|
Picometer
The picometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: pm) or picometer ( American spelling) is a unit of length in the International System of Units (SI), equal to , or one trillionth of a metre, which is the SI base unit of length. The picometre is one thousand femtometres, one thousandth of a nanometre ( nm), one millionth of a micrometre (also known as a micron), one billionth of a millimetre, and one trillionth of a metre. The symbol μμ was once used for it. It is also one hundredth of an ångström, an internationally known (but non-SI) unit of length. Use The picometre's length is of an order so small that its application is almost entirely confined to particle physics, quantum physics, chemistry, and acoustics. Atoms are between 62 and 520 pm in diameter, and the typical length of a carbon–carbon single bond is 154 pm. Smaller units still may be used to describe smaller particles (some of which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between atoms of fixed types, relatively independent of the rest of the molecule. Explanation Bond length is related to bond order: when more electrons participate in bond formation the bond is shorter. Bond length is also inversely related to bond strength and the bond dissociation energy: all other factors being equal, a stronger bond will be shorter. In a bond between two identical atoms, half the bond distance is equal to the covalent radius. Bond lengths are measured in the solid phase by means of X-ray diffraction, or approximated in the gas phase by microwave spectroscopy. A bond between a given pair of atoms may vary between different molecules. For example, the carbon to hydrogen bonds in methane are different from those in methyl chlori ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unit Of Length
A unit of length refers to any arbitrarily chosen and accepted reference standard for measurement of length. The most common units in modern use are the metric units, used in every country globally. In the United States the U.S. customary units are also in use. British Imperial units are still used for some purposes in the United Kingdom and some other countries. The metric system is sub-divided into SI and non-SI units. History Metric system SI The base unit in the International System of Units (SI) is the meter, defined as "the length of the path travelled by light in vacuum during a time interval of seconds." It is approximately equal to . Other SI units are derived from the meter by adding prefixes, as in millimeter or kilometer, thus producing systematic decimal multiples and submultiples of the base unit that span many orders of magnitude. For example, a kilometer is . Non-SI In the centimeter–gram–second system of units, the basic unit of length is th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. Atoms with the same number of protons but a different number of neutrons are called isotopes of the same element. Atoms are extremely small, typically around 100 picometers across. A human hair is about a million carbon atoms wide. Atoms are smaller than the shortest wavelength of visible light, which means humans cannot see atoms with conventional microscopes. They are so small that accurately predicting their behavior using classical physics is not possible due to quantum mechanics, quantum effects. More than 99.94% of an atom's mass is in the nucleus. Protons hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanometre
330px, Different lengths as in respect to the Molecule">molecular scale. The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm), or nanometer (American spelling), is a unit of length in the International System of Units (SI), equal to one billionth ( short scale) or one thousand million (long scale) of a meter (0.000000001 m) and to 1000 picometres. One nanometre can be expressed in scientific notation as 1 × 10−9 m and as m. History The nanometre was formerly known as the "''millimicrometre''" – or, more commonly, the "''millimicron''" for short – since it is of a micrometer. It was often denoted by the symbol ''mμ'' or, more rarely, as ''μμ'' (however, ''μμ'' should refer to a ''millionth'' of a micron). Etymology The name combines the SI prefix '' nano-'' (from the Ancient Greek , ', "dwarf") with the parent unit name ''metre'' (from Greek , ', "unit of measurement"). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gravitational Wave
Gravitational waves are oscillations of the gravitational field that Wave propagation, travel through space at the speed of light; they are generated by the relative motion of gravity, gravitating masses. They were proposed by Oliver Heaviside in 1893 and then later by Henri Poincaré in 1905 as the gravitational equivalent of Electromagnetic radiation, electromagnetic waves. In 1916, Albert Einstein demonstrated that gravitational waves result from his general theory of relativity as ripples in spacetime. Gravitational waves transport energy as gravitational radiation, a form of radiant energy similar to electromagnetic radiation. Newton's law of universal gravitation, part of classical mechanics, does not provide for their existence, instead asserting that gravity has instantaneous effect everywhere. Gravitational waves therefore stand as an important relativistic phenomenon that is absent from Newtonian physics. Gravitational-wave astronomy has the advantage that, unlike elec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
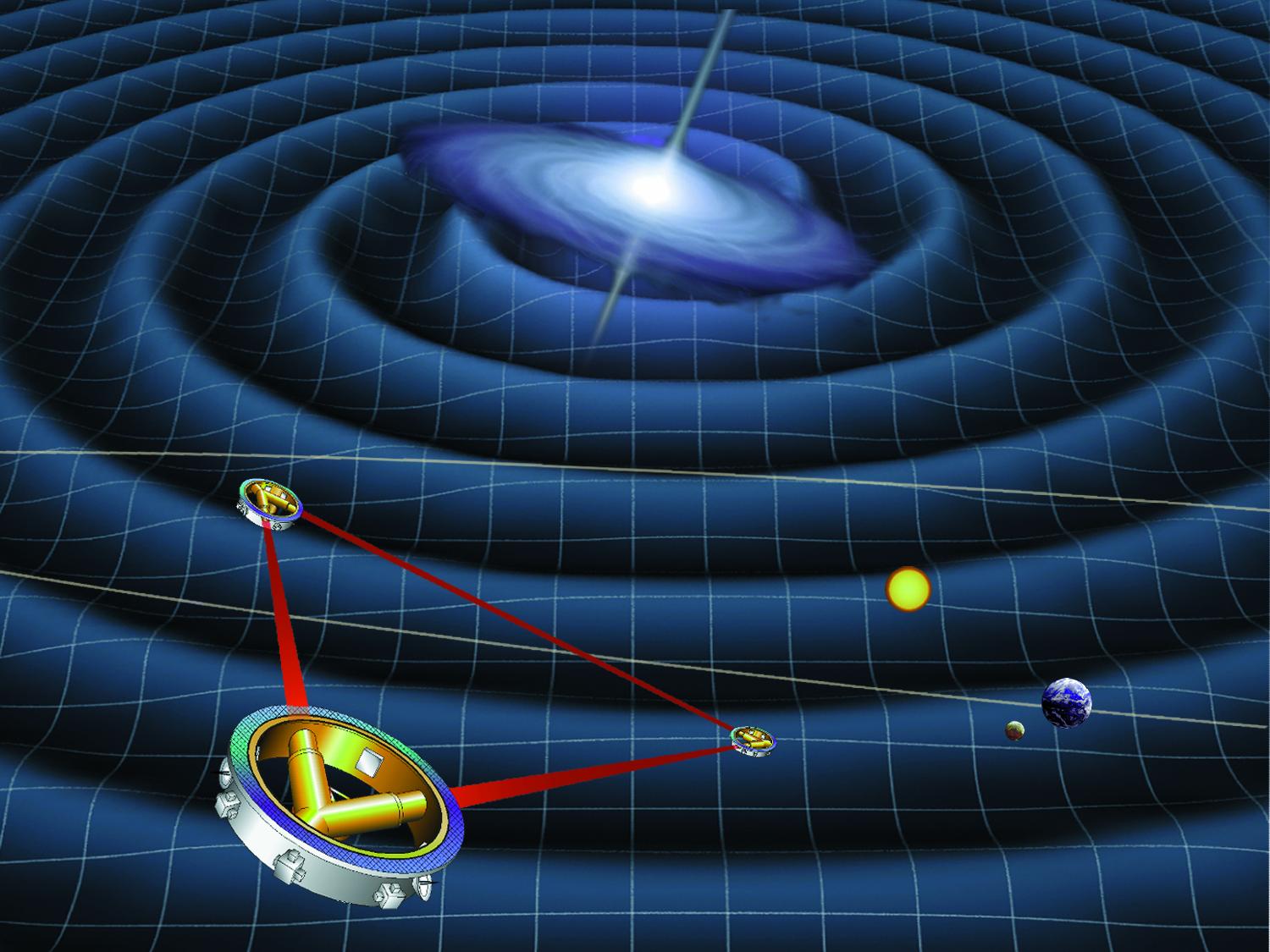
Laser Interferometer Space Antenna
The Laser Interferometer Space Antenna (LISA) is a planned space probe to detect and measure gravitational waves—tiny ripples in the fabric of spacetime—from astronomical sources. LISA will be the first dedicated space-based gravitational-wave observatory. It aims to measure gravitational waves directly by using laser interferometry. The LISA concept features three spacecraft arranged in an equilateral triangle with each side 2.5 million kilometers long, flying in an Earth-like heliocentric orbit. The distance between the satellites is precisely monitored to detect a passing gravitational wave. The LISA project started out as a joint effort between NASA and the European Space Agency (ESA). However, in 2011, NASA announced that it would be unable to continue its original LISA partnership with the European Space Agency due to funding limitations. In response, ESA continued developing the mission and in 2017, NASA re-engaged with LISA, contributing technology and scientific e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermion
In particle physics, a fermion is a subatomic particle that follows Fermi–Dirac statistics. Fermions have a half-integer spin (spin 1/2, spin , Spin (physics)#Higher spins, spin , etc.) and obey the Pauli exclusion principle. These particles include all quarks and leptons and all composite particles made of an even and odd, odd number of these, such as all baryons and many atoms and atomic nucleus, nuclei. Fermions differ from bosons, which obey Bose–Einstein statistics. Some fermions are elementary particles (such as electrons), and some are composite particles (such as protons). For example, according to the spin-statistics theorem in Theory of relativity, relativistic quantum field theory, particles with integer Spin (physics), spin are bosons. In contrast, particles with half-integer spin are fermions. In addition to the spin characteristic, fermions have another specific property: they possess conserved baryon or lepton quantum numbers. Therefore, what is usually referr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hadron
In particle physics, a hadron is a composite subatomic particle made of two or more quarks held together by the strong nuclear force. Pronounced , the name is derived . They are analogous to molecules, which are held together by the electric force. Most of the mass of ordinary matter comes from two hadrons: the proton and the neutron, while most of the mass of the protons and neutrons is in turn due to the binding energy of their constituent quarks, due to the strong force. Hadrons are categorized into two broad families: baryons, made of an odd number of quarks (usually three) and mesons, made of an even number of quarks (usually two: one quark and one antiquark). Protons and neutrons (which make the majority of the mass of an atom) are examples of baryons; pions are an example of a meson. A tetraquark state (an exotic meson), named the Z(4430), was discovered in 2007 by the Belle Collaboration and confirmed as a resonance in 2014 by the LHCb collaboration. Two pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalent Bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term "covalence" was introduced by Irving Langmuir in 1919, with Nevil Sidgwick using "co-valent link" in the 1920s. Merriam-Webster dates the specific phrase ''covalent bond'' to 1939, recognizing its first known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acoustics
Acoustics is a branch of physics that deals with the study of mechanical waves in gases, liquids, and solids including topics such as vibration, sound, ultrasound and infrasound. A scientist who works in the field of acoustics is an acoustician while someone working in the field of acoustics technology may be called an Acoustical engineering, acoustical engineer. The application of acoustics is present in almost all aspects of modern society with the most obvious being the audio and noise control industries. Hearing (sense), Hearing is one of the most crucial means of survival in the animal world and speech is one of the most distinctive characteristics of human development and culture. Accordingly, the science of acoustics spreads across many facets of human society—music, medicine, architecture, industrial production, warfare and more. Likewise, animal species such as songbirds and frogs use sound and hearing as a key element of mating rituals or for marking territories. Art, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Physics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is the foundation of all quantum physics, which includes quantum chemistry, quantum field theory, quantum technology, and quantum information science. Quantum mechanics can describe many systems that classical physics cannot. Classical physics can describe many aspects of nature at an ordinary (macroscopic and Microscopic scale, (optical) microscopic) scale, but is not sufficient for describing them at very small submicroscopic (atomic and subatomic) scales. Classical mechanics can be derived from quantum mechanics as an approximation that is valid at ordinary scales. Quantum systems have Bound state, bound states that are Quantization (physics), quantized to Discrete mathematics, discrete values of energy, momentum, angular momentum, and ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Particle Physics
Particle physics or high-energy physics is the study of Elementary particle, fundamental particles and fundamental interaction, forces that constitute matter and radiation. The field also studies combinations of elementary particles up to the scale of protons and neutrons, while the study of combinations of protons and neutrons is called nuclear physics. The fundamental particles in the universe are classified in the Standard Model as fermions (matter particles) and bosons (force-carrying particles). There are three Generation (particle physics), generations of fermions, although ordinary matter is made only from the first fermion generation. The first generation consists of Up quark, up and down quarks which form protons and neutrons, and electrons and electron neutrinos. The three fundamental interactions known to be mediated by bosons are electromagnetism, the weak interaction, and the strong interaction. Quark, Quarks cannot exist on their own but form hadrons. Hadrons that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |