The alkali metals consist of the
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
s
lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
(Li),
sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
(Na),
potassium
Potassium is a chemical element; it has Symbol (chemistry), symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to ...
(K),
[The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names for the elements in some languages, such as German and Russian.] rubidium (Rb),
caesium
Caesium (IUPAC spelling; also spelled cesium in American English) is a chemical element; it has Symbol (chemistry), symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only f ...
(Cs), and
francium
Francium is a chemical element; it has symbol Fr and atomic number 87. It is extremely radioactive; its most stable isotope, francium-223 (originally called '' actinium K'' after the natural decay chain in which it appears), has a half-l ...
(Fr). Together with
hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
they constitute
group 1, which lies in the
s-block of the
periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
. All alkali metals have their outermost electron in an
s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of
group trends in properties in the periodic table, with elements exhibiting well-characterised
homologous behaviour.
This family of elements is also known as the lithium family after its leading element.
The alkali metals are all shiny,
soft, highly
reactive metals at
standard temperature and pressure and readily lose their
outermost electron to form
cations with
charge +1. They can all be cut easily with a knife due to their softness, exposing a shiny surface that tarnishes rapidly in air due to
oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
by atmospheric moisture and
oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
(and in the case of lithium,
nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
). Because of their high reactivity, they must be stored under oil to prevent reaction with air, and are found naturally only in
salts and never as the free elements. Caesium, the fifth alkali metal, is the most reactive of all the metals. All the alkali metals react with water, with the heavier alkali metals reacting more vigorously than the lighter ones.
All of the discovered alkali metals occur in nature as their compounds: in order of
abundance, sodium is the most abundant, followed by potassium, lithium, rubidium, caesium, and finally francium, which is very rare due to its extremely high
radioactivity
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
; francium occurs only in minute
traces in nature as an intermediate step in some obscure side branches of the natural
decay chain
In nuclear science a decay chain refers to the predictable series of radioactive disintegrations undergone by the nuclei of certain unstable chemical elements.
Radioactive isotopes do not usually decay directly to stable isotopes, but rather ...
s. Experiments have been conducted to attempt the synthesis of
element 119, which is likely to be the next member of the group; none were successful. However, ununennium may not be an alkali metal due to
relativistic effects, which are predicted to have a large influence on the chemical properties of
superheavy element
Superheavy elements, also known as transactinide elements, transactinides, or super-heavy elements, or superheavies for short, are the chemical elements with atomic number greater than 104. The superheavy elements are those beyond the actinides in ...
s; even if it does turn out to be an alkali metal, it is predicted to have some differences in physical and chemical properties from its lighter homologues.
Most alkali metals have many different applications. One of the best-known applications of the pure elements is the use of rubidium and caesium in
atomic clock
An atomic clock is a clock that measures time by monitoring the resonant frequency of atoms. It is based on atoms having different energy levels. Electron states in an atom are associated with different energy levels, and in transitions betwee ...
s, of which caesium atomic clocks form the basis of the second. A common application of the compounds of sodium is the
sodium-vapour lamp, which emits light very efficiently.
Table salt, or sodium chloride, has been used since antiquity.
Lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
finds use as a psychiatric medication and as an
anode
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the devic ...
in
lithium batteries. Sodium, potassium and possibly lithium are
essential elements, having major biological roles as
electrolytes
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble salts, acids, and bases, dissolved in a polar solvent like water. Upon dissolving, t ...
, and although the other alkali metals are not essential, they also have various effects on the body, both beneficial and harmful.
__TOC__
History

Sodium compounds have been known since ancient times; salt (
sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
) has been an important commodity in human activities. While
potash
Potash ( ) includes various mined and manufactured salts that contain potassium in water- soluble form. has been used since ancient times, it was not understood for most of its history to be a fundamentally different substance from sodium mineral salts.
Georg Ernst Stahl obtained experimental evidence which led him to suggest the fundamental difference of sodium and potassium salts in 1702,
and
Henri-Louis Duhamel du Monceau was able to prove this difference in 1736. The exact chemical composition of potassium and sodium compounds, and the status as chemical element of potassium and sodium, was not known then, and thus
Antoine Lavoisier
Antoine-Laurent de Lavoisier ( ; ; 26 August 17438 May 1794), When reduced without charcoal, it gave off an air which supported respiration and combustion in an enhanced way. He concluded that this was just a pure form of common air and that i ...
did not include either alkali in his list of chemical elements in 1789.
Pure potassium was first isolated in 1807 in England by
Humphry Davy
Sir Humphry Davy, 1st Baronet (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several Chemical element, e ...
, who derived it from
caustic potash (KOH, potassium hydroxide) by the use of electrolysis of the molten salt with the newly invented
voltaic pile. Previous attempts at electrolysis of the aqueous salt were unsuccessful due to potassium's extreme reactivity.
Potassium was the first metal that was isolated by electrolysis.
Later that same year, Davy reported extraction of sodium from the similar substance
caustic soda (NaOH, lye) by a similar technique, demonstrating the elements, and thus the salts, to be different.
Petalite
Petalite, also known as castorite, is a lithium aluminum phyllosilicate mineral Li Al Si4 O10, crystallizing in the monoclinic system. Petalite occurs as colorless, pink, grey, yellow, yellow grey, to white tabular crystals and columnar masses. ...
() was discovered in 1800 by the Brazilian chemist
José Bonifácio de Andrada in a mine on the island of
Utö, Sweden.
However, it was not until 1817 that
Johan August Arfwedson, then working in the laboratory of the chemist
Jöns Jacob Berzelius,
detected the presence of a new element while analysing petalite
ore.
This new element was noted by him to form compounds similar to those of sodium and potassium, though its
carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
and
hydroxide were less
soluble in water and more
alkaline
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The ...
than the other alkali metals.
Berzelius gave the unknown material the name ''lithion''/''lithina'', from the
Greek word ''λιθoς'' (transliterated as ''lithos'', meaning "stone"), to reflect its discovery in a solid mineral, as opposed to potassium, which had been discovered in plant ashes, and sodium, which was known partly for its high abundance in animal blood. He named the metal inside the material ''lithium''.
Lithium, sodium, and potassium were part of the discovery of
periodicity, as they are among a series of triads of elements in the same
group that were noted by
Johann Wolfgang Döbereiner in 1850 as having similar properties.

Rubidium and caesium were the first elements to be discovered using the
spectroscope, invented in 1859 by
Robert Bunsen and
Gustav Kirchhoff
Gustav Robert Kirchhoff (; 12 March 1824 – 17 October 1887) was a German chemist, mathematician, physicist, and spectroscopist who contributed to the fundamental understanding of electrical circuits, spectroscopy and the emission of black-body ...
.
The next year, they discovered caesium in the
mineral water from
Bad Dürkheim
Bad Dürkheim () is a spa town in the Rhine-Neckar urban agglomeration. It is the seat of the Bad Dürkheim (district), Bad Dürkheim district in Rhineland-Palatinate, Germany, and the site of the discovery of the element caesium, in 1860.
Geogra ...
, Germany. Their discovery of rubidium came the following year in
Heidelberg
Heidelberg (; ; ) is the List of cities in Baden-Württemberg by population, fifth-largest city in the States of Germany, German state of Baden-Württemberg, and with a population of about 163,000, of which roughly a quarter consists of studen ...
, Germany, finding it in the mineral
lepidolite.
The names of rubidium and caesium come from the most prominent lines in their
emission spectra: a bright red line for rubidium (from the
Latin
Latin ( or ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally spoken by the Latins (Italic tribe), Latins in Latium (now known as Lazio), the lower Tiber area aroun ...
word ''rubidus'', meaning dark red or bright red), and a sky-blue line for caesium (derived from the Latin word ''caesius'', meaning sky-blue).
Around 1865
John Newlands produced a series of papers where he listed the elements in order of increasing atomic weight and similar physical and chemical properties that recurred at intervals of eight; he likened such periodicity to the
octave
In music, an octave (: eighth) or perfect octave (sometimes called the diapason) is an interval between two notes, one having twice the frequency of vibration of the other. The octave relationship is a natural phenomenon that has been referr ...
s of music, where notes an octave apart have similar musical functions. His version put all the alkali metals then known (lithium to caesium), as well as copper, silver, and
thallium
Thallium is a chemical element; it has Symbol (chemistry), symbol Tl and atomic number 81. It is a silvery-white post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Che ...
(which show the +1 oxidation state characteristic of the alkali metals), together into a group. His table placed hydrogen with the
halogens.

After 1869,
Dmitri Mendeleev
Dmitri Ivanovich Mendeleev ( ; ) was a Russian chemist known for formulating the periodic law and creating a version of the periodic table of elements. He used the periodic law not only to correct the then-accepted properties of some known ele ...
proposed his periodic table placing lithium at the top of a group with sodium, potassium, rubidium, caesium, and thallium. Two years later, Mendeleev revised his table, placing hydrogen in group 1 above lithium, and also moving thallium to the
boron group
The boron group are the chemical elements in periodic table group, group 13 of the periodic table, consisting of boron (B), aluminium (Al), gallium (Ga), indium (In), thallium (Tl) and nihonium (Nh). This group lies in the p-block of the perio ...
. In this 1871 version, copper, silver, and gold were placed twice, once as part of
group IB, and once as part of a "group VIII" encompassing today's groups
8 to 11.
[In the 1869 version of Mendeleev's periodic table, copper and silver were placed in their own group, aligned with hydrogen and mercury, while gold was tentatively placed under ]uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
and the undiscovered eka-aluminium in the boron group
The boron group are the chemical elements in periodic table group, group 13 of the periodic table, consisting of boron (B), aluminium (Al), gallium (Ga), indium (In), thallium (Tl) and nihonium (Nh). This group lies in the p-block of the perio ...
. After the introduction of the 18-column table, the group IB elements were moved to their current position in the
d-block, while alkali metals were left in ''group IA''. Later the group's name was changed to ''group 1'' in 1988.
[ The trivial name "alkali metals" comes from the fact that the hydroxides of the group 1 elements are all strong alkalis when dissolved in water.]actinium
Actinium is a chemical element; it has chemical symbol, symbol Ac and atomic number 89. It was discovered by Friedrich Oskar Giesel in 1902, who gave it the name ''emanium''; the element got its name by being wrongly identified with a substa ...
-227. Various tests eliminated the possibility of the unknown element being thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
, radium, lead, bismuth, or thallium
Thallium is a chemical element; it has Symbol (chemistry), symbol Tl and atomic number 81. It is a silvery-white post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Che ...
. The new product exhibited chemical properties of an alkali metal (such as coprecipitating with caesium salts), which led Perey to believe that it was element 87, caused by the alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an a ...
of actinium-227.[Adloff, Jean-Pierre; Kaufman, George B. (25 September 2005)]
Francium (Atomic Number 87), the Last Discovered Natural Element
. ''The Chemical Educator'' 10 (5). Retrieved 26 March 2007. Perey then attempted to determine the proportion of beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
to alpha decay in actinium-227. Her first test put the alpha branching at 0.6%, a figure that she later revised to 1%.ununennium
Ununennium, also known as eka-francium or element 119, is a hypothetical chemical element; it has symbol Uue and atomic number 119. ''Ununennium'' and ''Uue'' are the temporary systematic element name, systematic IUPAC name and symbol respectivel ...
(Uue), element 119.calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
-48 ions at the superHILAC accelerator at the Lawrence Berkeley National Laboratory in Berkeley, California. No atoms were identified, leading to a limiting yield of 300 nb.[The ]asterisk
The asterisk ( ), from Late Latin , from Ancient Greek , , "little star", is a Typography, typographical symbol. It is so called because it resembles a conventional image of a star (heraldry), heraldic star.
Computer scientists and Mathematici ...
denotes an excited state
In quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Add ...
.
It is highly unlikely
Occurrence
In the Solar System
 The Oddo–Harkins rule holds that elements with even atomic numbers are more common that those with odd atomic numbers, with the exception of hydrogen. This rule argues that elements with odd atomic numbers have one unpaired proton and are more likely to capture another, thus increasing their atomic number. In elements with even atomic numbers, protons are paired, with each member of the pair offsetting the spin of the other, enhancing stability.
The Oddo–Harkins rule holds that elements with even atomic numbers are more common that those with odd atomic numbers, with the exception of hydrogen. This rule argues that elements with odd atomic numbers have one unpaired proton and are more likely to capture another, thus increasing their atomic number. In elements with even atomic numbers, protons are paired, with each member of the pair offsetting the spin of the other, enhancing stability.noble gas
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of Group (periodic table), group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some ...
es and the alkaline earth metal
The alkaline earth metals are six chemical elements in group (periodic table), group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar p ...
s) in the Solar System. The heavier alkali metals are also less abundant than the lighter ones as the alkali metals from rubidium onward can only be synthesised in supernova
A supernova (: supernovae or supernovas) is a powerful and luminous explosion of a star. A supernova occurs during the last stellar evolution, evolutionary stages of a massive star, or when a white dwarf is triggered into runaway nuclear fusion ...
e and not in stellar nucleosynthesis
In astrophysics, stellar nucleosynthesis is the creation of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a ...
. Lithium is also much less abundant than sodium and potassium as it is poorly synthesised in both Big Bang nucleosynthesis
In physical cosmology, Big Bang nucleosynthesis (also known as primordial nucleosynthesis, and abbreviated as BBN) is a model for the production of light nuclei, deuterium, 3He, 4He, 7Li, between 0.01s and 200s in the lifetime of the universe ...
and in stars: the Big Bang could only produce trace quantities of lithium, beryllium
Beryllium is a chemical element; it has Symbol (chemistry), symbol Be and atomic number 4. It is a steel-gray, hard, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with ...
and boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
due to the absence of a stable nucleus with 5 or 8 nucleons, and stellar nucleosynthesis could only pass this bottleneck by the triple-alpha process, fusing three helium nuclei to form carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
, and skipping over those three elements.
On Earth
 The Earth formed from the same cloud of matter that formed the Sun, but the planets acquired different compositions during the formation and evolution of the Solar System. In turn, the natural history of the Earth caused parts of this planet to have differing concentrations of the elements. The mass of the Earth is approximately 5.98 kg. It is composed mostly of iron (32.1%),
The Earth formed from the same cloud of matter that formed the Sun, but the planets acquired different compositions during the formation and evolution of the Solar System. In turn, the natural history of the Earth caused parts of this planet to have differing concentrations of the elements. The mass of the Earth is approximately 5.98 kg. It is composed mostly of iron (32.1%), oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
(30.1%), silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
(15.1%), magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
(13.9%), sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
(2.9%), nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
(1.8%), calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
(1.5%), and aluminium (1.4%); with the remaining 1.2% consisting of trace amounts of other elements. Due to planetary differentiation, the core region is believed to be primarily composed of iron (88.8%), with smaller amounts of nickel (5.8%), sulfur (4.5%), and less than 1% trace elements.oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
and so associate strongly with silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
, forming relatively low-density minerals that do not sink down into the Earth's core. Potassium, rubidium and caesium are also incompatible elements due to their large ionic radii.Utah
Utah is a landlocked state in the Mountain states, Mountain West subregion of the Western United States. It is one of the Four Corners states, sharing a border with Arizona, Colorado, and New Mexico. It also borders Wyoming to the northea ...
's Great Salt Lake and the Dead Sea
The Dead Sea (; or ; ), also known by #Names, other names, is a landlocked salt lake bordered by Jordan to the east, the Israeli-occupied West Bank to the west and Israel to the southwest. It lies in the endorheic basin of the Jordan Rift Valle ...
.gallium
Gallium is a chemical element; it has Chemical symbol, symbol Ga and atomic number 31. Discovered by the French chemist Paul-Émile Lecoq de Boisbaudran in 1875,
elemental gallium is a soft, silvery metal at standard temperature and pressure. ...
and niobium
Niobium is a chemical element; it has chemical symbol, symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and Ductility, ductile transition metal. Pure niobium has a Mohs scale of mineral hardness, Mohs h ...
. Commercially, the most important lithium mineral is spodumene, which occurs in large deposits worldwide.zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
and more abundant than copper. It occurs naturally in the minerals leucite, pollucite, carnallite
Carnallite (also carnalite) is an evaporite mineral, a hydrated potassium magnesium chloride with formula KCl.MgCl2·6(H2O). It is variably colored yellow to white, reddish, and sometimes colorless or blue. It is usually massive to fibrous with r ...
, zinnwaldite, and lepidolite, although none of these contain only rubidium and no other alkali metals.antimony
Antimony is a chemical element; it has chemical symbol, symbol Sb () and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (). Antimony compounds have been known since ancient t ...
, cadmium
Cadmium is a chemical element; it has chemical symbol, symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12 element, group 12, zinc and mercury (element), mercury. Like z ...
, tin, and tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
, but is much less abundant than rubidium.alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an a ...
of actinium-227 and can be found in trace amounts in uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
minerals.earth's crust
Earth's crust is its thick outer shell of rock, referring to less than one percent of the planet's radius and volume. It is the top component of the lithosphere, a solidified division of Earth's layers that includes the crust and the upper ...
at any time, due to its extremely short half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 22 minutes.
Properties
Physical and chemical
The physical and chemical properties of the alkali metals can be readily explained by their having an ns1 valence electron configuration, which results in weak metallic bonding
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be desc ...
. Hence, all the alkali metals are soft and have low densities,melting
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which inc ...
boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
s,electrical conductivity
Electrical resistivity (also called volume resistivity or specific electrical resistance) is a fundamental specific property of a material that measures its electrical resistance or how strongly it resists electric current. A low resistivity in ...
.radioactivity
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
;electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
,alkaline earth metal
The alkaline earth metals are six chemical elements in group (periodic table), group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar p ...
s calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
, strontium
Strontium is a chemical element; it has symbol Sr and atomic number 38. An alkaline earth metal, it is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to ...
, and barium, as well as the divalent lanthanides europium
Europium is a chemical element; it has symbol Eu and atomic number 63. It is a silvery-white metal of the lanthanide series that reacts readily with air to form a dark oxide coating. Europium is the most chemically reactive, least dense, and soft ...
and ytterbium, are pale yellow, though the colour is much less prominent than it is for caesium.mineral oil
Mineral oil is any of various colorless, odorless, light mixtures of higher alkanes from a mineral source, particularly a distillate of petroleum, as distinct from usually edible vegetable oils.
The name 'mineral oil' by itself is imprecise, ...
or kerosene
Kerosene, or paraffin, is a combustibility, combustible hydrocarbon liquid which is derived from petroleum. It is widely used as a fuel in Aviation fuel, aviation as well as households. Its name derives from the Greek (''kērós'') meaning " ...
(paraffin oil).periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
effective nuclear charge
In atomic physics, the effective nuclear charge of an electron in a multi-electron atom or ion is the number of elementary charges (e) an electron experiences by the nucleus. It is denoted by ''Z''eff. The term "effective" is used because the shi ...
noble gas
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of Group (periodic table), group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some ...
configuration by losing just one electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
.alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s and phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (− O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds ar ...
, gaseous ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
, and alkynes, the last demonstrating the phenomenal degree of their reactivity. Their great power as reducing agents makes them very useful in liberating other metals from their oxides or halides.anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s and were thought to be able to appear in salts only as cations. The alkalide anions have filled s-subshells, which gives them enough stability to exist. All the stable alkali metals except lithium are known to be able to form alkalides, and the alkalides have much theoretical interest due to their unusual stoichiometry
Stoichiometry () is the relationships between the masses of reactants and Product (chemistry), products before, during, and following chemical reactions.
Stoichiometry is based on the law of conservation of mass; the total mass of reactants must ...
and low ionisation potentials. Alkalides are chemically similar to the electrides, which are salts with trapped electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s acting as anions.metastable
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball is onl ...
or stable.coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion ...
s and shapes agree well with those expected from their ionic radii. In aqueous solution the water molecules directly attached to the metal ion are said to belong to the first coordination sphere, also known as the first, or primary, solvation shell. The bond between a water molecule and the metal ion is a dative covalent bond, with the oxygen atom donating both electrons to the bond. Each coordinated water molecule may be attached by hydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
s to other water molecules. The latter are said to reside in the second coordination sphere. However, for the alkali metal cations, the second coordination sphere is not well-defined as the +1 charge on the cation is not high enough to polarise the water molecules in the primary solvation shell enough for them to form strong hydrogen bonds with those in the second coordination sphere, producing a more stable entity.
Lithium
The chemistry of lithium shows several differences from that of the rest of the group as the small Li+ cation polarises anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s and gives its compounds a more covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
character.magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
have a diagonal relationship due to their similar atomic radii,alkaline earth metal
The alkaline earth metals are six chemical elements in group (periodic table), group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar p ...
s (magnesium's group) but unique among the alkali metals.deliquescent
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption (chemistry), absorption or adsorption from the surrounding Natural environment, environment, which is usually at normal or room temperature. If water mol ...
.hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption (chemistry), absorption or adsorption from the surrounding Natural environment, environment, which is usually at normal or room temperature. If water mol ...
: this allows salts like lithium chloride and lithium bromide to be used in dehumidifiers and air-conditioners.
Francium
Francium is also predicted to show some differences due to its high atomic weight, causing its electrons to travel at considerable fractions of the speed of light and thus making relativistic effects more prominent. In contrast to the trend of decreasing electronegativities and ionisation energies of the alkali metals, francium's electronegativity and ionisation energy are predicted to be higher than caesium's due to the relativistic stabilisation of the 7s electrons; also, its atomic radius is expected to be abnormally low. Thus, contrary to expectation, caesium is the most reactive of the alkali metals, not francium.
Nuclear
All the alkali metals have odd atomic numbers; hence, their isotopes must be either odd–odd (both proton and neutron number are odd) or odd–even ( proton number is odd, but neutron number is even). Odd–odd nuclei have even mass number
The mass number (symbol ''A'', from the German word: ''Atomgewicht'', "atomic weight"), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is appro ...
s, whereas odd–even nuclei have odd mass numbers. Odd–odd primordial nuclides are rare because most odd–odd nuclei are highly unstable with respect to beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
, because the decay products are even–even, and are therefore more strongly bound, due to nuclear pairing effects.beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
toward the lowest-mass nuclide. An effect of the instability of an odd number of either type of nucleons is that odd-numbered elements, such as the alkali metals, tend to have fewer stable isotopes than even-numbered elements. Of the 26 monoisotopic elements that have only a single stable isotope, all but one have an odd atomic number and all but one also have an even number of neutrons. Beryllium
Beryllium is a chemical element; it has Symbol (chemistry), symbol Be and atomic number 4. It is a steel-gray, hard, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with ...
is the single exception to both rules, due to its low atomic number.radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
.medium-lived fission product
Long-lived fission products (LLFPs) are radioactive materials with a long half-life (more than 200,000 years) produced by nuclear fission of uranium and plutonium. Because of their persistent Ionizing radiation, radiotoxicity, it is necessary to is ...
s, along with strontium-90
Strontium-90 () is a radioactive isotope of strontium produced by nuclear fission, with a half-life of 28.79 years. It undergoes β− decay into yttrium-90, with a decay energy of 0.546 MeV. Strontium-90 has applications in medicine a ...
, which are responsible for most of the radioactivity
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
of spent nuclear fuel after several years of cooling, up to several hundred years after use. It constitutes most of the radioactivity still left from the Chernobyl accident. Caesium-137 undergoes high-energy beta decay and eventually becomes stable barium-137. It is a strong emitter of gamma radiation. Caesium-137 has a very low rate of neutron capture and cannot be feasibly disposed of in this way, but must be allowed to decay.Chernobyl disaster
On 26 April 1986, the no. 4 reactor of the Chernobyl Nuclear Power Plant, located near Pripyat, Ukrainian Soviet Socialist Republic, Ukrainian SSR, Soviet Union (now Ukraine), exploded. With dozens of direct casualties, it is one of only ...
. As of 2005, caesium-137 is the principal source of radiation in the zone of alienation around the Chernobyl nuclear power plant.
Periodic trends
The alkali metals are more similar to each other than the elements in any other group are to each other.electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
,
Atomic and ionic radii
 The atomic radii of the alkali metals increase going down the group.
The atomic radii of the alkali metals increase going down the group.electron shell
In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (o ...
, each electron feels electric repulsion from the other electrons as well as electric attraction from the nucleus.atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
) is cancelled by the inner electrons; the number of inner electrons of an alkali metal is always one less than the nuclear charge. Therefore, the only factor which affects the atomic radius of the alkali metals is the number of electron shells. Since this number increases down the group, the atomic radius must also increase down the group.electron shell
In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (o ...
than the inner electrons, and thus when it is removed the resulting atom has one fewer electron shell and is smaller. Additionally, the effective nuclear charge
In atomic physics, the effective nuclear charge of an electron in a multi-electron atom or ion is the number of elementary charges (e) an electron experiences by the nucleus. It is denoted by ''Z''eff. The term "effective" is used because the shi ...
has increased, and thus the electrons are attracted more strongly towards the nucleus and the ionic radius decreases.
First ionisation energy
 The first ionisation energy of an element or
The first ionisation energy of an element or molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
is the energy required to move the most loosely held electron from one mole of gaseous atoms of the element or molecules to form one mole of gaseous ions with electric charge
Electric charge (symbol ''q'', sometimes ''Q'') is a physical property of matter that causes it to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative''. Like charges repel each other and ...
+1. The factors affecting the first ionisation energy are the nuclear charge, the amount of shielding by the inner electrons and the distance from the most loosely held electron from the nucleus, which is always an outer electron in main group elements. The first two factors change the effective nuclear charge the most loosely held electron feels. Since the outermost electron of alkali metals always feels the same effective nuclear charge (+1), the only factor which affects the first ionisation energy is the distance from the outermost electron to the nucleus. Since this distance increases down the group, the outermost electron feels less attraction from the nucleus and thus the first ionisation energy decreases.electron shell
In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (o ...
and is thus difficult to remove.
Reactivity
The reactivities of the alkali metals increase going down the group. This is the result of a combination of two factors: the first ionisation energies and atomisation energies of the alkali metals. Because the first ionisation energy of the alkali metals decreases down the group, it is easier for the outermost electron to be removed from the atom and participate in chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
s, thus increasing reactivity down the group. The atomisation energy measures the strength of the metallic bond of an element, which falls down the group as the atoms increase in radius
In classical geometry, a radius (: radii or radiuses) of a circle or sphere is any of the line segments from its Centre (geometry), center to its perimeter, and in more modern usage, it is also their length. The radius of a regular polygon is th ...
and thus the metallic bond must increase in length, making the delocalised electrons further away from the attraction of the nuclei of the heavier alkali metals. Adding the atomisation and first ionisation energies gives a quantity closely related to (but not equal to) the activation energy of the reaction of an alkali metal with another substance. This quantity decreases going down the group, and so does the activation energy; thus, chemical reactions can occur faster and the reactivity increases down the group.
Electronegativity

Electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
is a chemical property that describes the tendency of an atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
or a functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
to attract electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s (or electron density
Electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial variables and is typical ...
) towards itself.sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
and chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
in sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
were covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
, the pair of shared electrons would be attracted to the chlorine because the effective nuclear charge on the outer electrons is +7 in chlorine but is only +1 in sodium. The electron pair is attracted so close to the chlorine atom that they are practically transferred to the chlorine atom (an ionic bond
Ionic bonding is a type of chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic ...
). However, if the sodium atom was replaced by a lithium atom, the electrons will not be attracted as close to the chlorine atom as before because the lithium atom is smaller, making the electron pair more strongly attracted to the closer effective nuclear charge from lithium. Hence, the larger alkali metal atoms (further down the group) will be less electronegative as the bonding pair is less strongly attracted towards them. As mentioned previously, francium is expected to be an exception.deliquescent
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption (chemistry), absorption or adsorption from the surrounding Natural environment, environment, which is usually at normal or room temperature. If water mol ...
.
Melting and boiling points
The melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
of a substance is the point where it changes state from solid to liquid while the boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
of a substance (in liquid state) is the point where the vapour pressure of the liquid equals the environmental pressure surrounding the liquid and all the liquid changes state to gas. As a metal is heated to its melting point, the metallic bonds keeping the atoms in place weaken so that the atoms can move around, and the metallic bonds eventually break completely at the metal's boiling point.
Density
The alkali metals all have the same crystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
( body-centred cubic)
Compounds
The alkali metals form complete series of compounds with all usually encountered anions, which well illustrate group trends. These compounds can be described as involving the alkali metals losing electrons to acceptor species and forming monopositive ions.ionic bond
Ionic bonding is a type of chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic ...
ing gives way to metallic bonding along the series NaCl, Na2O, Na2S, Na3P, Na3As, Na3Sb, Na3Bi, Na.
Hydroxides
 All the alkali metals react vigorously or explosively with cold water, producing an
All the alkali metals react vigorously or explosively with cold water, producing an aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water ...
of a strongly basic
Basic or BASIC may refer to:
Science and technology
* BASIC, a computer programming language
* Basic (chemistry), having the properties of a base
* Basic access authentication, in HTTP
Entertainment
* Basic (film), ''Basic'' (film), a 2003 film
...
alkali metal hydroxide and releasing hydrogen gas.hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
gas; this takes place faster for the more reactive heavier alkali metals. Second, the heat generated by the first part of the reaction often ignites the hydrogen gas, causing it to burn explosively into the surrounding air. This secondary hydrogen gas explosion produces the visible flame above the bowl of water, lake or other body of water, not the initial reaction of the metal with water (which tends to happen mostly under water).carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
to form carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
s or bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
s, or with hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
to form sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
s or bisulfides, and may be used to separate thiols from petroleum. They react with amphoteric oxides: for example, the oxides of aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
, zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
, tin, and lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
react with the alkali metal hydroxides to give aluminates, zincates, stannates, and plumbates. Silicon dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundan ...
is acidic, and thus the alkali metal hydroxides can also attack silicate glass.
Intermetallic compounds
 The alkali metals form many intermetallic compounds with each other and the elements from groups 2 to 13 in the periodic table of varying stoichiometries,
The alkali metals form many intermetallic compounds with each other and the elements from groups 2 to 13 in the periodic table of varying stoichiometries,
Compounds with the group 13 elements
The intermetallic compounds of the alkali metals with the heavier group 13 elements (aluminium, gallium
Gallium is a chemical element; it has Chemical symbol, symbol Ga and atomic number 31. Discovered by the French chemist Paul-Émile Lecoq de Boisbaudran in 1875,
elemental gallium is a soft, silvery metal at standard temperature and pressure. ...
, indium
Indium is a chemical element; it has Symbol (chemistry), symbol In and atomic number 49. It is a silvery-white post-transition metal and one of the softest elements. Chemically, indium is similar to gallium and thallium, and its properties are la ...
, and thallium
Thallium is a chemical element; it has Symbol (chemistry), symbol Tl and atomic number 81. It is a silvery-white post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Che ...
), such as NaTl, are poor conductors or semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
s, unlike the normal alloys with the preceding elements, implying that the alkali metal involved has lost an electron to the Zintl anions involved.[S.M. Kauzlarich, Encyclopedia of Inorganic chemistry, 1994, John Wiley & Sons, ]
Boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
is a special case, being the only nonmetal in group 13. The alkali metal borides tend to be boron-rich, involving appreciable boron–boron bonding involving deltahedral structures,
Compounds with the group 14 elements
Lithium and sodium react with carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
to form acetylides, Li2C2 and Na2C2, which can also be obtained by reaction of the metal with acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is u ...
. Potassium, rubidium, and caesium react with graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
; their atoms are intercalated between the hexagonal graphite layers, forming graphite intercalation compounds of formulae MC60 (dark grey, almost black), MC48 (dark grey, almost black), MC36 (blue), MC24 (steel blue), and MC8 (bronze) (M = K, Rb, or Cs). These compounds are over 200 times more electrically conductive than pure graphite, suggesting that the valence electron of the alkali metal is transferred to the graphite layers (e.g. ).reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are common reducing agents include hydrogen, carbon ...
and is pyrophoric and explodes on contact with water.carbon group
The carbon group is a group (periodic table), periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block.
In modern International Union of Pure and Applied Ch ...
(silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
, germanium
Germanium is a chemical element; it has Symbol (chemistry), symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid or a nonmetal in the carbon group that is chemically ...
, tin, and lead), ionic substances with cage-like structures are formed, such as the silicides M4 Si4 (M = K, Rb, or Cs), which contains M+ and tetrahedral ions.
Nitrides and pnictides
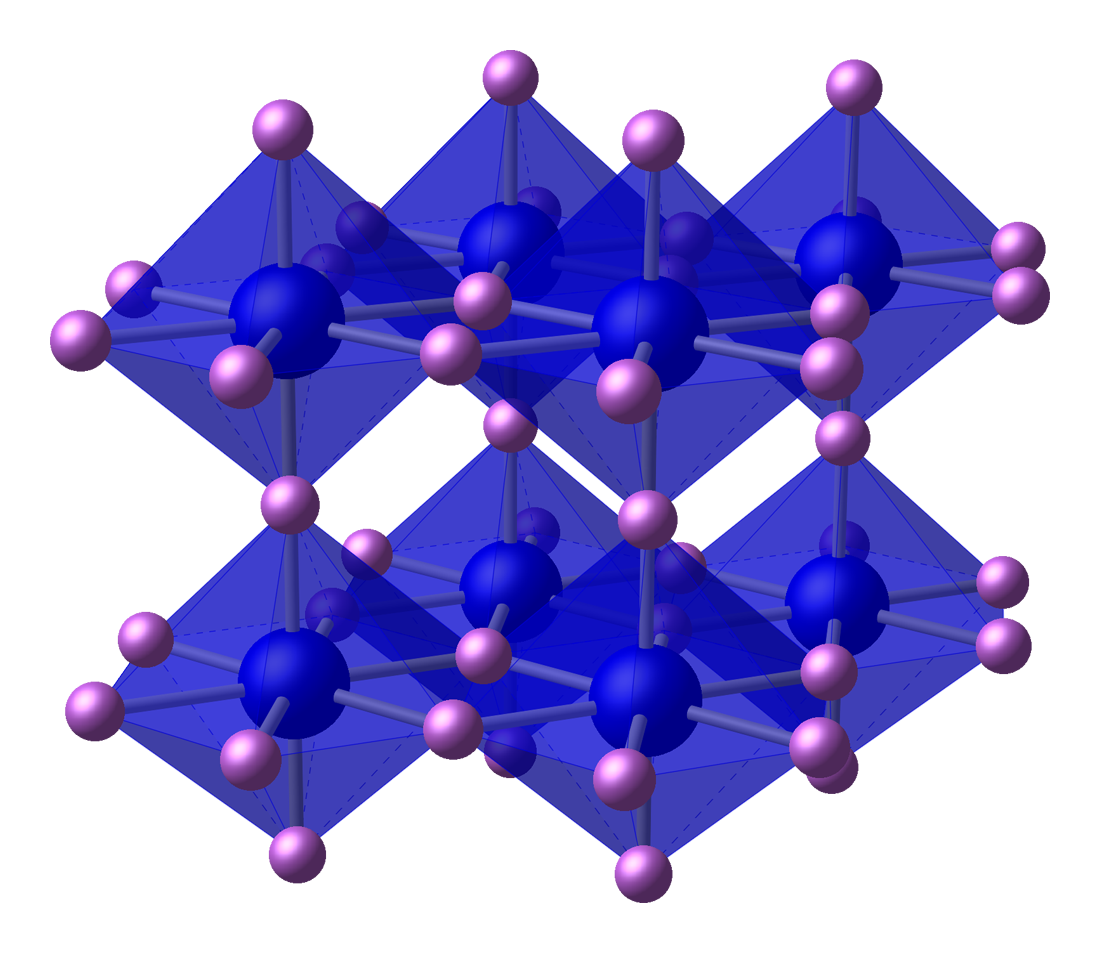 Lithium, the lightest of the alkali metals, is the only alkali metal which reacts with
Lithium, the lightest of the alkali metals, is the only alkali metal which reacts with nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
at standard conditions, and its nitride is the only stable alkali metal nitride. Nitrogen is an unreactive
In chemistry, reactivity is the impulse for which a chemical substance undergoes a chemical reaction, either by itself or with other materials, with an overall release of energy.
''Reactivity'' refers to:
* the chemical reactions of a single sub ...
gas because breaking the strong triple bond in the dinitrogen
Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh ...
molecule (N2) requires a lot of energy. The formation of an alkali metal nitride would consume the ionisation energy of the alkali metal (forming M+ ions), the energy required to break the triple bond in N2 and the formation of N3− ions, and all the energy released from the formation of an alkali metal nitride is from the lattice energy of the alkali metal nitride. The lattice energy is maximised with small, highly charged ions; the alkali metals do not form highly charged ions, only forming ions with a charge of +1, so only lithium, the smallest alkali metal, can release enough lattice energy to make the reaction with nitrogen exothermic, forming lithium nitride. The reactions of the other alkali metals with nitrogen would not release enough lattice energy and would thus be endothermic
An endothermic process is a chemical or physical process that absorbs heat from its surroundings. In terms of thermodynamics, it is a thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, ...
, so they do not form nitrides at standard conditions.
'Elusive Binary Compound Prepared'
''Chemical & Engineering News'' 80 No. 20 (20 May 2002)phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
and arsenic
Arsenic is a chemical element; it has Symbol (chemistry), symbol As and atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is not ...
to form phosphides and arsenide
In chemistry, an arsenide is a compound of arsenic with a less electronegative element or elements. Many metals form binary compounds containing arsenic, and these are called arsenides. They exist with many Stoichiometry, stoichiometries, and in t ...
s with the formula M3Pn (where M represents an alkali metal and Pn represents a pnictogen
, -
! colspan=2 style="text-align:left;" , ↓ Period
, -
! 2
,
, -
! 3
,
, -
! 4
,
, -
! 5
,
, -
! 6
,
, -
! 7
,
, -
, colspan="2",
----
''Legend''
A pnictogen ( or ; from "to choke" and -gen, "generator") is any ...
– phosphorus, arsenic, antimony
Antimony is a chemical element; it has chemical symbol, symbol Sb () and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (). Antimony compounds have been known since ancient t ...
, or bismuth). This is due to the greater size of the P3− and As3− ions, so that less lattice energy needs to be released for the salts to form.[H.G. Von Schnering, W. Hönle ''Phosphides – Solid-state Chemistry'' Encyclopedia of Inorganic Chemistry Ed. R. Bruce King (1994) John Wiley & Sons ] While most metals form arsenides, only the alkali and alkaline earth metals form mostly ionic arsenides. The structure of Na3As is complex with unusually short Na–Na distances of 328–330 pm which are shorter than in sodium metal, and this indicates that even with these electropositive metals the bonding cannot be straightforwardly ionic.
Oxides and chalcogenides
All the alkali metals react vigorously with oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
at standard conditions. They form various types of oxides, such as simple oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
s (containing the O2− ion), peroxide
In chemistry, peroxides are a group of Chemical compound, compounds with the structure , where the R's represent a radical (a portion of a complete molecule; not necessarily a free radical) and O's are single oxygen atoms. Oxygen atoms are joined ...
s (containing the ion, where there is a single bond between the two oxygen atoms), superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of t ...
s (containing the ion), and many others. Lithium burns in air to form lithium oxide, but sodium reacts with oxygen to form a mixture of sodium oxide and sodium peroxide. Potassium forms a mixture of potassium peroxide
Potassium peroxide is an inorganic compound with the molecular formula K2O2. It is formed as potassium reacts with oxygen in the air, along with potassium oxide (K2O) and potassium superoxide (KO2).
Potassium peroxide reacts with water to for ...
and potassium superoxide, while rubidium and caesium form the superoxide exclusively. Their reactivity increases going down the group: while lithium, sodium and potassium merely burn in air, rubidium and caesium are pyrophoric (spontaneously catch fire in air).carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
to form the alkali metal carbonate and oxygen gas, which allows them to be used in submarine
A submarine (often shortened to sub) is a watercraft capable of independent operation underwater. (It differs from a submersible, which has more limited underwater capability.) The term "submarine" is also sometimes used historically or infor ...
air purifiers; the presence of water vapour
Water vapor, water vapour, or aqueous vapor is the gaseous phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Water vapor ...
, naturally present in breath, makes the removal of carbon dioxide by potassium superoxide even more efficient.ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
: the ozonides may be then extracted using liquid ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
. They slowly decompose at standard conditions to the superoxides and oxygen, and hydrolyse immediately to the hydroxides when in contact with water.sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
, selenium
Selenium is a chemical element; it has symbol (chemistry), symbol Se and atomic number 34. It has various physical appearances, including a brick-red powder, a vitreous black solid, and a grey metallic-looking form. It seldom occurs in this elem ...
, tellurium, and polonium), and all the alkali metal chalcogenides are known (with the exception of francium's). Reaction with an excess of the chalcogen can similarly result in lower chalcogenides, with chalcogen ions containing chains of the chalcogen atoms in question. For example, sodium can react with sulfur to form the sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
( Na2S) and various polysulfide
Polysulfides are a class of chemical compounds derived from anionic chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. The inorganic polysulfides have the general formula . These anions are the conjugate bas ...
s with the formula Na2S''x'' (''x'' from 2 to 6), containing the ions.
Halides, hydrides, and pseudohalides
The alkali metals are among the most electropositive elements on the periodic table and thus tend to bond ionically to the most electronegative elements on the periodic table, the halogens (fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
, chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
, bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
, iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a vi ...
, and astatine
Astatine is a chemical element; it has Symbol (chemistry), symbol At and atomic number 85. It is the abundance of elements in Earth's crust, rarest naturally occurring element in the Earth's crust, occurring only as the Decay chain, decay product ...
), forming salts known as the alkali metal halides. The reaction is very vigorous and can sometimes result in explosions.sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
, otherwise known as common salt. All of the stable alkali metal halides have the formula MX where M is an alkali metal and X is a halogen. They are all white ionic crystalline solids that have high melting points.hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
anion acts as a pseudohalide: these are often used as reducing agents, producing hydrides, complex metal hydrides, or hydrogen gas.
Coordination complexes
Alkali metal cations do not usually form coordination complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
es with simple Lewis bases due to their low charge of just +1 and their relatively large size; thus the Li+ ion forms most complexes and the heavier alkali metal ions form less and less (though exceptions occur for weak complexes).coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion ...
s from 1 to 12, although octahedral hexacoordination is its preferred mode.aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water ...
, the alkali metal ions exist as octahedral hexahydrate complexes (H2O)6sup>+, with the exception of the lithium ion, which due to its small size forms tetrahedral tetrahydrate complexes i(H2O)4sup>+; the alkali metals form these complexes because their ions are attracted by electrostatic forces of attraction to the polar water molecules. Because of this, anhydrous salts containing alkali metal cations are often used as desiccants.
Ammonia solutions
The alkali metals dissolve slowly in liquid ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
, forming ammoniacal solutions of solvated metal cation M+ and solvated electron e−, which react to form hydrogen gas and the alkali metal amide (MNH2, where M represents an alkali metal): this was first noted by Humphry Davy
Sir Humphry Davy, 1st Baronet (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several Chemical element, e ...
in 1809 and rediscovered by W. Weyl in 1864. The process may be speeded up by a catalyst. Similar solutions are formed by the heavy divalent alkaline earth metal
The alkaline earth metals are six chemical elements in group (periodic table), group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar p ...
s calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
, strontium
Strontium is a chemical element; it has symbol Sr and atomic number 38. An alkaline earth metal, it is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to ...
, barium, as well as the divalent lanthanides, europium
Europium is a chemical element; it has symbol Eu and atomic number 63. It is a silvery-white metal of the lanthanide series that reacts readily with air to form a dark oxide coating. Europium is the most chemically reactive, least dense, and soft ...
and ytterbium. The amide salt is quite insoluble and readily precipitates out of solution, leaving intensely coloured ammonia solutions of the alkali metals. In 1907, Charles A. Kraus identified the colour as being due to the presence of solvated electrons, which contribute to the high electrical conductivity of these solutions. At low concentrations (below 3 M), the solution is dark blue and has ten times the conductivity of aqueous sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
; at higher concentrations (above 3 M), the solution is copper-coloured and has approximately the conductivity of liquid metals like mercury.diatomic
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear mol ...
alkali metal molecules (M2) and alkali metal anions (M−). These are unstable and eventually become the more thermodynamically stable alkali metal amide and hydrogen gas. Solvated electrons are powerful reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are common reducing agents include hydrogen, carbon ...
s and are often used in chemical synthesis.
Organometallic
Organolithium
 Being the smallest alkali metal, lithium forms the widest variety of and most stable organometallic compounds, which are bonded covalently. Organolithium compounds are electrically non-conducting volatile solids or liquids that melt at low temperatures, and tend to form oligomers with the structure (RLi)''x'' where R is the organic group. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful bases and nucleophiles. For use as bases, butyllithiums are often used and are commercially available. An example of an organolithium compound is
Being the smallest alkali metal, lithium forms the widest variety of and most stable organometallic compounds, which are bonded covalently. Organolithium compounds are electrically non-conducting volatile solids or liquids that melt at low temperatures, and tend to form oligomers with the structure (RLi)''x'' where R is the organic group. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful bases and nucleophiles. For use as bases, butyllithiums are often used and are commercially available. An example of an organolithium compound is methyllithium
Methyllithium is the simplest organolithium reagent, with the empirical formula LiCH3. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used i ...
((CH3Li)''x''), which exists in tetrameric (''x'' = 4, tetrahedral) and hexameric (''x'' = 6, octahedral) forms.ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s by reaction with metal carbonyls. The reaction with nickel tetracarbonyl, for example, proceeds through an unstable acyl nickel carbonyl complex which then undergoes electrophilic substitution to give the desired aldehyde (using H+ as the electrophile) or ketone (using an alkyl halide) product.amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
s to give aldehydes and ketones, and symmetrical ketones by reacting with carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
. They thermally decompose to eliminate a β-hydrogen, producing alkenes and lithium hydride: another route is the reaction of ethers with alkyl- and aryllithiums that act as strong bases.carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
s (ArCO2H) and aryl ketones to tertiary carbinols (Ar'2C(Ar)OH). Finally, they may be used to synthesise other organometallic compounds through metal-halogen exchange.
Heavier alkali metals
Unlike the organolithium compounds, the organometallic compounds of the heavier alkali metals are predominantly ionic. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compound
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
s, which are commercially available and exhibit more convenient reactivity. The principal organosodium compound of commercial importance is sodium cyclopentadienide
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pin ...
. Sodium tetraphenylborate can also be classified as an organosodium compound since in the solid state sodium is bound to the aryl groups. Organometallic compounds of the higher alkali metals are even more reactive than organosodium compounds and of limited utility. A notable reagent is Schlosser's base, a mixture of ''n''-butyllithium and potassium ''tert''-butoxide. This reagent reacts with propene to form the compound allylpotassium (KCH2CHCH2). ''cis''-2-Butene and ''trans''-2-butene equilibrate when in contact with alkali metals. Whereas isomerisation is fast with lithium and sodium, it is slow with the heavier alkali metals. The heavier alkali metals also favour the sterically congested conformation. Several crystal structures of organopotassium compounds have been reported, establishing that they, like the sodium compounds, are polymeric.tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
. The most important of these compounds is sodium cyclopentadienide
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pin ...
, NaC5H5, an important precursor to many transition metal cyclopentadienyl derivatives.uranocene
Uranocene, U(C8H8)2, is an organouranium compound composed of a uranium atom sandwiched between two cyclooctatetraene, cyclooctatetraenide rings. It was one of the first Organoactinide chemistry, organoactinide compounds to be synthesized. It is a ...
.sodium naphthalenide
Sodium naphthalene is an organic salt (chemistry), salt with the chemical formula . In the research laboratory, it is used as a reductant in the synthesis of organic, organometallic, and inorganic chemistry. It is usually generated in situ. When is ...
, Na+ 10H8•sup>−, a strong reducing agent.
Representative reactions of alkali metals
Reaction with oxygen
Upon reacting with oxygen, alkali metals form oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
s, peroxide
In chemistry, peroxides are a group of Chemical compound, compounds with the structure , where the R's represent a radical (a portion of a complete molecule; not necessarily a free radical) and O's are single oxygen atoms. Oxygen atoms are joined ...
s, superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of t ...
s and suboxides. However, the first three are more common. The table below["Inorganic Chemistry" by Gary L. Miessler and Donald A. Tar, 6th edition, Pearson] shows the types of compounds formed in reaction with oxygen. The compound in brackets represents the minor product of combustion.
The alkali metal peroxides are ionic compounds that are unstable in water. The peroxide anion is weakly bound to the cation, and it is hydrolysed, forming stronger covalent bonds.
:Na2O2 + 2H2O → 2NaOH + H2O2
The other oxygen compounds are also unstable in water.
:2KO2 + 2H2O → 2KOH + H2O2 + O2
:Li2O + H2O → 2LiOH
Reaction with sulfur
With sulfur, they form sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
s and polysulfide
Polysulfides are a class of chemical compounds derived from anionic chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. The inorganic polysulfides have the general formula . These anions are the conjugate bas ...
s.
:2Na + 1/8S8 → Na2S + 1/8S8 → Na2S2...Na2S7
Because alkali metal sulfides are essentially salts of a weak acid and a strong base, they form basic solutions.
:S2- + H2O → HS− + HO−
:HS− + H2O → H2S + HO−
Reaction with nitrogen
Lithium is the only metal that combines directly with nitrogen at room temperature.
:3Li + 1/2N2 → Li3N
Li3N can react with water to liberate ammonia.
:Li3N + 3H2O → 3LiOH + NH3
Reaction with hydrogen
With hydrogen, alkali metals form saline hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
s that hydrolyse in water.
:2 Na \ + H2 \ -> ce\ 2 NaH
:2 NaH \ + \ 2 H2O \ \longrightarrow \ 2 NaOH \ + \ H2 \uparrow
Reaction with carbon
Lithium is the only metal that reacts directly with carbon to give dilithium acetylide. Na and K can react with acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is u ...
to give acetylides.
:2 Li \ + \ 2 C \ \longrightarrow \ Li2C2
:2 Na \ + \ 2 C2H2 \ -> ce\ 2 NaC2H \ + \ H2
:2 Na \ + \ 2 NaC2H \ -> ce\ 2 Na2C2 \ + \ H2
Reaction with water
On reaction with water, they generate hydroxide ions and hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
gas. This reaction is vigorous and highly exothermic and the hydrogen resulted may ignite in air or even explode in the case of Rb and Cs.
Reaction with other salts
The alkali metals are very good reducing agents. They can reduce metal cations that are less electropositive. Titanium
Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in ...
is produced industrially by the reduction of titanium tetrachloride with Na at 400 °C ( van Arkel–de Boer process).
:TiCl4 + 4Na → 4NaCl + Ti
Reaction with organohalide compounds
Alkali metals react with halogen derivatives to generate hydrocarbon via the Wurtz reaction.
:2CH3-Cl + 2Na → H3C-CH3 + 2NaCl
Alkali metals in liquid ammonia
Alkali metals dissolve in liquid ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
or other donor solvents like aliphatic amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
s or hexamethylphosphoramide to give blue solutions. These solutions are believed to contain free electrons. Reaction 1) is known as Birch reduction.
Other reductions
Reaction 1) is known as Birch reduction.
Other reductions
Extensions
 Although francium is the heaviest alkali metal that has been discovered, there has been some theoretical work predicting the physical and chemical characteristics of hypothetical heavier alkali metals. Being the first period 8 element, the undiscovered element
Although francium is the heaviest alkali metal that has been discovered, there has been some theoretical work predicting the physical and chemical characteristics of hypothetical heavier alkali metals. Being the first period 8 element, the undiscovered element ununennium
Ununennium, also known as eka-francium or element 119, is a hypothetical chemical element; it has symbol Uue and atomic number 119. ''Ununennium'' and ''Uue'' are the temporary systematic element name, systematic IUPAC name and symbol respectivel ...
(element 119) is predicted to be the next alkali metal after francium and behave much like their lighter congeners; however, it is also predicted to differ from the lighter alkali metals in some properties.periodic trends
In chemistry, periodic trends are specific patterns present in the periodic table that illustrate different aspects of certain Chemical element, elements when grouped by period (periodic table), period and/or Group (periodic table), group. They w ...
, ignoring relativistic effects would predict ununennium to be even more reactive than caesium and francium. This lowered reactivity is due to the relativistic stabilisation of ununennium's valence electron, increasing ununennium's first ionisation energy and decreasing the metallic and ionic radii;electron affinity
The electron affinity (''E''ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.
::X(g) + e− → X−(g) + energy
This differs by si ...
far beyond that of caesium and francium; indeed, ununennium is expected to have an electron affinity higher than all the alkali metals lighter than it. Relativistic effects also cause a very large drop in the polarisability of ununennium. The stabilisation of ununennium's valence electron and thus the contraction of the 8s orbital cause its atomic radius to be lowered to 240 pm,
The stabilisation of ununennium's valence electron and thus the contraction of the 8s orbital cause its atomic radius to be lowered to 240 pm,oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
s, which are not seen in any other alkali metal,covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
character, due to the involvement of the 7p3/2 electrons in the bonding. Not as much work has been done predicting the properties of the alkali metals beyond ununennium. Although a simple extrapolation of the periodic table (by the
Not as much work has been done predicting the properties of the alkali metals beyond ununennium. Although a simple extrapolation of the periodic table (by the Aufbau principle
In atomic physics and quantum chemistry, the Aufbau principle (, from ), also called the Aufbau rule, states that in the ground state of an atom or ion, electrons first fill Electron shell#Subshells, subshells of the lowest available energy, the ...
) would put element 169, unhexennium, under ununennium, Dirac-Fock calculations predict that the next element after ununennium with alkali-metal-like properties may be element 165, unhexpentium, which is predicted to have the electron configuration g5g18 6f14 7d10 8s2 8p1/22 9s1.group 11 element
Group 11, by modern IUPAC numbering, is a group (periodic table), group of chemical elements in the periodic table, consisting of copper (Cu), silver (Ag), gold (Au), and roentgenium (Rg), although no chemical experiments have yet been carried ...
, and while its physical and atomic properties would be closer to the former, its chemistry may be closer to that of the latter. Further calculations show that unhexpentium would follow the trend of increasing ionisation energy beyond caesium, having an ionisation energy comparable to that of sodium, and that it should also continue the trend of decreasing atomic radii beyond caesium, having an atomic radius comparable to that of potassium.alkaline earth metal
The alkaline earth metals are six chemical elements in group (periodic table), group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar p ...
s both being s-block elements, these predictions for the trends and properties of ununennium and unhexpentium also mostly hold quite similarly for the corresponding alkaline earth metals unbinilium (Ubn) and unhexhexium (Uhh).
Pseudo-alkali metals
Many other substances are similar to the alkali metals in their tendency to form monopositive cations. Analogously to the pseudohalogen
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorgani ...
s, they have sometimes been called "pseudo-alkali metals". These substances include some elements and many more polyatomic ion
A polyatomic ion (also known as a molecular ion) is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that usually has a net charge that is not zero, or in special c ...
s; the polyatomic ions are especially similar to the alkali metals in their large size and weak polarising power.
Hydrogen
The element hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
, with one electron per neutral atom, is usually placed at the top of Group 1 of the periodic table because of its electron configuration. But hydrogen is not normally considered to be an alkali metal.diatomic
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear mol ...
gas consisting of two atoms per molecule (H2); however, the alkali metals form diatomic molecules (such as dilithium, Li2) only at high temperatures, when they are in the gaseous state.
Hydrogen, like the alkali metals, has one valence electron[ and reacts easily with the halogens,][ but the similarities mostly end there because of the small size of a bare proton H+ compared to the alkali metal cations.][ Its placement above lithium is primarily due to its electron configuration.]fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
due to their similar chemical properties, though the resemblance is likewise not absolute.[Huheey, J.E.; Keiter, E.A. and Keiter, R.L. (1993) ''Inorganic Chemistry: Principles of Structure and Reactivity'', 4th edition, HarperCollins, New York, USA.][James, A.M. and Lord, M.P. (1992) ''Macmillan's Chemical and Physical Data'', Macmillan, London, UK.] As only one additional electron is required to fill in the outermost shell of the hydrogen atom, hydrogen often behaves like a halogen, forming the negative hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
ion, and is very occasionally considered to be a halogen on that basis. (The alkali metals can also form negative ions, known as alkalides, but these are little more than laboratory curiosities, being unstable.)pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and eve ...
s, such as those found at the cores of Jupiter
Jupiter is the fifth planet from the Sun and the List of Solar System objects by size, largest in the Solar System. It is a gas giant with a Jupiter mass, mass more than 2.5 times that of all the other planets in the Solar System combined a ...
and Saturn
Saturn is the sixth planet from the Sun and the second largest in the Solar System, after Jupiter. It is a gas giant, with an average radius of about 9 times that of Earth. It has an eighth the average density of Earth, but is over 95 tim ...
, hydrogen does become metallic and behaves like an alkali metal; in this phase, it is known as metallic hydrogen. The electrical resistivity
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by ...
of liquid metallic hydrogen at 3000 K is approximately equal to that of liquid rubidium and caesium
Caesium (IUPAC spelling; also spelled cesium in American English) is a chemical element; it has Symbol (chemistry), symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only f ...
at 2000 K at the respective pressures when they undergo a nonmetal-to-metal transition.
The 1s1 electron configuration of hydrogen, while analogous to that of the alkali metals (ns1), is unique because there is no 1p subshell. Hence it can lose an electron to form the hydron H+, or gain one to form the hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
ion H−.electron affinity
The electron affinity (''E''ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.
::X(g) + e− → X−(g) + energy
This differs by si ...
, but hydrogen cannot be tetravalent. Thus none of the three placements are entirely satisfactory, although group 1 is the most common placement (if one is chosen) because of the electron configuration and the fact that the hydron is by far the most important of all monatomic hydrogen species, being the foundation of acid-base chemistry.hydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
s, which are an effect of charge-transfer, electrostatic, and electron correlative contributing phenomena.
Ammonium and derivatives
 The
The ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleu ...
ion () has very similar properties to the heavier alkali metals, acting as an alkali metal intermediate between potassium and rubidium,Uranus
Uranus is the seventh planet from the Sun. It is a gaseous cyan-coloured ice giant. Most of the planet is made of water, ammonia, and methane in a Supercritical fluid, supercritical phase of matter, which astronomy calls "ice" or Volatile ( ...
and Neptune
Neptune is the eighth and farthest known planet from the Sun. It is the List of Solar System objects by size, fourth-largest planet in the Solar System by diameter, the third-most-massive planet, and the densest giant planet. It is 17 t ...
, which may have significant impacts on their interior magnetic fields.ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
and dihydrogen molecules to metallic ammonium may occur at pressures just below 25 GPa.pnictogen
, -
! colspan=2 style="text-align:left;" , ↓ Period
, -
! 2
,
, -
! 3
,
, -
! 4
,
, -
! 5
,
, -
! 6
,
, -
! 7
,
, -
, colspan="2",
----
''Legend''
A pnictogen ( or ; from "to choke" and -gen, "generator") is any ...
s), creating a phosphonium () or arsonium () cation that can itself be substituted similarly; while stibonium () itself is not known, some of its organic derivatives are characterised.
Cobaltocene and derivatives
Cobaltocene, Co(C5H5)2, is a metallocene, the cobalt
Cobalt is a chemical element; it has Symbol (chemistry), symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. ...
analogue of ferrocene
Ferrocene is an organometallic chemistry, organometallic compound with the formula . The molecule is a Cyclopentadienyl complex, complex consisting of two Cyclopentadienyl anion, cyclopentadienyl rings sandwiching a central iron atom. It is an o ...
. It is a dark purple solid. Cobaltocene has 19 valence electrons, one more than usually found in organotransition metal complexes, such as its very stable relative, ferrocene, in accordance with the 18-electron rule. This additional electron occupies an orbital that is antibonding with respect to the Co–C bonds. Consequently, many chemical reactions of Co(C5H5)2 are characterized by its tendency to lose this "extra" electron, yielding a very stable 18-electron cation known as cobaltocenium. Many cobaltocenium salts coprecipitate with caesium salts, and cobaltocenium hydroxide is a strong base that absorbs atmospheric carbon dioxide to form cobaltocenium carbonate.
Thallium

Thallium
Thallium is a chemical element; it has Symbol (chemistry), symbol Tl and atomic number 81. It is a silvery-white post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Che ...
is the heaviest stable element in group 13 of the periodic table. At the bottom of the periodic table, the inert-pair effect is quite strong, because of the relativistic stabilisation of the 6s orbital and the decreasing bond energy as the atoms increase in size so that the amount of energy released in forming two more bonds is not worth the high ionisation energies of the 6s electrons.oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
closely resemble the corresponding potassium or silver compounds stoichiometrically due to the similar ionic radii of the Tl+ (164 pm), K+ (152 pm) and Ag+ (129 pm) ions.continental Europe
Continental Europe or mainland Europe is the contiguous mainland of Europe, excluding its surrounding islands. It can also be referred to ambiguously as the European continent, – which can conversely mean the whole of Europe – and, by som ...
(but not in England) in the years immediately following its discovery,Dmitri Mendeleev
Dmitri Ivanovich Mendeleev ( ; ) was a Russian chemist known for formulating the periodic law and creating a version of the periodic table of elements. He used the periodic law not only to correct the then-accepted properties of some known ele ...
's 1869 periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
and Julius Lothar Meyer's 1868 periodic table.boron group
The boron group are the chemical elements in periodic table group, group 13 of the periodic table, consisting of boron (B), aluminium (Al), gallium (Ga), indium (In), thallium (Tl) and nihonium (Nh). This group lies in the p-block of the perio ...
and left the space below caesium blank.[.] While Tl+ is stabilised by the inert-pair effect, this inert pair of 6s electrons is still able to participate chemically, so that these electrons are stereochemically active in aqueous solution. Additionally, the thallium halides (except TlF) are quite insoluble in water, and TlI has an unusual structure because of the presence of the stereochemically active inert pair in thallium.
Copper, silver, and gold
The group 11 metals (or coinage metals), copper, silver, and gold, are typically categorised as transition metals given they can form ions with incomplete d-shells. Physically, they have the relatively low melting points and high electronegativity values associated with post-transition metal
The metallic elements in the periodic table located between the transition metals to their left and the chemically weak nonmetallic metalloids to their right have received many names in the literature, such as post-transition metals, poor metal ...
s. "The filled ''d'' subshell and free ''s'' electron of Cu, Ag, and Au contribute to their high electrical and thermal conductivity. Transition metals to the left of group 11 experience interactions between ''s'' electrons and the partially filled ''d'' subshell that lower electron mobility." Chemically, the group 11 metals behave like main-group metals in their +1 valence states, and are hence somewhat related to the alkali metals: this is one reason for their previously being labelled as "group IB", paralleling the alkali metals' "group IA". They are occasionally classified as post-transition metals. Their spectra are analogous to those of the alkali metals.ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
, as well as having more covalent character in their compounds.transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
character.pseudohalogen
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorgani ...
because its 5d106s1 configuration has one electron less than the quasi-closed shell 5d106s2 configuration of mercury.
Production and isolation
The production of pure alkali metals is somewhat complicated due to their extreme reactivity with commonly used substances, such as water.silicate
A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used ...
ores, all the stable alkali metals may be obtained the same way: sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
is first used to dissolve the desired alkali metal ion and aluminium(III) ions from the ore (leaching), whereupon basic precipitation removes aluminium ions from the mixture by precipitating it as the hydroxide. The remaining insoluble alkali metal carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
is then precipitated selectively; the salt is then dissolved in hydrochloric acid to produce the chloride. The result is then left to evaporate and the alkali metal can then be isolated.magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
or calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
for the heaviest alkali metals) is used to reduce the alkali metal chloride. The liquid or gaseous product (the alkali metal) then undergoes fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation ...
for purification.pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology
The word ''pyrolysis'' is coined from the Gree ...
of the corresponding alkali metal azide, which yields the metal for sodium, potassium, rubidium, and caesium and the nitride for lithium.[
Lithium salts have to be extracted from the water of mineral springs, brine pools, and brine deposits. The metal is produced electrolytically from a mixture of fused lithium chloride and potassium chloride.]seabed
The seabed (also known as the seafloor, sea floor, ocean floor, and ocean bottom) is the bottom of the ocean. All floors of the ocean are known as seabeds.
The structure of the seabed of the global ocean is governed by plate tectonics. Most of ...
,electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of c ...
of sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
by lowering the melting point of the substance to below 700 °C through the use of a Downs cell.potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which utili ...
, found extensively in places such as Canada, Russia, Belarus, Germany, Israel, United States, and Jordan, in a method similar to how sodium was produced in the late 1800s and early 1900s.seawater
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximat ...
.fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation ...
technique. Because metallic caesium is too reactive to handle, it is normally offered as caesium azide (CsN3). Caesium hydroxide is formed when caesium interacts aggressively with water and ice (CsOH).
Rubidium is the 16th most abundant element in the earth's crust; however, it is quite rare. Some minerals found in North America, South Africa, Russia, and Canada contain rubidium. Some potassium minerals ( lepidolites, biotites, feldspar
Feldspar ( ; sometimes spelled felspar) is a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the ''plagiocl ...
, carnallite
Carnallite (also carnalite) is an evaporite mineral, a hydrated potassium magnesium chloride with formula KCl.MgCl2·6(H2O). It is variably colored yellow to white, reddish, and sometimes colorless or blue. It is usually massive to fibrous with r ...
) contain it, together with caesium. Pollucite, carnallite
Carnallite (also carnalite) is an evaporite mineral, a hydrated potassium magnesium chloride with formula KCl.MgCl2·6(H2O). It is variably colored yellow to white, reddish, and sometimes colorless or blue. It is usually massive to fibrous with r ...
, leucite, and lepidolite are all minerals that contain rubidium. As a by-product of lithium extraction, it is commercially obtained from lepidolite. Rubidium is also found in potassium rocks and brines, which is a commercial supply. The majority of rubidium is now obtained as a byproduct of refining lithium. Rubidium is used in vacuum tube
A vacuum tube, electron tube, thermionic valve (British usage), or tube (North America) is a device that controls electric current flow in a high vacuum between electrodes to which an electric voltage, potential difference has been applied. It ...
s as a getter, a material that combines with and removes trace gases from vacuum tubes. For several years in the 1950s and 1960s, a by-product of the potassium production called Alkarb was a main source for rubidium. Alkarb contained 21% rubidium while the rest was potassium and a small fraction of caesium. Today the largest producers of caesium, for example the Tanco Mine in Manitoba, Canada, produce rubidium as by-product from pollucite.
For several years in the 1950s and 1960s, a by-product of the potassium production called Alkarb was a main source for rubidium. Alkarb contained 21% rubidium while the rest was potassium and a small fraction of caesium. Today the largest producers of caesium, for example the Tanco Mine in Manitoba, Canada, produce rubidium as by-product from pollucite.alum
An alum () is a type of chemical compound, usually a hydrated double salt, double sulfate salt (chemistry), salt of aluminium with the general chemical formula, formula , such that is a valence (chemistry), monovalent cation such as potassium ...
( Cs, Rb) Al( SO4)2·12 H2O, which yields pure rubidium alum after approximately 30 recrystallisations.tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1,000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton in the United States to distinguish it from the non-metric units of the s ...
s per year.calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
metal at 750 °C and low pressure.
Applications
Lithium, sodium, and potassium have many useful applications, while rubidium and caesium are very notable in academic contexts but do not have many applications yet.carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
in space capsules and submarines.fatty acid
In chemistry, in particular in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated and unsaturated compounds#Organic chemistry, saturated or unsaturated. Most naturally occurring fatty acids have an ...
s are used as soap. Pure sodium metal also has many applications, including use in sodium-vapour lamps, which produce very efficient light compared to other types of lighting,titanium
Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in ...
and zirconium, from their chlorides. Furthermore, it is very useful as a heat-exchange liquid in fast breeder nuclear reactors due to its low melting point, viscosity, and cross-section towards neutron absorption.fertiliser
A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrition, plant nutrients. Fertilizers may be distinct from Liming (soil), liming materials or other non- ...
sPotassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which utili ...
is a very strong base, and is used to control the pH of various substances. Potassium nitrate and potassium permanganate are often used as powerful oxidising agents.atomic clock
An atomic clock is a clock that measures time by monitoring the resonant frequency of atoms. It is based on atoms having different energy levels. Electron states in an atom are associated with different energy levels, and in transitions betwee ...
s.spectroscopy
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.
Spectro ...
experiments, leading to more information regarding energy level
A quantum mechanics, quantum mechanical system or particle that is bound state, bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical mechanics, classical pa ...
s and the coupling constants of the weak interaction
In nuclear physics and particle physics, the weak interaction, weak force or the weak nuclear force, is one of the four known fundamental interactions, with the others being electromagnetism, the strong interaction, and gravitation. It is th ...
. Studies on the light emitted by laser-trapped francium-210 ions have provided accurate data on transitions between atomic energy levels, similar to those predicted by quantum theory.
Biological role and precautions
Metals
Pure alkali metals are dangerously reactive with air and water and must be kept away from heat, fire, oxidising agents, acids, most organic compounds, halocarbons, plastics, and moisture. They also react with carbon dioxide and carbon tetrachloride, so that normal fire extinguishers are counterproductive when used on alkali metal fires.isopropanol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable, organic compound with a pungent alcoholic odor.
Isopropyl alcohol, an organic polar molecule, is miscible in water, ethanol, an ...
.mineral oil
Mineral oil is any of various colorless, odorless, light mixtures of higher alkanes from a mineral source, particularly a distillate of petroleum, as distinct from usually edible vegetable oils.
The name 'mineral oil' by itself is imprecise, ...
or an inert atmosphere. The inert atmosphere used may be argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
or nitrogen gas, except for lithium, which reacts with nitrogen.
Ions
 The bioinorganic chemistry of the alkali metal ions has been extensively reviewed.
Solid state crystal structures have been determined for many complexes of alkali metal ions in small peptides, nucleic acid constituents, carbohydrates and ionophore complexes.
Lithium naturally only occurs in traces in biological systems and has no known biological role, but does have effects on the body when ingested.
The bioinorganic chemistry of the alkali metal ions has been extensively reviewed.
Solid state crystal structures have been determined for many complexes of alkali metal ions in small peptides, nucleic acid constituents, carbohydrates and ionophore complexes.
Lithium naturally only occurs in traces in biological systems and has no known biological role, but does have effects on the body when ingested.psychiatry
Psychiatry is the medical specialty devoted to the diagnosis, treatment, and prevention of deleterious mental disorder, mental conditions. These include matters related to cognition, perceptions, Mood (psychology), mood, emotion, and behavior.
...
to treat bipolar disorder
Bipolar disorder (BD), previously known as manic depression, is a mental disorder characterized by periods of Depression (mood), depression and periods of abnormally elevated Mood (psychology), mood that each last from days to weeks, and in ...
( manic-depression) in daily doses of about 0.5 to 2 grams, although there are side-effects.poison
A poison is any chemical substance that is harmful or lethal to living organisms. The term is used in a wide range of scientific fields and industries, where it is often specifically defined. It may also be applied colloquially or figurati ...
s the central nervous system
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain, spinal cord and retina. The CNS is so named because the brain integrates the received information and coordinates and influences the activity o ...
,electrolytes
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble salts, acids, and bases, dissolved in a polar solvent like water. Upon dissolving, t ...
inside and outside cells.Sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
(also known as common salt) is the principal source of sodium in the diet, and is used as seasoning and preservative, such as for pickling and jerky; most of it comes from processed foods. The Dietary Reference Intake for sodium is 1.5 grams per day, but most people in the United States consume more than 2.3 grams per day, the minimum amount that promotes hypertension; this in turn causes 7.6 million premature deaths worldwide.
Potassium is the major cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
(positive ion) inside animal cells,concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', '' number concentration'', ...
differences of these charged particles causes a difference in electric potential
Electric potential (also called the ''electric field potential'', potential drop, the electrostatic potential) is defined as electric potential energy per unit of electric charge. More precisely, electric potential is the amount of work (physic ...
between the inside and outside of cells, known as the membrane potential
Membrane potential (also transmembrane potential or membrane voltage) is the difference in electric potential between the interior and the exterior of a biological cell. It equals the interior potential minus the exterior potential. This is th ...
. The balance between potassium and sodium is maintained by ion transporter proteins in the cell membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
.action potential
An action potential (also known as a nerve impulse or "spike" when in a neuron) is a series of quick changes in voltage across a cell membrane. An action potential occurs when the membrane potential of a specific Cell (biology), cell rapidly ri ...
—a "spike" of electrical discharge. The ability of cells to produce electrical discharge is critical for body functions such as neurotransmission, muscle contraction, and heart function.metabolism
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the co ...
,[ The median lethal dose (LD50) value for ]caesium chloride
Caesium chloride or cesium chloride is the inorganic compound with the formula Caesium, CsChloride, Cl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural ...
in mice is 2.3 g per kilogram, which is comparable to the LD50 values of potassium chloride and sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
. Caesium chloride has been promoted as an alternative cancer therapy, but has been linked to the deaths of over 50 patients, on whom it was used as part of a scientifically unvalidated cancer treatment.[Wood, Leonie. ]
Radioisotopes of caesium require special precautions: the improper handling of caesium-137 gamma ray
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of electromagnetic radiation arising from high energy interactions like the radioactive decay of atomic nuclei or astronomical events like solar flares. It consists o ...
sources can lead to release of this radioisotope and radiation injuries. Perhaps the best-known case is the Goiânia accident of 1987, in which an improperly-disposed-of radiation therapy system from an abandoned clinic in the city of Goiânia
Goiânia ( ; ) is the capital and largest city of the Brazilian federative units of Brazil, state of Goiás. With a population of 1,536,097, it is the second-largest city in the Central-West Region, Brazil, Central-West Region and the 10th-larges ...
, Brazil, was scavenged from a junkyard, and the glowing caesium salt sold to curious, uneducated buyers. This led to four deaths and serious injuries from radiation exposure. Together with caesium-134, iodine-131, and strontium-90
Strontium-90 () is a radioactive isotope of strontium produced by nuclear fission, with a half-life of 28.79 years. It undergoes β− decay into yttrium-90, with a decay energy of 0.546 MeV. Strontium-90 has applications in medicine a ...
, caesium-137 was among the isotopes distributed by the Chernobyl disaster
On 26 April 1986, the no. 4 reactor of the Chernobyl Nuclear Power Plant, located near Pripyat, Ukrainian Soviet Socialist Republic, Ukrainian SSR, Soviet Union (now Ukraine), exploded. With dozens of direct casualties, it is one of only ...
which constitute the greatest risk to health.
Notes
References
{{Authority control
A
Groups (periodic table)
Periodic table
Articles containing video clips
 Sodium compounds have been known since ancient times; salt (
Sodium compounds have been known since ancient times; salt ( Rubidium and caesium were the first elements to be discovered using the spectroscope, invented in 1859 by Robert Bunsen and
Rubidium and caesium were the first elements to be discovered using the spectroscope, invented in 1859 by Robert Bunsen and  After 1869,
After 1869,  The Earth formed from the same cloud of matter that formed the Sun, but the planets acquired different compositions during the formation and evolution of the Solar System. In turn, the natural history of the Earth caused parts of this planet to have differing concentrations of the elements. The mass of the Earth is approximately 5.98 kg. It is composed mostly of iron (32.1%),
The Earth formed from the same cloud of matter that formed the Sun, but the planets acquired different compositions during the formation and evolution of the Solar System. In turn, the natural history of the Earth caused parts of this planet to have differing concentrations of the elements. The mass of the Earth is approximately 5.98 kg. It is composed mostly of iron (32.1%),  All the alkali metals react vigorously or explosively with cold water, producing an
All the alkali metals react vigorously or explosively with cold water, producing an  The alkali metals form many intermetallic compounds with each other and the elements from groups 2 to 13 in the periodic table of varying stoichiometries, such as the sodium amalgams with mercury, including Na5Hg8 and Na3Hg. Some of these have ionic characteristics: taking the alloys with gold, the most electronegative of metals, as an example, NaAu and KAu are metallic, but RbAu and CsAu are semiconductors. NaK is an alloy of sodium and potassium that is very useful because it is liquid at room temperature, although precautions must be taken due to its extreme reactivity towards water and air. The eutectic mixture melts at −12.6 °C. An alloy of 41% caesium, 47% sodium, and 12% potassium has the lowest known melting point of any metal or alloy, −78 °C.
The alkali metals form many intermetallic compounds with each other and the elements from groups 2 to 13 in the periodic table of varying stoichiometries, such as the sodium amalgams with mercury, including Na5Hg8 and Na3Hg. Some of these have ionic characteristics: taking the alloys with gold, the most electronegative of metals, as an example, NaAu and KAu are metallic, but RbAu and CsAu are semiconductors. NaK is an alloy of sodium and potassium that is very useful because it is liquid at room temperature, although precautions must be taken due to its extreme reactivity towards water and air. The eutectic mixture melts at −12.6 °C. An alloy of 41% caesium, 47% sodium, and 12% potassium has the lowest known melting point of any metal or alloy, −78 °C.
 Lithium, the lightest of the alkali metals, is the only alkali metal which reacts with
Lithium, the lightest of the alkali metals, is the only alkali metal which reacts with  Being the smallest alkali metal, lithium forms the widest variety of and most stable organometallic compounds, which are bonded covalently. Organolithium compounds are electrically non-conducting volatile solids or liquids that melt at low temperatures, and tend to form oligomers with the structure (RLi)''x'' where R is the organic group. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful bases and nucleophiles. For use as bases, butyllithiums are often used and are commercially available. An example of an organolithium compound is
Being the smallest alkali metal, lithium forms the widest variety of and most stable organometallic compounds, which are bonded covalently. Organolithium compounds are electrically non-conducting volatile solids or liquids that melt at low temperatures, and tend to form oligomers with the structure (RLi)''x'' where R is the organic group. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful bases and nucleophiles. For use as bases, butyllithiums are often used and are commercially available. An example of an organolithium compound is  Reaction 1) is known as Birch reduction.
Other reductions that can be carried by these solutions are:
:S8 + 2e− → S82-
:Fe(CO)5 + 2e− → Fe(CO)42- + CO
Reaction 1) is known as Birch reduction.
Other reductions that can be carried by these solutions are:
:S8 + 2e− → S82-
:Fe(CO)5 + 2e− → Fe(CO)42- + CO
 The
The 
 For several years in the 1950s and 1960s, a by-product of the potassium production called Alkarb was a main source for rubidium. Alkarb contained 21% rubidium while the rest was potassium and a small fraction of caesium. Today the largest producers of caesium, for example the Tanco Mine in Manitoba, Canada, produce rubidium as by-product from pollucite. Today, a common method for separating rubidium from potassium and caesium is the fractional crystallisation of a rubidium and caesium
For several years in the 1950s and 1960s, a by-product of the potassium production called Alkarb was a main source for rubidium. Alkarb contained 21% rubidium while the rest was potassium and a small fraction of caesium. Today the largest producers of caesium, for example the Tanco Mine in Manitoba, Canada, produce rubidium as by-product from pollucite. Today, a common method for separating rubidium from potassium and caesium is the fractional crystallisation of a rubidium and caesium  The bioinorganic chemistry of the alkali metal ions has been extensively reviewed.
Solid state crystal structures have been determined for many complexes of alkali metal ions in small peptides, nucleic acid constituents, carbohydrates and ionophore complexes.
Lithium naturally only occurs in traces in biological systems and has no known biological role, but does have effects on the body when ingested. Lithium carbonate is used as a mood stabiliser in
The bioinorganic chemistry of the alkali metal ions has been extensively reviewed.
Solid state crystal structures have been determined for many complexes of alkali metal ions in small peptides, nucleic acid constituents, carbohydrates and ionophore complexes.
Lithium naturally only occurs in traces in biological systems and has no known biological role, but does have effects on the body when ingested. Lithium carbonate is used as a mood stabiliser in