiphasic liquids exist: Triphasic systems * Nonpolar solvent / aqueous biphasic mixture *: ''e.g. using hexane, heptane, cyclohexane, or mineral oil as the nonpolar solvent'' ** Nonpolar solvent / polar solvent / salt / water **: ''e.g. 100 ml mineral oil, 100 ml isopropanol, 75 ml water, 35 g calcium chloride'' ** Nonpolar solvent / water-soluble polymer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...-insoluble liquid with the odor associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) attached to a phenyl group by a single bond. As such, its systematic IUPAC nomenclature of organic chemistry, IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent. As the solvent in some types of paint thinner, permanent markers, contact cement and certain types of glue, toluene is sometimes used as a recreational inhalant and has the potential of causin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes: as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odorless, colorless, flammable, viscous liquid. It has a sweet taste but is toxic in high concentrations. This molecule has been observed in outer space. Production Industrial routes Ethylene glycol is produced from ethylene (ethene), via the intermediate ethylene oxide. Ethylene oxide reacts with water to produce ethylene glycol according to the chemical equation : This reaction can be catalyzed by either acids or bases or can occur at neutral pH under elevated temperatures. The highest yields of ethylene glycol occur at acidic or neutral pH with a large excess of water. Under these conditions, ethylene glycol yields of 90% can be achieved. The major byproducts are the oligomers diethylene glycol, triethylene glycol, and tetra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organofluorine Chemistry
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from Lipophobicity, oil and hydrophobe, water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents. The carbon–fluorine bond Fluorine has several distinctive differences from all other substituents encountered in organic molecules. As a result, the physical and chemical properties of organofluorines can be distinctive in comparison to other organohalogens. # The carbon–fluorine bond is one of the strongest in organic chemistry (an average bond energy around 480 kJ/mol). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluorocarbon
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics. Nomenclature Perfluorocarbons or PFCs, are organofluorine compounds with the formula CxFy, meaning they contain only carbon and fluorine. The terminology is not strictly followed and many fluorine-containing organic compounds are also called fluorocarbons. Compounds with the prefix perfluoro- are hydrocarbons, including those with heteroatoms, wherein all C-H bonds have been replaced by C-F bonds. Fluorocarbons includes perfluoroalkanes, fluoroalkenes, fluoroalkynes, and perfluoroaromatic compounds. Perfluoroalkanes Chemical properties Perfluoroalkanes are very stable because of the strength of the carbon–fluorine bond, one of the strongest in organic chemistry. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wiley-VCH
Wiley-VCH is a German publisher owned by John Wiley & Sons. It was founded in 1921 as Verlag Chemie (meaning "Chemistry Press": VCH stands for ''Verlag Chemie'') by two German learned societies A learned society ( ; also scholarly, intellectual, or academic society) is an organization that exists to promote an academic discipline, profession, or a group of related disciplines such as the arts and sciences. Membership may be open to al .... Later, it was merged into the German Chemical Society (GDCh). In 1991, VCH acquired Akademie Verlag. It has been owned by John Wiley & Sons since 1996. The humanities section of Akademie Verlag and the Akademie brand were sold in 1997 to R. Oldenbourg Verlag, while VCH retained the natural sciences catalog. References External links * Wiley (publisher) Publishing companies of Germany Publishing companies established in 1921 Weinheim German companies established in 1921 {{publish-company-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury (element)
Mercury is a chemical element; it has Symbol (chemistry), symbol Hg and atomic number 80. It is commonly known as quicksilver. A Heavy metal element, heavy, silvery d-block element, mercury is the only metallic element that is known to be liquid at standard temperature and pressure; the only other element that is liquid under these conditions is the halogen bromine, though metals such as caesium, gallium, and rubidium melt just above room temperature. Mercury occurs in deposits throughout the world mostly as cinnabar (mercuric sulfide). The red pigment vermilion is obtained by Mill (grinding), grinding natural cinnabar or synthetic mercuric sulfide. Exposure to mercury and mercury-containing organic compounds is toxic to the nervous system, immune system and kidneys of humans and other animals; mercury poisoning can result from exposure to water-soluble forms of mercury (such as mercuric chloride or methylmercury) either directly or through mechanisms of biomagnification. Mercu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium
Gallium is a chemical element; it has Chemical symbol, symbol Ga and atomic number 31. Discovered by the French chemist Paul-Émile Lecoq de Boisbaudran in 1875, elemental gallium is a soft, silvery metal at standard temperature and pressure. In its liquid state, it becomes silvery white. If enough force is applied, solid gallium may fracture conchoidal fracture, conchoidally. Since its discovery in 1875, gallium has widely been used to make alloys with low melting points. It is also used in semiconductors, as a dopant in semiconductor substrates. The melting point of gallium, , is used as a temperature reference point. Gallium alloys are used in thermometers as a non-toxic and environmentally friendly alternative to Mercury (element), mercury, and can withstand higher temperatures than mercury. A melting point of , well below the freezing point of water, is claimed for the alloy galinstan (62–95% gallium, 5–22% indium, and 0–16% tin by weight), but that may be t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
White Phosphorus
White phosphorus, yellow phosphorus, or simply tetraphosphorus (P4) is an allotrope of phosphorus. It is a translucent waxy solid that quickly yellows in light (due to its photochemical conversion into red phosphorus), and impure white phosphorus is for this reason called yellow phosphorus. White phosphorus is the first allotrope of phosphorus, and in fact the first elementary substance to be discovered that was not known since ancient times. It glows greenish in the dark (when exposed to oxygen) and is highly flammable and pyrophoric (self-igniting) upon contact with air. It is toxic, causing severe liver damage on ingestion and phossy jaw from chronic ingestion or inhalation. The odour of combustion of this form has a characteristic garlic odor, and samples are commonly coated with white " diphosphorus pentoxide", which consists of tetrahedra with oxygen inserted between the phosphorus atoms and at their vertices. White phosphorus is only slightly soluble in water and can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluoro(dimethylcyclohexane)
A perfluorinated compound (PFC) or perfluoro compound is an organofluorine compound that lacks C-H bonds. Many perfluorinated compounds have properties that are quite different from their C-H containing analogues. Common functional groups in PFCs are OH, CO2H, chlorine, O, and SO3H. Electrofluorination is the predominant method for PFC production. Due to their chemical stability, some of these perfluorinated compounds bioaccumulate. Applications One class of perfluorinated compounds, the fluorosurfactants, are widely used in the production of teflon (PTFE) and related fluorinated polymers. They also have been used to confer hydrophobicity and stain-resistance to fabrics. They are components of fire-fighting foam. Fluorosurfactants (PFAS) reduce surface tension by concentrating at the liquid-air interface due to the ''lipo''phobicity of polyfluorocarbons. Chlorofluorocarbons are also perfluorinated compounds, many of which were formerly used as refrigerants (Freon) until th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans. Relative to benzene, aniline is "electron-rich". It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone to oxidation: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening to yellow or red, due to the formation of strongly colored, oxidized impurities. Ani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |