|
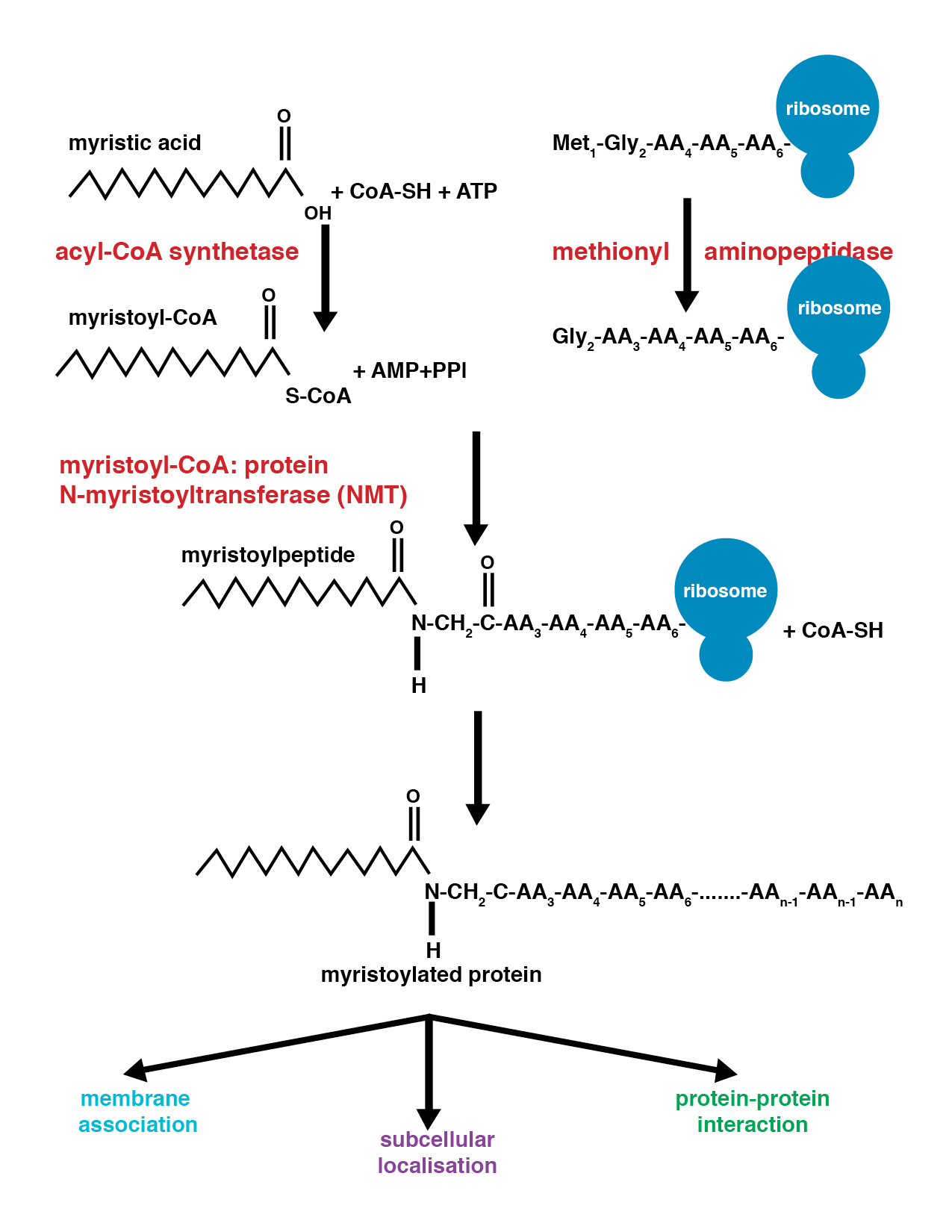
Myristoylation
Myristoylation is a lipidation modification where a myristoyl group, derived from myristic acid, is covalently attached by an amide bond to the alpha-amino group of an N-terminal glycine residue. Myristic acid is a 14-carbon saturated fatty acid (14:0) with the systematic name of ''n''-Tetradecanoic acid. This modification can be added either co-translationally or post-translationally. N-myristoyltransferase (NMT) catalyzes the myristic acid addition reaction in the cytoplasm of cells. This lipidation event is the most found type of fatty acylation and is common among many organisms including animals, plants, fungi, protozoans and viruses. Myristoylation allows for weak protein–protein and protein–lipid interactions and plays an essential role in membrane targeting, protein–protein interactions and functions widely in a variety of signal transduction pathways. Discovery In 1982, Koiti Titani's lab identified an "N-terminal blocking group" on the catalytic subunit of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-myristoyltransferase 1
Glycylpeptide N-tetradecanoyltransferase 1 also known as myristoyl-CoA:protein N-myristoyltransferase 1 (NMT-1) is an enzyme that in humans is encoded by the ''NMT1'' gene. It belongs to the protein N-terminal methyltransferase and glycylpeptide N-tetradecanoyltransferase family of enzymes. References Further reading * * * * * * * * * * * * * * * * * * * See also * Myristoylation Myristoylation is a lipidation modification where a myristoyl group, derived from myristic acid, is covalently attached by an amide bond to the alpha-amino group of an N-terminus, N-terminal glycine residue. Myristic acid is a 14-carbon saturat ... * N-myristoyltransferase 2 External links * EC 2.3.1 Human proteins {{gene-17-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Posttranslational Modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signaling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini. They can extend the chemical repertoire of the 20 standard amino acids by modifying an existing functional group or introducing a new one such as phosphate. Phosphorylation is a highly effective mechanism for regulating the activity of enzymes and is the most common post-translational modification. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a process called glycosyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NMT2
Glycylpeptide N-tetradecanoyltransferase 2 known also as N-myristoyltransferase, is an enzyme (EC: 2.3.1.97) that in humans is encoded by the ''NMT2'' gene. Function N-myristoyltransferase (NMT) catalyzes the reaction of N-terminal myristoylation of many signaling proteins. It transfers myristic acid from myristoyl coenzyme A to the amino group of a protein's N-terminal glycine residue. Biochemical evidence indicates the presence of several distinct NMTs, varying in apparent molecular weight and /or subcellular distribution. The 496-amino acid of human NMT2 protein shares 77% and 96% sequence identity with human NMT1 and mouse Nmt2 comprise two distinct families of N-myristoyltransferases. Interactions NMT2 has been shown to interact with: * caspase 3 * MARCKS See also * N-myristoyltransferase 1 Glycylpeptide N-tetradecanoyltransferase 1 also known as myristoyl-CoA:protein N-myristoyltransferase 1 (NMT-1) is an enzyme that in humans is encoded by the ''NMT1'' gene. It b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Post-translational Modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signaling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini. They can extend the chemical repertoire of the 20 standard amino acids by modifying an existing functional group or introducing a new one such as phosphate. Phosphorylation is a highly effective mechanism for regulating the activity of enzymes and is the most common post-translational modification. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a process calle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycylpeptide N-tetradecanoyltransferase
In enzymology, a glycylpeptide N-tetradecanoyltransferase () is an enzyme that catalyzes the chemical reaction :tetradecanoyl-CoA + glycylpeptide \rightleftharpoons CoA + N-tetradecanoylglycylpeptide Thus, the two substrates of this enzyme are tetradecanoyl-CoA and glycylpeptide, whereas its two products are CoA and N-tetradecanoylglycylpeptide. It participates in the N-Myristoylation of proteins, and in vertebrates there are two isoenzymes NMT1 and NMT2. Besides tetradecanoyl-CoA, this enzyme is also capable of using modified versions of this substrate. In human retina, an even wider range of fatty acids, including 14:1 n–9, 14:2n–6, and 12:0, are accepted by the enzyme and grafted onto guanylate cyclase activators. This is mainly a result of a special set of fatty-acid-CoA substrates available in the retina. Nomenclature This enzyme belongs to the family of transferases, specifically those N-acyltransferases transferring groups other than aminoacyl groups (cd0430 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myristic Acid
Myristic acid (IUPAC name: tetradecanoic acid) is a common saturated fatty acid with the molecular formula CH3(CH2)12COOH. Its salts and esters are commonly referred to as myristates or tetradecanoates. It is named after the binomial name for nutmeg ('' Myristica fragrans''), from which it was first isolated in 1841 by Lyon Playfair. Occurrence Nutmeg butter has 75% trimyristin, the triglyceride of myristic acid. Besides nutmeg, myristic acid is found in palm kernel oil, coconut oil, butterfat, 8–14% of bovine milk, and 8.6% of breast milk as well as being a minor component of many other animal fats. It is found in spermaceti, the crystallized fraction of oil from the sperm whale. It is also found in the rhizomes of the Iris, including Orris root. Chemical behaviour Myristic acid acts as a lipid anchor in biomembranes. Reduction of myristic acid yields myristyl aldehyde Myristyl aldehyde, also known as tetradecanal, is a reduced form of myristic acid. It is na ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Kinase A
In cell biology, protein kinase A (PKA) is a family of enzymes whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase (). PKA has several functions in the cell, including regulation of glycogen, sugar, and lipid metabolism. It should not be confused with 5'-AMP-activated protein kinase ( AMP-activated protein kinase). History Protein kinase A, more precisely known as adenosine 3',5'-monophosphate (cyclic AMP)-dependent protein kinase, abbreviated to PKA, was discovered by chemists Edmond H. Fischer and Edwin G. Krebs in 1968. They won the Nobel Prize in Physiology or Medicine in 1992 for their work on phosphorylation and dephosphorylation and how it relates to PKA activity. PKA is one of the most widely researched protein kinases, in part because of its uniqueness; out of 540 different protein kinase genes that make up the human kinome, only one other protein kinase, casein kinase 2, is known to exist in a p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Addition Reaction
In organic chemistry, an addition reaction is, in simplest terms, an organic reaction where two or more molecules combine to form a larger one (the adduct).. Addition reactions are limited to chemical compounds that have multiple bonds, such as molecules with carbon–carbon double bonds (alkenes), or with triple bonds (alkynes), and compounds that have rings, which are also considered points of unsaturation. Molecules containing carbon—hetero double bonds like carbonyl () groups, or imine () groups, can undergo addition, as they too have double-bond character. An addition reaction is the reverse of an elimination reaction. For instance, the hydration of an alkene to an alcohol is reversed by dehydration. There are two main types of polar addition reactions: electrophilic addition and nucleophilic addition. Two non-polar addition reactions exist as well, called free-radical addition and cycloadditions. Addition reactions are also encountered in polymerizations and cal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
α-helix
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues earlier along the protein sequence. The alpha helix is also called a classic Pauling–Corey–Branson α-helix. The name 3.613-helix is also used for this type of helix, denoting the average number of residues per helical turn, with 13 atoms being involved in the ring formed by the hydrogen bond. Among types of local structure in proteins, the α-helix is the most extreme and the most predictable from sequence, as well as the most prevalent. Discovery In the early 1930s, William Astbury showed that there were drastic changes in the X-ray fiber diffraction of moist wool or hair fibers upon significant stretching. The data suggested that the unstretched fibers had a coiled molecular structure with a characteristic repeat of ≈. Astbur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
β-sheet
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet. A β-strand is a stretch of polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformation. The supramolecular association of β-sheets has been implicated in the formation of the fibrils and protein aggregates observed in amyloidosis, notably Alzheimer's disease. History The first β-sheet structure was proposed by William Astbury in the 1930s. He proposed the idea of hydrogen bonding between the peptide bonds of parallel or antiparallel extended β-strands. However, Astbury did not have the necessary data on the bond geometry of the amino acids in order to build accurate models, especially since he did not then know that the peptide bond was planar. A refined version wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Structure
Protein structure is the molecular geometry, three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, the monomers of the polymer. A single amino acid monomer may also be called a ''residue'' indicating a repeating unit of a polymer. Proteins form by amino acids undergoing condensation reactions, in which the amino acids lose one water molecule per chemical reaction, reaction in order to attach to one another with a peptide bond. By convention, a chain under 30 amino acids is often identified as a peptide, rather than a protein. To be able to perform their biological function, proteins fold into one or more specific spatial conformations driven by a number of non-covalent interactions such as hydrogen bonding, ionic interactions, Van der Waals forces, and hydrophobic packing. To understand the functions of proteins at a molecular level, it is often necessary to determine their Pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |