Fluorine on:
[Wikipedia]
[Google]
[Amazon]
Fluorine is a
 The bond energy of
The bond energy of
 At room temperature, fluorine is a gas of diatomic molecules, pale yellow when pure (sometimes described as yellow-green). It has a characteristic halogen-like pungent and biting odor detectable at 20 ppb. Fluorine condenses into a bright yellow liquid at −188 °C (−306 °F), a transition temperature similar to those of oxygen and nitrogen.
Fluorine has two solid forms, α- and β-fluorine. The latter crystallizes at −220 °C (−364 °F) and is transparent and soft, with the same disordered cubic structure of freshly crystallized solid oxygen, unlike the orthorhombic systems of other solid halogens. Further cooling to −228 °C (−378 °F) induces a
At room temperature, fluorine is a gas of diatomic molecules, pale yellow when pure (sometimes described as yellow-green). It has a characteristic halogen-like pungent and biting odor detectable at 20 ppb. Fluorine condenses into a bright yellow liquid at −188 °C (−306 °F), a transition temperature similar to those of oxygen and nitrogen.
Fluorine has two solid forms, α- and β-fluorine. The latter crystallizes at −220 °C (−364 °F) and is transparent and soft, with the same disordered cubic structure of freshly crystallized solid oxygen, unlike the orthorhombic systems of other solid halogens. Further cooling to −228 °C (−378 °F) induces a
, the Scientific Committee on Problems of the Environment (SCOPE), 1993. See table 1.9 in Section 1.4.5.2. Other radioisotopes have half-lives less than 70 seconds; most decay in less than half a second.. The isotopes and undergo β+ decay and
File:Fluorite-270246.jpg, Fluorite: Pink globular mass with crystal facets
File:Apatite Canada.jpg, Fluorapatite: Long prism-like crystal, without luster, at an angle coming out of aggregate-like rock
File:Ivigtut cryolite edit.jpg, Cryolite: A parallelogram-shaped outline with diatomic molecules arranged in two layers
Other minerals such as topaz contain fluorine. Fluorides, unlike other halides, are insoluble and do not occur in commercially favorable concentrations in saline waters. Trace quantities of organofluorines of uncertain origin have been detected in volcanic eruptions and geothermal springs. The existence of gaseous fluorine in crystals, suggested by the smell of crushed
 In 1529, Georgius Agricola described fluorite as an additive used to lower the melting point of metals during
In 1529, Georgius Agricola described fluorite as an additive used to lower the melting point of metals during
 The Frigidaire division of
The Frigidaire division of
 Hydrogen and fluorine combine to yield hydrogen fluoride, in which discrete molecules form clusters by hydrogen bonding, resembling water more than
Hydrogen and fluorine combine to yield hydrogen fluoride, in which discrete molecules form clusters by hydrogen bonding, resembling water more than
 Binary fluorides of metalloids and p-block nonmetals are generally covalent and volatile, with varying reactivities. Period 3 and heavier nonmetals can form hypervalent fluorides..
Boron trifluoride is planar and possesses an incomplete octet. It functions as a Lewis acid and combines with Lewis bases like ammonia to form adducts. Carbon tetrafluoride is tetrahedral and inert; its group analogues, silicon and germanium tetrafluoride, are also tetrahedral but behave as Lewis acids. The pnictogens form trifluorides that increase in reactivity and basicity with higher molecular weight, although
Binary fluorides of metalloids and p-block nonmetals are generally covalent and volatile, with varying reactivities. Period 3 and heavier nonmetals can form hypervalent fluorides..
Boron trifluoride is planar and possesses an incomplete octet. It functions as a Lewis acid and combines with Lewis bases like ammonia to form adducts. Carbon tetrafluoride is tetrahedral and inert; its group analogues, silicon and germanium tetrafluoride, are also tetrahedral but behave as Lewis acids. The pnictogens form trifluorides that increase in reactivity and basicity with higher molecular weight, although
 Noble gases, having complete electron shells, defied reaction with other elements until 1962 when Neil Bartlett reported synthesis of xenon hexafluoroplatinate;
Noble gases, having complete electron shells, defied reaction with other elements until 1962 when Neil Bartlett reported synthesis of xenon hexafluoroplatinate;
 The carbon–fluorine bond is
The carbon–fluorine bond is
 Moissan's method is used to produce industrial quantities of fluorine, via the electrolysis of a potassium fluoride/ hydrogen fluoride mixture: hydrogen and fluoride ions are reduced and oxidized at a steel container
Moissan's method is used to produce industrial quantities of fluorine, via the electrolysis of a potassium fluoride/ hydrogen fluoride mixture: hydrogen and fluoride ions are reduced and oxidized at a steel container
Image:The fluorine economy.svg, 675px, center, Clickable diagram of the fluorochemical industry according to mass flows
rect 9 6 81 34 Fluorite
rect 9 172 81 199 Fluorapatite
rect 142 5 244 34 Hydrogen fluoride
rect 142 65 245 97 Metal smelting
rect 142 121 244 154 Glass production
rect 309 5 411 33 Fluorocarbons
rect 310 63 413 92 Sodium hexafluoroaluminate
rect 311 121 414 154 Pickling (metal)
rect 310 171 412 200 Fluorosilicic acid
rect 309 211 412 243 Alkane cracking
rect 483 6 585 34
 At least 17,000 metric tons of fluorine are produced each year. It costs only $5–8 per kilogram as uranium or sulfur hexafluoride, but many times more as an element because of handling challenges. Most processes using free fluorine in large amounts employ ''in situ'' generation under
At least 17,000 metric tons of fluorine are produced each year. It costs only $5–8 per kilogram as uranium or sulfur hexafluoride, but many times more as an element because of handling challenges. Most processes using free fluorine in large amounts employ ''in situ'' generation under
 As with other iron alloys, around 3 kg (6.5 lb) metspar is added to each metric ton of steel; the fluoride ions lower its melting point and
As with other iron alloys, around 3 kg (6.5 lb) metspar is added to each metric ton of steel; the fluoride ions lower its melting point and
 About 180,000 metric tons of fluoropolymers were produced in 2006 and 2007, generating over $3.5 billion revenue per year.. The global market was estimated at just under $6 billion in 2011 and was predicted to grow by 6.5% per year up to 2016. Fluoropolymers can only be formed by
About 180,000 metric tons of fluoropolymers were produced in 2006 and 2007, generating over $3.5 billion revenue per year.. The global market was estimated at just under $6 billion in 2011 and was predicted to grow by 6.5% per year up to 2016. Fluoropolymers can only be formed by
 Twenty percent of modern pharmaceuticals contain fluorine.. One of these, the cholesterol-reducer
Twenty percent of modern pharmaceuticals contain fluorine.. One of these, the cholesterol-reducer
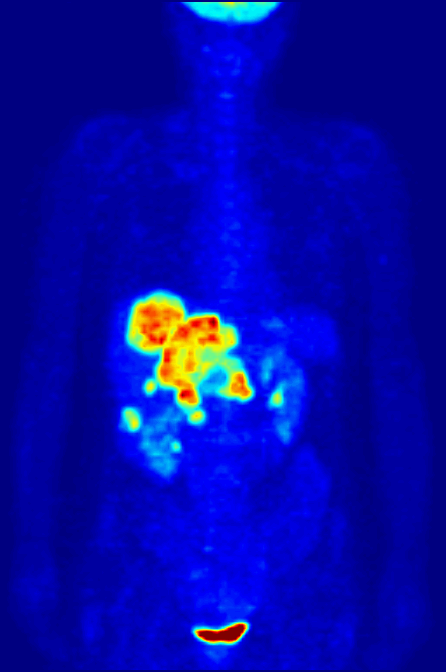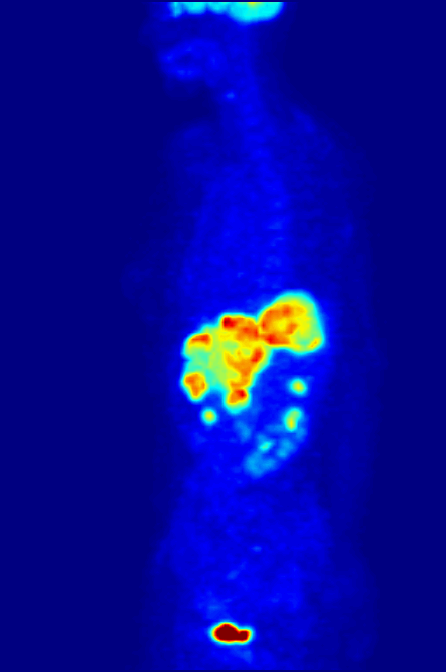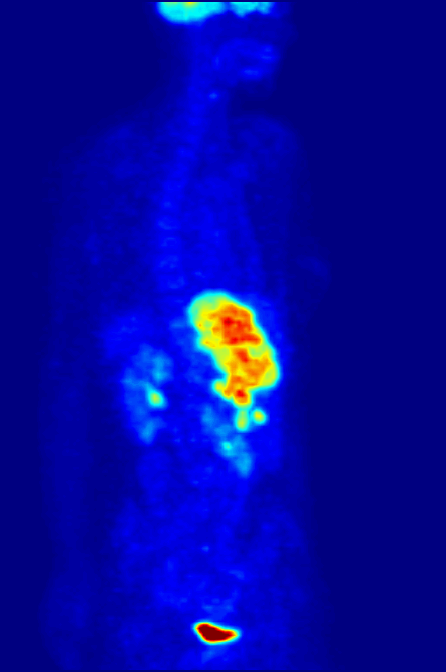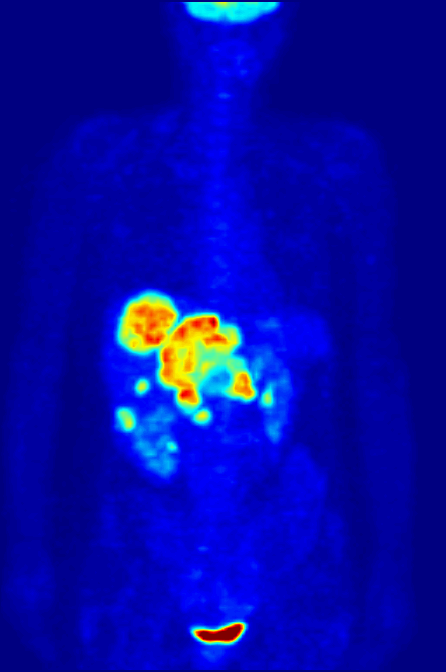 Fluorine-18 is often found in
Fluorine-18 is often found in
 Fluorine is not essential for humans and other mammals, but small amounts are known to be beneficial for the strengthening of dental enamel (where the formation of fluorapatite makes the enamel more resistant to attack, from acids produced by bacterial fermentation of sugars). Small amounts of fluorine may be beneficial for bone strength, but the latter has not been definitively established. Both the WHO and the Institute of Medicine of the US National Academies publish recommended daily allowance (RDA) and upper tolerated intake of fluorine, which varies with age and gender.
Natural organofluorines have been found in microorganisms and plants but not animals. The most common is fluoroacetate, which is used as a defense against herbivores by at least 40 plants in Africa, Australia and Brazil. Other examples include terminally fluorinated
Fluorine is not essential for humans and other mammals, but small amounts are known to be beneficial for the strengthening of dental enamel (where the formation of fluorapatite makes the enamel more resistant to attack, from acids produced by bacterial fermentation of sugars). Small amounts of fluorine may be beneficial for bone strength, but the latter has not been definitively established. Both the WHO and the Institute of Medicine of the US National Academies publish recommended daily allowance (RDA) and upper tolerated intake of fluorine, which varies with age and gender.
Natural organofluorines have been found in microorganisms and plants but not animals. The most common is fluoroacetate, which is used as a defense against herbivores by at least 40 plants in Africa, Australia and Brazil. Other examples include terminally fluorinated
 Hydrofluoric acid is the weakest of the hydrohalic acids, having a
Hydrofluoric acid is the weakest of the hydrohalic acids, having a
 The
The
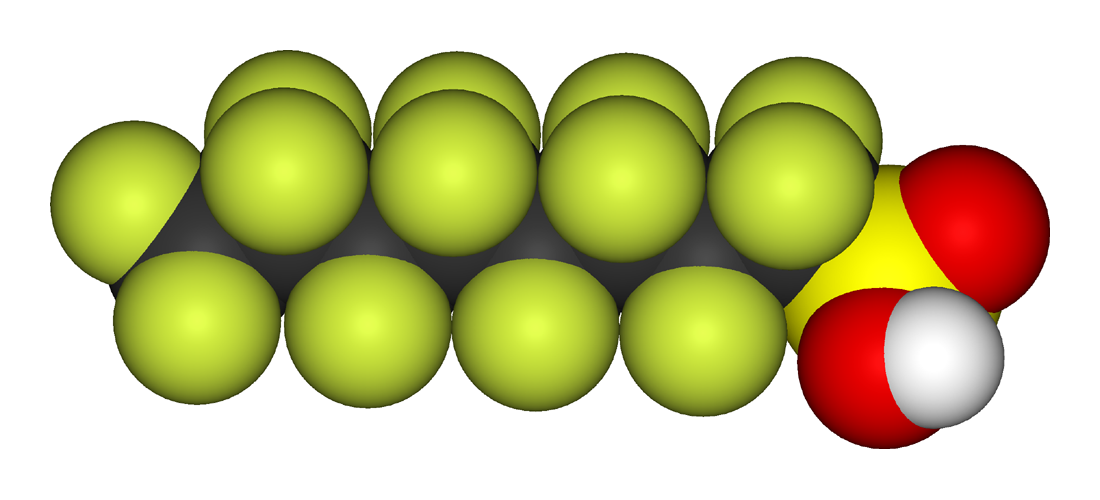 Organofluorines exhibit biopersistence due to the strength of the carbon–fluorine bond. Perfluoroalkyl acids (PFAAs), which are sparingly water-soluble owing to their acidic functional groups, are noted persistent organic pollutants; perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) are most often researched. PFAAs have been found in trace quantities worldwide from polar bears to humans, with PFOS and PFOA known to reside in breast milk and the blood of newborn babies. A 2013 review showed a slight correlation between groundwater and soil PFAA levels and human activity; there was no clear pattern of one chemical dominating, and higher amounts of PFOS were correlated to higher amounts of PFOA.. In the body, PFAAs bind to proteins such as serum albumin; they tend to concentrate within humans in the liver and blood before excretion through the kidneys. Dwell time in the body varies greatly by species, with half-lives of days in rodents, and years in humans.... High doses of PFOS and PFOA cause cancer and death in newborn rodents but human studies have not established an effect at current exposure levels.
Organofluorines exhibit biopersistence due to the strength of the carbon–fluorine bond. Perfluoroalkyl acids (PFAAs), which are sparingly water-soluble owing to their acidic functional groups, are noted persistent organic pollutants; perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) are most often researched. PFAAs have been found in trace quantities worldwide from polar bears to humans, with PFOS and PFOA known to reside in breast milk and the blood of newborn babies. A 2013 review showed a slight correlation between groundwater and soil PFAA levels and human activity; there was no clear pattern of one chemical dominating, and higher amounts of PFOS were correlated to higher amounts of PFOA.. In the body, PFAAs bind to proteins such as serum albumin; they tend to concentrate within humans in the liver and blood before excretion through the kidneys. Dwell time in the body varies greatly by species, with half-lives of days in rodents, and years in humans.... High doses of PFOS and PFOA cause cancer and death in newborn rodents but human studies have not established an effect at current exposure levels.
chemical element
A chemical element is a species of atoms that have a given number of protons in their atomic nucleus, nuclei, including the pure Chemical substance, substance consisting only of that species. Unlike chemical compounds, chemical elements canno ...
with the symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
F and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of ever ...
9. It is the lightest halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this grou ...
and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases.
Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting
Smelting is a process of applying heat to ore, to extract a base metal. It is a form of extractive metallurgy. It is used to extract many metals from their ores, including silver, iron, copper, and other base metals. Smelting uses heat and a c ...
, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
, a process still employed for modern production. Industrial production of fluorine gas for uranium enrichment, its largest application, began during the Manhattan Project
The Manhattan Project was a research and development undertaking during World War II that produced the first nuclear weapons. It was led by the United States with the support of the United Kingdom and Canada. From 1942 to 1946, the project w ...
in World War II
World War II or the Second World War, often abbreviated as WWII or WW2, was a world war that lasted from 1939 to 1945. It involved the vast majority of the world's countries—including all of the great powers—forming two opposi ...
.
Owing to the expense of refining pure fluorine, most commercial applications use fluorine compounds, with about half of mined fluorite used in steelmaking. The rest of the fluorite is converted into corrosive hydrogen fluoride en route to various organic fluorides, or into cryolite, which plays a key role in aluminium refining. Molecules containing a carbon–fluorine bond often have very high chemical and thermal stability; their major uses are as refrigerant
A refrigerant is a working fluid used in the refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are heavily regulated ...
s, electrical insulation and cookware, and PTFE
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene that has numerous applications. It is one of the best-known and widely applied PFAS. The commonly known brand name of PTFE-based composition is Teflon by Chem ...
(Teflon). Pharmaceuticals such as atorvastatin
Atorvastatin is a statin medication used to prevent cardiovascular disease in those at high risk and to treat abnormal lipid levels. For the prevention of cardiovascular disease, statins are a first-line treatment. It is taken by mouth.
Common ...
and fluoxetine contain C−F bonds. The fluoride ion from dissolved fluoride salts inhibits dental cavities, and so finds use in toothpaste
Toothpaste is a paste or gel dentifrice used with a toothbrush to clean and maintain the aesthetics and health of teeth. Toothpaste is used to promote oral hygiene: it is an abrasive that aids in removing dental plaque and food from the teeth, ...
and water fluoridation. Global fluorochemical sales amount to more than US$69 billion a year.
Fluorocarbon
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often has distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Fluorocarbons and their derivatives are commerci ...
gases are generally greenhouse gases with global-warming potentials 100 to 23,500 times that of carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
, and SF6 has the highest global warming potential of any known substance. Organofluorine compounds
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refr ...
often persist in the environment due to the strength of the carbon–fluorine bond. Fluorine has no known metabolic role in mammals; a few plant
Plants are predominantly photosynthetic eukaryotes of the kingdom Plantae. Historically, the plant kingdom encompassed all living things that were not animals, and included algae and fungi; however, all current definitions of Plantae excl ...
s and sea sponges
Sponges, the members of the phylum Porifera (; meaning 'pore bearer'), are a basal animal clade as a sister of the diploblasts. They are multicellular organisms that have bodies full of pores and channels allowing water to circulate through ...
synthesize organofluorine poisons (most often monofluoroacetates) that help deter predation.
Characteristics
Electron configuration
Fluorine atoms have nine electrons, one fewer than neon, andelectron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon ato ...
1s22s22p5: two electrons in a filled inner shell and seven in an outer shell requiring one more to be filled. The outer electrons are ineffective at nuclear shielding, and experience a high effective nuclear charge In atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an electron in a multi-electron atom. The term "effective" is used because the shielding effect of negatively charged electrons prevent ...
of 9 − 2 = 7; this affects the atom's physical properties.
Fluorine's first ionization energy is third-highest among all elements, behind helium and neon, which complicates the removal of electrons from neutral fluorine atoms. It also has a high electron affinity, second only to chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
, and tends to capture an electron to become isoelectronic with the noble gas neon; it has the highest electronegativity of any reactive element. Fluorine atoms have a small covalent radius of around 60 picometers, similar to those of its period neighbors oxygen and neon..
Reactivity
 The bond energy of
The bond energy of difluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, ...
is much lower than that of either or and similar to the easily cleaved peroxide
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable.
The most common peroxide is hydrogen p ...
bond; this, along with high electronegativity, accounts for fluorine's easy dissociation, high reactivity, and strong bonds to non-fluorine atoms. Conversely, bonds to other atoms are very strong because of fluorine's high electronegativity. Unreactive substances like powdered steel, glass fragments, and asbestos
Asbestos () is a naturally occurring fibrous silicate mineral. There are six types, all of which are composed of long and thin fibrous crystals, each fibre being composed of many microscopic "fibrils" that can be released into the atmosphere b ...
fibers react quickly with cold fluorine gas; wood and water spontaneously combust under a fluorine jet..
Reactions of elemental fluorine with metals require varying conditions. Alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
s cause explosions and alkaline earth metals display vigorous activity in bulk; to prevent passivation from the formation of metal fluoride layers, most other metals such as aluminium and iron must be powdered, and noble metals require pure fluorine gas at 300–450 °C (575–850 °F). Some solid nonmetals (sulfur, phosphorus) react vigorously in liquid fluorine. Hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The under ...
and sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic a ...
combine readily with fluorine, the latter sometimes explosively; sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
exhibits much less activity, requiring elevated temperatures.
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
, like some of the alkali metals, reacts explosively with fluorine. Carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
, as lamp black, reacts at room temperature to yield fluoromethane
Fluoromethane, also known as methyl fluoride, Freon 41, Halocarbon-41 and HFC-41, is a non-toxic, liquefiable, and flammable gas at standard temperature and pressure. It is made of carbon, hydrogen, and fluorine. The name stems from the fact th ...
. Graphite combines with fluorine above 400 °C (750 °F) to produce non-stoichiometric carbon monofluoride; higher temperatures generate gaseous fluorocarbon
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often has distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Fluorocarbons and their derivatives are commerci ...
s, sometimes with explosions.. Carbon dioxide and carbon monoxide react at or just above room temperature,. whereas paraffins
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in whic ...
and other organic chemicals generate strong reactions: even completely substituted haloalkanes such as carbon tetrachloride, normally incombustible, may explode.. Although nitrogen trifluoride
Nitrogen trifluoride () is an inorganic, colorless, non- flammable, toxic gas with a slightly musty odor. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics. Nitrogen trif ...
is stable, nitrogen requires an electric discharge at elevated temperatures for reaction with fluorine to occur, due to the very strong triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
in elemental nitrogen; ammonia may react explosively... Oxygen does not combine with fluorine under ambient conditions, but can be made to react using electric discharge at low temperatures and pressures; the products tend to disintegrate into their constituent elements when heated.. Heavier halogens react readily with fluorine as does the noble gas radon
Radon is a chemical element with the symbol Rn and atomic number 86. It is a radioactive, colourless, odourless, tasteless noble gas. It occurs naturally in minute quantities as an intermediate step in the normal radioactive decay chains th ...
; of the other noble gases, only xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
and krypton
Krypton (from grc, κρυπτός, translit=kryptos 'the hidden one') is a chemical element with the symbol Kr and atomic number 36. It is a colorless, odorless, tasteless noble gas that occurs in trace amounts in the atmosphere and is of ...
react, and only under special conditions..
Phases
phase transition
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states ...
into opaque and hard α-fluorine, which has a monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic ...
structure with dense, angled layers of molecules. The transition from β- to α-fluorine is more exothermic than the condensation of fluorine, and can be violent..
Isotopes
Only oneisotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass num ...
of fluorine occurs naturally in abundance, the stable isotope . It has a high magnetogyric ratio and exceptional sensitivity to magnetic fields; because it is also the only stable isotope, it is used in magnetic resonance imaging
Magnetic resonance imaging (MRI) is a medical imaging technique used in radiology to form pictures of the anatomy and the physiological processes of the body. MRI scanners use strong magnetic fields, magnetic field gradients, and radio wave ...
. Eighteen radioisotopes with mass numbers from 13 to 31 have been synthesized, of which is the most stable with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
of 109.77 minutes. is a natural trace radioisotope produced by cosmic ray spallation of atmospheric argon as well as by reaction of protons with natural oxygen: 18O + p → 18F + n.SCOPE 50 - Radioecology after Chernobyl, the Scientific Committee on Problems of the Environment (SCOPE), 1993. See table 1.9 in Section 1.4.5.2. Other radioisotopes have half-lives less than 70 seconds; most decay in less than half a second.. The isotopes and undergo β+ decay and
electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. ...
, lighter isotopes decay by proton emission, and those heavier than undergo β− decay (the heaviest ones with delayed neutron emission). Two metastable isomers
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have h ...
of fluorine are known, , with a half-life of 162(7) nanoseconds, and , with a half-life of 2.2(1) milliseconds.
Occurrence
Universe
Among the lighter elements, fluorine's abundance value of 400 ppb (parts per billion) – 24th among elements in the universe – is exceptionally low: other elements from carbon to magnesium are twenty or more times as common. This is becausestellar nucleosynthesis
Stellar nucleosynthesis is the creation (nucleosynthesis) of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. A ...
processes bypass fluorine, and any fluorine atoms otherwise created have high nuclear cross sections, allowing collisions with hydrogen or helium to generate oxygen or neon respectively.
Beyond this transient existence, three explanations have been proposed for the presence of fluorine:..
* during type II supernova
A Type II supernova (plural: ''supernovae'' or ''supernovas'') results from the rapid collapse and violent explosion of a massive star. A star must have at least 8 times, but no more than 40 to 50 times, the mass of the Sun () to undergo this ...
e, bombardment of neon atoms by neutrinos could transmute them to fluorine;
* the solar wind of Wolf–Rayet star
Wolf–Rayet stars, often abbreviated as WR stars, are a rare heterogeneous set of stars with unusual spectra showing prominent broad emission lines of ionised helium and highly ionised nitrogen or carbon. The spectra indicate very high surfa ...
s could blow fluorine away from any hydrogen or helium atoms; or
* fluorine is borne out on convection currents arising from fusion in asymptotic giant branch stars.
Earth
Fluorine is the thirteenth most common element in Earth's crust at 600–700 ppm (parts per million) by mass. Though believed not to occur naturally, elemental fluorine has been shown to be present as an occlusion in antozonite, a variant of fluorite. Most fluorine exists as fluoride-containing minerals. Fluorite, fluorapatite and cryolite are the most industrially significant. Fluorite (), also known as fluorspar, abundant worldwide, is the main source of fluoride, and hence fluorine. China and Mexico are the major suppliers.... Fluorapatite (Ca5(PO4)3F), which contains most of the world's fluoride, is an inadvertent source of fluoride as a byproduct of fertilizer production. Cryolite (), used in the production of aluminium, is the most fluorine-rich mineral. Economically viable natural sources of cryolite have been exhausted, and most is now synthesised commercially.antozonite
Antozonite (historically known as Stinkspat, Stinkfluss, Stinkstein, Stinkspar and fetid fluorite) is a radioactive fluorite variety first found in Wölsendorf, Bavaria, in 1841, and named in 1862.
It is characterized by the presence of multip ...
, is contentious; a 2012 study reported the presence of 0.04% by weight in antozonite, attributing these inclusions to radiation from the presence of tiny amounts of uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
.
History
Early discoveries
 In 1529, Georgius Agricola described fluorite as an additive used to lower the melting point of metals during
In 1529, Georgius Agricola described fluorite as an additive used to lower the melting point of metals during smelting
Smelting is a process of applying heat to ore, to extract a base metal. It is a form of extractive metallurgy. It is used to extract many metals from their ores, including silver, iron, copper, and other base metals. Smelting uses heat and a c ...
.. He penned the Latin word ''fluorēs'' (''fluor,'' flow) for fluorite rocks. The name later evolved into ''fluorspar'' (still commonly used) and then ''fluorite''.. The composition of fluorite was later determined to be calcium difluoride..
Hydrofluoric acid was used in glass etching from 1720 onward. Andreas Sigismund Marggraf
Andreas Sigismund Marggraf (; 3 March 1709 – 7 August 1782) was a German chemist from Berlin, then capital of the Margraviate of Brandenburg, and a pioneer of analytical chemistry. He isolated zinc in 1746 by heating calamine and carbon. Tho ...
first characterized it in 1764 when he heated fluorite with sulfuric acid, and the resulting solution corroded its glass container. Swedish chemist Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hydr ...
repeated the experiment in 1771, and named the acidic product ''fluss-spats-syran'' (fluorspar acid). In 1810, the French physicist André-Marie Ampère suggested that hydrogen and an element analogous to chlorine constituted hydrofluoric acid. He also proposed in a letter to Sir Humphry Davy dated August 26, 1812 that this then-unknown substance may be named ''fluorine'' from fluoric acid and the ''-ine'' suffix of other halogens. This word, often with modifications, is used in most European languages; however, Greek, Russian, and some others, following Ampère's later suggestion, use the name ''ftor'' or derivatives, from the Greek φθόριος (''phthorios'', destructive). The New Latin name ''fluorum'' gave the element its current symbol F; Fl was used in early papers.
Isolation
upleft, 1887 drawing of Moissan's apparatus Initial studies on fluorine were so dangerous that several 19th-century experimenters were deemed "fluorine martyrs" after misfortunes with hydrofluoric acid. Isolation of elemental fluorine was hindered by the extreme corrosiveness of both elemental fluorine itself and hydrogen fluoride, as well as the lack of a simple and suitable electrolyte. Edmond Frémy postulated thatelectrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
of pure hydrogen fluoride to generate fluorine was feasible and devised a method to produce anhydrous samples from acidified potassium bifluoride; instead, he discovered that the resulting (dry) hydrogen fluoride did not conduct electricity.. Frémy's former student Henri Moissan persevered, and after much trial and error found that a mixture of potassium bifluoride and dry hydrogen fluoride was a conductor, enabling electrolysis. To prevent rapid corrosion of the platinum in his electrochemical cells, he cooled the reaction to extremely low temperatures in a special bath and forged cells from a more resistant mixture of platinum and iridium
Iridium is a chemical element with the symbol Ir and atomic number 77. A very hard, brittle, silvery-white transition metal of the platinum group, it is considered the second-densest naturally occurring metal (after osmium) with a density o ...
, and used fluorite stoppers. In 1886, after 74 years of effort by many chemists, Moissan isolated elemental fluorine..
In 1906, two months before his death, Moissan received the Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
,. with the following citation:.
Later uses
 The Frigidaire division of
The Frigidaire division of General Motors
The General Motors Company (GM) is an American Multinational corporation, multinational Automotive industry, automotive manufacturing company headquartered in Detroit, Michigan, United States. It is the largest automaker in the United States and ...
(GM) experimented with chlorofluorocarbon refrigerants in the late 1920s, and Kinetic Chemicals was formed as a joint venture between GM and DuPont
DuPont de Nemours, Inc., commonly shortened to DuPont, is an American multinational chemical company first formed in 1802 by French-American chemist and industrialist Éleuthère Irénée du Pont de Nemours. The company played a major role in ...
in 1930 hoping to market Freon-12 () as one such refrigerant
A refrigerant is a working fluid used in the refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are heavily regulated ...
. It replaced earlier and more toxic compounds, increased demand for kitchen refrigerators, and became profitable; by 1949 DuPont had bought out Kinetic and marketed several other Freon compounds... Polytetrafluoroethylene
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene that has numerous applications. It is one of the best-known and widely applied PFAS. The commonly known brand name of PTFE-based composition is Teflon by Chemo ...
(Teflon) was serendipitously discovered in 1938 by Roy J. Plunkett
Roy J. Plunkett (June 26, 1910 – May 12, 1994) was an American chemist. He discovered polytetrafluoroethylene (PTFE), better known as Teflon, in 1938.
Personal life and education
Plunkett was born in New Carlisle, Ohio and attended Newton High ...
while working on refrigerants at Kinetic, and its superlative chemical and thermal resistance lent it to accelerated commercialization and mass production by 1941.
Large-scale production of elemental fluorine began during World War II. Germany used high-temperature electrolysis to make tons of the planned incendiary chlorine trifluoride and the Manhattan Project
The Manhattan Project was a research and development undertaking during World War II that produced the first nuclear weapons. It was led by the United States with the support of the United Kingdom and Canada. From 1942 to 1946, the project w ...
used huge quantities to produce uranium hexafluoride for uranium enrichment. Since is as corrosive as fluorine, gaseous diffusion plants required special materials: nickel for membranes, fluoropolymers for seals, and liquid fluorocarbons as coolants and lubricants. This burgeoning nuclear industry later drove post-war fluorochemical development.
Compounds
Fluorine has a rich chemistry, encompassing organic and inorganic domains. It combines with metals, nonmetals, metalloids, and most noble gases,. and almost exclusively assumes anoxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
of −1. Fluorine's high electron affinity results in a preference for ionic bonding; when it forms covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between ato ...
s, these are polar, and almost always single..The metastable boron and nitrogen monofluoride
Nitrogen monofluoride (fluoroimidogen) is a metastable species that has been observed in laser studies. It is isoelectronic with O2. Like boron monofluoride, it is an instance of the rare multiply-bonded fluorine atom. It is unstable with respec ...
have higher-order fluorine bonds, and some metal complexes use it as a bridging ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually ...
. Hydrogen bonding is another possibility.
Metals
Alkali metals form ionic and highly soluble monofluorides; these have the cubic arrangement of sodium chloride and analogous chlorides. Alkaline earth difluorides possess strong ionic bonds but are insoluble in water,. with the exception of beryllium difluoride, which also exhibits some covalent character and has aquartz
Quartz is a hard, crystalline mineral composed of silica ( silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical f ...
-like structure.. Rare earth element
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides ( yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous sil ...
s and many other metals form mostly ionic trifluorides..
Covalent bonding first comes to prominence in the tetrafluorides: those of zirconium, hafnium
Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri M ...
. and several actinides are ionic with high melting points, while those of titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion i ...
, vanadium, and niobium are polymeric, melting or decomposing at no more than 350 °C (660 °F). Pentafluorides continue this trend with their linear polymers and oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relati ...
ic complexes. Thirteen metal hexafluorides are known, all octahedral, and are mostly volatile solids but for liquid and , and gaseous .. Rhenium heptafluoride
Rhenium heptafluoride is the compound with the formula ReF7. It is a yellow low melting solid and is the only thermally stable metal heptafluoride. It has a distorted pentagonal bipyramidal structure similar to IF7, which was confirmed by neutron ...
, the only characterized metal heptafluoride, is a low-melting molecular solid with pentagonal bipyramidal molecular geometry
In chemistry, a pentagonal bipyramid is a molecular geometry with one atom at the centre with seven ligands at the corners of a pentagonal bipyramid. A perfect pentagonal bipyramid belongs to the molecular point group ''D5h''.
The pentagona ...
. Metal fluorides with more fluorine atoms are particularly reactive.
Hydrogen
hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chlorid ...
.... It boils at a much higher temperature than heavier hydrogen halides and unlike them is miscible with water. Hydrogen fluoride readily hydrates on contact with water to form aqueous hydrogen fluoride, also known as hydrofluoric acid. Unlike the other hydrohalic acids, which are strong
Strong may refer to:
Education
* The Strong, an educational institution in Rochester, New York, United States
* Strong Hall (Lawrence, Kansas), an administrative hall of the University of Kansas
* Strong School, New Haven, Connecticut, United S ...
, hydrofluoric acid is a weak acid
Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions ...
at low concentrations. However, it can attack glass, something the other acids cannot do..
Other reactive nonmetals
 Binary fluorides of metalloids and p-block nonmetals are generally covalent and volatile, with varying reactivities. Period 3 and heavier nonmetals can form hypervalent fluorides..
Boron trifluoride is planar and possesses an incomplete octet. It functions as a Lewis acid and combines with Lewis bases like ammonia to form adducts. Carbon tetrafluoride is tetrahedral and inert; its group analogues, silicon and germanium tetrafluoride, are also tetrahedral but behave as Lewis acids. The pnictogens form trifluorides that increase in reactivity and basicity with higher molecular weight, although
Binary fluorides of metalloids and p-block nonmetals are generally covalent and volatile, with varying reactivities. Period 3 and heavier nonmetals can form hypervalent fluorides..
Boron trifluoride is planar and possesses an incomplete octet. It functions as a Lewis acid and combines with Lewis bases like ammonia to form adducts. Carbon tetrafluoride is tetrahedral and inert; its group analogues, silicon and germanium tetrafluoride, are also tetrahedral but behave as Lewis acids. The pnictogens form trifluorides that increase in reactivity and basicity with higher molecular weight, although nitrogen trifluoride
Nitrogen trifluoride () is an inorganic, colorless, non- flammable, toxic gas with a slightly musty odor. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics. Nitrogen trif ...
resists hydrolysis and is not basic.. The pentafluorides of phosphorus, arsenic, and antimony are more reactive than their respective trifluorides, with antimony pentafluoride the strongest neutral Lewis acid known..
Chalcogens have diverse fluorides: unstable difluorides have been reported for oxygen (the only known compound with oxygen in an oxidation state of +2), sulfur, and selenium; tetrafluorides and hexafluorides exist for sulfur, selenium, and tellurium. The latter are stabilized by more fluorine atoms and lighter central atoms, so sulfur hexafluoride
Sulfur hexafluoride or sulphur hexafluoride (British spelling) is an inorganic compound with the formula SF6. It is a colorless, odorless, non- flammable, and non-toxic gas. has an octahedral geometry, consisting of six fluorine atoms attach ...
is especially inert.. Chlorine, bromine, and iodine can each form mono-, tri-, and pentafluorides, but only iodine heptafluoride
Iodine heptafluoride, also known as iodine(VII) fluoride or iodine fluoride, is an interhalogen compound with the chemical formula I F7. It has an unusual pentagonal bipyramidal structure, as predicted by VSEPR theory. The molecule can undergo ...
has been characterized among possible interhalogen
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms (fluorine, chlorine, bromine, iodine, or astatine) and no atoms of elements from any other group.
Most interhalogen compounds known are binar ...
heptafluorides. Many of them are powerful sources of fluorine atoms, and industrial applications using chlorine trifluoride require precautions similar to those using fluorine..
Noble gases
 Noble gases, having complete electron shells, defied reaction with other elements until 1962 when Neil Bartlett reported synthesis of xenon hexafluoroplatinate;
Noble gases, having complete electron shells, defied reaction with other elements until 1962 when Neil Bartlett reported synthesis of xenon hexafluoroplatinate; xenon difluoride
Xenon difluoride is a powerful fluorinating agent with the chemical formula , and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherwis ...
, tetrafluoride, hexafluoride, and multiple oxyfluorides have been isolated since then. Among other noble gases, krypton forms a difluoride, and radon and fluorine generate a solid suspected to be radon difluoride.. Binary fluorides of lighter noble gases are exceptionally unstable: argon and hydrogen fluoride combine under extreme conditions to give argon fluorohydride. Helium and neon have no long-lived fluorides,. and no neon fluoride has ever been observed; helium fluorohydride has been detected for milliseconds at high pressures and low temperatures.
Organic compounds
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J ...
's strongest,. and gives stability to organofluorines. It is almost non-existent in nature, but is used in artificial compounds. Research in this area is usually driven by commercial applications; the compounds involved are diverse and reflect the complexity inherent in organic chemistry.
Discrete molecules
The substitution of hydrogen atoms in analkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in wh ...
by progressively more fluorine atoms gradually alters several properties: melting and boiling points are lowered, density increases, solubility in hydrocarbons decreases and overall stability increases. Perfluorocarbons, in which all hydrogen atoms are substituted, are insoluble in most organic solvents, reacting at ambient conditions only with sodium in liquid ammonia.
The term '' perfluorinated compound'' is used for what would otherwise be a perfluorocarbon if not for the presence of a functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the r ...
, often a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyli ...
. These compounds share many properties with perfluorocarbons such as stability and hydrophobicity, while the functional group augments their reactivity, enabling them to adhere to surfaces or act as surfactants;. Fluorosurfactant
Per- and polyfluoroalkyl substances (PFASs) are synthetic organofluorine chemical compounds that have multiple fluorine atoms attached to an alkyl chain. An early definition, from 2011, required that they contain at least one perfluoroalkyl ...
s, in particular, can lower the surface tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) t ...
of water more than their hydrocarbon-based analogues. Fluorotelomers, which have some unfluorinated carbon atoms near the functional group, are also regarded as perfluorinated..
Polymers
Polymers exhibit the same stability increases afforded by fluorine substitution (for hydrogen) in discrete molecules; their melting points generally increase too.Polytetrafluoroethylene
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene that has numerous applications. It is one of the best-known and widely applied PFAS. The commonly known brand name of PTFE-based composition is Teflon by Chemo ...
(PTFE), the simplest fluoropolymer and perfluoro analogue of polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging ( plastic bags, plastic films, geomembranes and containers including b ...
with structural unit
In polymer chemistry, a structural unit is a building block of a polymer chain. It is the result of a monomer which has been polymerized into a long chain.
There may be more than one structural unit in the repeat unit. When different monomers are ...
––, demonstrates this change as expected, but its very high melting point makes it difficult to mold. Various PTFE derivatives are less temperature-tolerant but easier to mold: fluorinated ethylene propylene replaces some fluorine atoms with trifluoromethyl groups, perfluoroalkoxy alkane
Perfluoroalkoxy alkanes (PFA) are fluoropolymers. They are copolymers of tetrafluoroethylene (C2F4) and perfluoroethers (C2F3ORf, where Rf is a perfluorinated group such as trifluoromethyl (CF3)). The properties of these polymers are similar to ...
s do the same with trifluoromethoxy groups, and Nafion contains perfluoroether side chains capped with sulfonic acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is k ...
groups. Other fluoropolymers retain some hydrogen atoms; polyvinylidene fluoride has half the fluorine atoms of PTFE and polyvinyl fluoride has a quarter, but both behave much like perfluorinated polymers.
Production
Elemental fluorine and virtually all fluorine compounds are produced from hydrogen fluoride or its aqueous solutions, hydrofluoric acid. Hydrogen fluoride is produced in kilns by theendothermic reaction
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. ...
of fluorite (CaF2) with sulfuric acid:
:CaF2 + H2SO4 → 2 HF(g) + CaSO4
The gaseous HF can then be absorbed in water or liquefied.
About 20% of manufactured HF is a byproduct of fertilizer production, which produces hexafluorosilicic acid (H2SiF6), which can be degraded to release HF thermally and by hydrolysis:
:H2SiF6 → 2 HF + SiF4
:SiF4 + 2 H2O → 4 HF + SiO2
Industrial routes to F2
 Moissan's method is used to produce industrial quantities of fluorine, via the electrolysis of a potassium fluoride/ hydrogen fluoride mixture: hydrogen and fluoride ions are reduced and oxidized at a steel container
Moissan's method is used to produce industrial quantities of fluorine, via the electrolysis of a potassium fluoride/ hydrogen fluoride mixture: hydrogen and fluoride ions are reduced and oxidized at a steel container cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in whi ...
and a carbon block anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic is ...
, under 8–12 volts, to generate hydrogen and fluorine gas respectively. Temperatures are elevated, KF•2HF melting at and being electrolyzed at . KF, which acts to provide electrical conductivity, is essential since pure HF cannot be electrolyzed because it is virtually non-conductive. Fluorine can be stored in steel cylinders that have passivated interiors, at temperatures below ; otherwise nickel can be used. Regulator valves and pipework are made of nickel, the latter possibly using Monel instead. Frequent passivation, along with the strict exclusion of water and greases, must be undertaken. In the laboratory, glassware may carry fluorine gas under low pressure and anhydrous conditions; some sources instead recommend nickel-Monel-PTFE systems..
Laboratory routes
While preparing for a 1986 conference to celebrate the centennial of Moissan's achievement, Karl O. Christe reasoned that chemical fluorine generation should be feasible since some metal fluoride anions have no stable neutral counterparts; their acidification potentially triggers oxidation instead. He devised a method which evolves fluorine at high yield and atmospheric pressure:. :2 KMnO4 + 2 KF + 10 HF + 3 H2O2 → 2 K2MnF6 + 8 H2O + 3 O2↑ :2 K2MnF6 + 4 SbF5 → 4 KSbF6 + 2 MnF3 + F2↑ Christe later commented that the reactants "had been known for more than 100 years and even Moissan could have come up with this scheme." As late as 2008, some references still asserted that fluorine was too reactive for any chemical isolation.Industrial applications
Fluorite mining, which supplies most global fluorine, peaked in 1989 when 5.6 millionmetric ton
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton (United States ...
s of ore were extracted. Chlorofluorocarbon restrictions lowered this to 3.6 million tons in 1994; production has since been increasing. Around 4.5 million tons of ore and revenue of US$550 million were generated in 2003; later reports estimated 2011 global fluorochemical sales at $15 billion and predicted 2016–18 production figures of 3.5 to 5.9 million tons, and revenue of at least $20 billion.. Froth flotation separates mined fluorite into two main metallurgical grades of equal proportion: 60–85% pure metspar is almost all used in iron smelting whereas 97%+ pure acidspar is mainly converted to the key industrial intermediate hydrogen fluoride..
Hydrofluorocarbon
Hydrofluorocarbons (HFCs) are man-made organic compounds that contain fluorine and hydrogen atoms, and are the most common type of organofluorine compounds. Most are gases at room temperature and pressure. They are frequently used in air condi ...
s
rect 484 47 585 76 Hydrochlorofluorocarbons
rect 483 88 586 116 Chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and p ...
rect 483 128 585 160 Teflon
rect 484 170 586 200 Water fluoridation
rect 483 210 586 238 Uranium enrichment
rect 484 258 586 287 Sulfur hexafluoride
Sulfur hexafluoride or sulphur hexafluoride (British spelling) is an inorganic compound with the formula SF6. It is a colorless, odorless, non- flammable, and non-toxic gas. has an octahedral geometry, consisting of six fluorine atoms attach ...
rect 484 297 585 357 Tungsten hexafluoride
Tungsten(VI) fluoride, also known as tungsten hexafluoride, is an inorganic compound with the formula W F6. It is a toxic, corrosive, colorless gas, with a density of about (roughly 11 times heavier than air). It is one of the densest known gase ...
rect 28 246 177 293 Phosphogypsum
desc bottom-left
 At least 17,000 metric tons of fluorine are produced each year. It costs only $5–8 per kilogram as uranium or sulfur hexafluoride, but many times more as an element because of handling challenges. Most processes using free fluorine in large amounts employ ''in situ'' generation under
At least 17,000 metric tons of fluorine are produced each year. It costs only $5–8 per kilogram as uranium or sulfur hexafluoride, but many times more as an element because of handling challenges. Most processes using free fluorine in large amounts employ ''in situ'' generation under vertical integration
In microeconomics, management and international political economy, vertical integration is a term that describes the arrangement in which the supply chain of a company is integrated and owned by that company. Usually each member of the suppl ...
.
The largest application of fluorine gas, consuming up to 7,000 metric tons annually, is in the preparation of for the nuclear fuel cycle. Fluorine is used to fluorinate uranium tetrafluoride, itself formed from uranium dioxide and hydrofluoric acid. Fluorine is monoisotopic, so any mass differences between molecules are due to the presence of or , enabling uranium enrichment via gaseous diffusion or gas centrifuge. About 6,000 metric tons per year go into producing the inert dielectric
In electromagnetism, a dielectric (or dielectric medium) is an electrical insulator that can be polarised by an applied electric field. When a dielectric material is placed in an electric field, electric charges do not flow through the ma ...
for high-voltage transformers and circuit breakers, eliminating the need for hazardous polychlorinated biphenyl
Polychlorinated biphenyls (PCBs) are highly carcinogenic chemical compounds, formerly used in industrial and consumer products, whose production was banned in the United States by the Toxic Substances Control Act of 1976, Toxic Substances Contro ...
s associated with devices. Several fluorine compounds are used in electronics: rhenium and tungsten hexafluoride in chemical vapor deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
In typical CVD, the wafer (subst ...
, tetrafluoromethane in plasma etching
Plasma etching is a form of plasma processing used to fabricate integrated circuits. It involves a high-speed stream of glow discharge ( plasma) of an appropriate gas mixture being shot (in pulses) at a sample. The plasma source, known as etch spec ...
and nitrogen trifluoride
Nitrogen trifluoride () is an inorganic, colorless, non- flammable, toxic gas with a slightly musty odor. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics. Nitrogen trif ...
in cleaning equipment. Fluorine is also used in the synthesis of organic fluorides, but its reactivity often necessitates conversion first to the gentler , , or , which together allow calibrated fluorination. Fluorinated pharmaceuticals use sulfur tetrafluoride instead.
Inorganic fluorides
viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the int ...
.. Alongside its role as an additive in materials like enamels and welding rod coats, most acidspar is reacted with sulfuric acid to form hydrofluoric acid, which is used in steel pickling, glass etching and alkane cracking. One-third of HF goes into synthesizing cryolite and aluminium trifluoride, both fluxes in the Hall–Héroult process for aluminium extraction; replenishment is necessitated by their occasional reactions with the smelting apparatus. Each metric ton of aluminium requires about 23 kg (51 lb) of flux. Fluorosilicates consume the second largest portion, with sodium fluorosilicate used in water fluoridation and laundry effluent treatment, and as an intermediate en route to cryolite and silicon tetrafluoride. Other important inorganic fluorides include those of cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow t ...
, and ammonium.
Organic fluorides
Organofluorides consume over 20% of mined fluorite and over 40% of hydrofluoric acid, with refrigerant gases dominating and fluoropolymers increasing their market share.. Surfactants are a minor application but generate over $1 billion in annual revenue.. Due to the danger from direct hydrocarbon–fluorine reactions above −150 °C (−240 °F), industrial fluorocarbon production is indirect, mostly through halogen exchange reactions such as Swarts fluorination, in which chlorocarbon chlorines are substituted for fluorines by hydrogen fluoride under catalysts.Electrochemical fluorination Electrochemical fluorination (ECF), or electrofluorination, is a foundational organofluorine chemistry method for the preparation of fluorocarbon-based organofluorine compounds.G. Siegemund, W. Schwertfeger, A. Feiring, B. Smart, F. Behr, H. Vogel, ...
subjects hydrocarbons to electrolysis in hydrogen fluoride, and the Fowler process The Fowler process is an industry and laboratory route to fluorocarbons, by fluorinating hydrocarbons or their partially fluorinated derivatives in the vapor phase over cobalt(III) fluoride.
Background
The Manhattan Project required the productio ...
treats them with solid fluorine carriers like cobalt trifluoride.
Refrigerant gases
Halogenated refrigerants, termed Freons in informal contexts, are identified by R-numbers that denote the amount of fluorine, chlorine, carbon, and hydrogen present. Chlorofluorocarbons (CFCs) like R-11, R-12, and R-114 once dominated organofluorines, peaking in production in the 1980s. Used for air conditioning systems, propellants and solvents, their production was below one-tenth of this peak by the early 2000s, after widespread international prohibition. Hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs) were designed as replacements; their synthesis consumes more than 90% of the fluorine in the organic industry. Important HCFCs include R-22, chlorodifluoromethane, and R-141b. The main HFC isR-134a
1,1,1,2-Tetrafluoroethane (also known as norflurane (INN), R-134a, Freon 134a, Forane 134a, Genetron 134a, Green Gas, Florasol 134a, Suva 134a, or HFC-134a) is a hydrofluorocarbon (HFC) and haloalkane refrigerant with thermodynamic properties ...
with a new type of molecule HFO-1234yf, a Hydrofluoroolefin (HFO) coming to prominence owing to its global warming potential of less than 1% that of HFC-134a..
Polymers
 About 180,000 metric tons of fluoropolymers were produced in 2006 and 2007, generating over $3.5 billion revenue per year.. The global market was estimated at just under $6 billion in 2011 and was predicted to grow by 6.5% per year up to 2016. Fluoropolymers can only be formed by
About 180,000 metric tons of fluoropolymers were produced in 2006 and 2007, generating over $3.5 billion revenue per year.. The global market was estimated at just under $6 billion in 2011 and was predicted to grow by 6.5% per year up to 2016. Fluoropolymers can only be formed by polymerizing
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many for ...
free radicals.
Polytetrafluoroethylene (PTFE), sometimes called by its DuPont name Teflon, represents 60–80% by mass of the world's fluoropolymer production. The largest application is in electrical insulation since PTFE is an excellent dielectric
In electromagnetism, a dielectric (or dielectric medium) is an electrical insulator that can be polarised by an applied electric field. When a dielectric material is placed in an electric field, electric charges do not flow through the ma ...
. It is also used in the chemical industry where corrosion resistance is needed, in coating pipes, tubing, and gaskets. Another major use is in PFTE-coated fiberglass cloth for stadium roofs. The major consumer application is for non-stick cookware.. Jerked PTFE film becomes expanded PTFE (ePTFE), a fine-pored membrane sometimes referred to by the brand name Gore-Tex and used for rainwear, protective apparel, and filters
Filter, filtering or filters may refer to:
Science and technology
Computing
* Filter (higher-order function), in functional programming
* Filter (software), a computer program to process a data stream
* Filter (video), a software component that ...
; ePTFE fibers may be made into seals
Seals may refer to:
* Pinniped, a diverse group of semi-aquatic marine mammals, many of which are commonly called seals, particularly:
** Earless seal, or "true seal"
** Fur seal
* Seal (emblem), a device to impress an emblem, used as a means of a ...
and dust filters. Other fluoropolymers, including fluorinated ethylene propylene, mimic PTFE's properties and can substitute for it; they are more moldable, but also more costly and have lower thermal stability. Films from two different fluoropolymers replace glass in solar cells..
The chemically resistant (but expensive) fluorinated ionomers are used as electrochemical cell membranes, of which the first and most prominent example is Nafion. Developed in the 1960s, it was initially deployed as fuel cell material in spacecraft and then replaced mercury-based chloralkali process cells. Recently, the fuel cell application has reemerged with efforts to install proton exchange membrane
A proton-exchange membrane, or polymer-electrolyte membrane (PEM), is a semipermeable membrane generally made from ionomers and designed to conduct protons while acting as an electronic insulator and reactant barrier, e.g. to oxygen and hydrogen g ...
fuel cells into automobiles.... Fluoroelastomer {{refimprove, date=June 2008
A fluoroelastomer is a fluorocarbon-based synthetic rubber. Fluroelastomers generally have wide chemical resistance.
Composition
Several compositions of fluoroelastomers exist including FKM (by ASTM D1418 standard, equi ...
s such as Viton are crosslink
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
ed fluoropolymer mixtures mainly used in O-rings; perfluorobutane (C4F10) is used as a fire-extinguishing agent.
Surfactants
Fluorosurfactants are small organofluorine molecules used for repelling water and stains. Although expensive (comparable to pharmaceuticals at $200–2000 per kilogram), they yielded over $1 billion in annual revenues by 2006;Scotchgard
Scotchgard is a 3M brand of products, a stain and durable water repellent applied to fabric, furniture, and carpets to protect them from stains. Scotchgard products typically rely on organofluorine chemicals as the main active ingredient along w ...
alone generated over $300 million in 2000... Fluorosurfactants are a minority in the overall surfactant market, most of which is taken up by much cheaper hydrocarbon-based products. Applications in paints are burdened by compounding
In the field of pharmacy, compounding (performed in compounding pharmacies) is preparation of a custom formulation of a medication to fit a unique need of a patient that cannot be met with commercially available products. This may be done for me ...
costs; this use was valued at only $100 million in 2006.
Agrichemicals
About 30% ofagrichemical
An agrochemical or agrichemical, a contraction of ''agricultural chemical'', is a chemical product used in industrial agriculture. Agrichemical refers to biocides (pesticides including insecticides, herbicides, fungicides and nematicides) and sy ...
s contain fluorine, most of them herbicide
Herbicides (, ), also commonly known as weedkillers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page f ...
s and fungicides with a few crop regulators. Fluorine substitution, usually of a single atom or at most a trifluoromethyl group, is a robust modification with effects analogous to fluorinated pharmaceuticals: increased biological stay time, membrane crossing, and altering of molecular recognition. Trifluralin
Trifluralin is a commonly used pre-emergence herbicide. With about used in the United States in 2001, it is one of the most widely used herbicides. Trifluralin is generally applied to the soil to provide control of a variety of annual grass and ...
is a prominent example, with large-scale use in the U.S. as a weedkiller,.. but it is a suspected carcinogen and has been banned in many European countries. Sodium monofluoroacetate (1080) is a mammalian poison in which two acetic acid hydrogens are replaced with fluorine and sodium; it disrupts cell metabolism by replacing acetate in the citric acid cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and prot ...
. First synthesized in the late 19th century, it was recognized as an insecticide in the early 20th, and was later deployed in its current use. New Zealand, the largest consumer of 1080, uses it to protect kiwis from the invasive Australian common brushtail possum.. Europe and the U.S. have banned 1080.
Medicinal applications
Dental care
Population studies from the mid-20th century onwards showtopical
A topical medication is a medication that is applied to a particular place on or in the body. Most often topical medication means application to body surfaces such as the skin or mucous membranes to treat ailments via a large range of classes ...
fluoride reduces dental caries. This was first attributed to the conversion of tooth enamel hydroxyapatite
Hydroxyapatite, also called hydroxylapatite (HA), is a naturally occurring mineral form of calcium apatite with the formula Ca5(PO4)3(OH), but it is usually written Ca10(PO4)6(OH)2 to denote that the crystal unit cell comprises two entities. ...
into the more durable fluorapatite, but studies on pre-fluoridated teeth refuted this hypothesis, and current theories involve fluoride aiding enamel growth in small caries.. After studies of children in areas where fluoride was naturally present in drinking water, controlled public water supply fluoridation to fight tooth decay began in the 1940s and is now applied to water supplying 6 percent of the global population, including two-thirds of Americans... Reviews of the scholarly literature in 2000 and 2007 associated water fluoridation with a significant reduction of tooth decay in children.; see for a summary. Despite such endorsements and evidence of no adverse effects other than mostly benign dental fluorosis, opposition
Opposition may refer to:
Arts and media
* ''Opposition'' (Altars EP), 2011 EP by Christian metalcore band Altars
* The Opposition (band), a London post-punk band
* '' The Opposition with Jordan Klepper'', a late-night television series on Com ...
still exists on ethical and safety grounds. The benefits of fluoridation have lessened, possibly due to other fluoride sources, but are still measurable in low-income groups. Sodium monofluorophosphate and sometimes sodium or tin(II) fluoride
Tin(II) fluoride, commonly referred to commercially as stannous fluoride (from Latin ', 'tin'), is a chemical compound with the formula SnF2. It is a colourless solid used as an ingredient in toothpastes.
Oral health benefits
Stannous fluoride wa ...
are often found in fluoride toothpaste
Toothpaste is a paste or gel dentifrice used with a toothbrush to clean and maintain the aesthetics and health of teeth. Toothpaste is used to promote oral hygiene: it is an abrasive that aids in removing dental plaque and food from the teeth, ...
s, first introduced in the U.S. in 1955 and now ubiquitous in developed countries, alongside fluoridated mouthwashes, gels, foams, and varnishes...
Pharmaceuticals
 Twenty percent of modern pharmaceuticals contain fluorine.. One of these, the cholesterol-reducer
Twenty percent of modern pharmaceuticals contain fluorine.. One of these, the cholesterol-reducer atorvastatin
Atorvastatin is a statin medication used to prevent cardiovascular disease in those at high risk and to treat abnormal lipid levels. For the prevention of cardiovascular disease, statins are a first-line treatment. It is taken by mouth.
Common ...
(Lipitor), made more revenue than any other drug until it became generic in 2011.. The combination asthma prescription Seretide, a top-ten revenue drug in the mid-2000s, contains two active ingredients, one of which – fluticasone – is fluorinated. Many drugs are fluorinated to delay inactivation and lengthen dosage periods because the carbon–fluorine bond is very stable.. Fluorination also increases lipophilicity because the bond is more hydrophobic than the carbon–hydrogen bond, and this often helps in cell membrane penetration and hence bioavailability..
Tricyclics and other pre-1980s antidepressants had several side effects due to their non-selective interference with neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, any main body part or target cell, may be another neuron, but could also be a gland or muscle cell.
Neu ...
s other than the serotonin
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and va ...
target; the fluorinated fluoxetine was selective and one of the first to avoid this problem. Many current antidepressants receive this same treatment, including the selective serotonin reuptake inhibitors: citalopram, its isomer escitalopram, and fluvoxamine and paroxetine. Quinolones
Quinolone may refer to:
* 2-Quinolone
* 4-Quinolone
4-Quinolone is an organic compound derived from quinoline. It and 2-quinolone are the two most important parent (meaning simplified) quinolones. 4-Quinolone exists in equilibrium with a mino ...
are artificial broad-spectrum antibiotics that are often fluorinated to enhance their effects. These include ciprofloxacin
Ciprofloxacin is a fluoroquinolone antibiotic used to treat a number of bacterial infections. This includes bone and joint infections, intra abdominal infections, certain types of infectious diarrhea, respiratory tract infections, skin i ...
and levofloxacin
Levofloxacin, sold under the brand name Levaquin among others, is an antibiotic medication. It is used to treat a number of bacterial infections including acute bacterial sinusitis, pneumonia, H. pylori (in combination with other medications), ...
.. Fluorine also finds use in steroids: fludrocortisone
Fludrocortisone, sold under the brand name Florinef, among others, is a corticosteroid used to treat adrenogenital syndrome, postural hypotension, and adrenal insufficiency. In adrenal insufficiency, it is generally taken together with hydroco ...
is a blood pressure-raising mineralocorticoid
Mineralocorticoids are a class of corticosteroids, which in turn are a class of steroid hormones. Mineralocorticoids are produced in the adrenal cortex and influence salt and water balances ( electrolyte balance and fluid balance). The primary ...
, and triamcinolone and dexamethasone
Dexamethasone is a glucocorticoid medication used to treat rheumatic problems, a number of skin diseases, severe allergies, asthma, chronic obstructive lung disease, croup, brain swelling, eye pain following eye surgery, superior vena ...
are strong glucocorticoids.. The majority of inhaled anesthetics are heavily fluorinated; the prototype halothane is much more inert and potent than its contemporaries. Later compounds such as the fluorinated ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again ...
s sevoflurane
Sevoflurane, sold under the brand name Sevorane, among others, is a sweet-smelling, nonflammable, highly fluorinated methyl isopropyl ether used as an inhalational anaesthetic for induction and maintenance of general anesthesia. After desfluran ...
and desflurane
Desflurane (1,2,2,2-tetrafluoroethyl difluoromethyl ether) is a highly fluorinated methyl ethyl ether used for maintenance of general anesthesia. Like halothane, enflurane, and isoflurane, it is a racemic mixture of (''R'') and (''S'') optical is ...
are better than halothane and are almost insoluble in blood, allowing faster waking times...
PET scanning
 Fluorine-18 is often found in
Fluorine-18 is often found in radioactive tracer
A radioactive tracer, radiotracer, or radioactive label is a chemical compound in which one or more atoms have been replaced by a radionuclide so by virtue of its radioactive decay it can be used to explore the mechanism of chemical reactions by ...
s for positron emission tomography, as its half-life of almost two hours is long enough to allow for its transport from production facilities to imaging centers.. The most common tracer is fluorodeoxyglucose which, after intravenous injection, is taken up by glucose-requiring tissues such as the brain and most malignant tumors; computer-assisted tomography can then be used for detailed imaging.
Oxygen carriers
Liquid fluorocarbons can hold large volumes of oxygen or carbon dioxide, more so than blood, and have attracted attention for their possible uses in artificial blood and in liquid breathing.. Because fluorocarbons do not normally mix with water, they must be mixed into emulsions (small droplets of perfluorocarbon suspended in water) to be used as blood.. One such product,Oxycyte
Perfluoro ''tert''-butylcyclohexane is a perfluorinated chemical compound (or perfluorocarbon, PFC). It is a component of the experimental therapeutic oxygen carrier called Oxycyte.
Chemical properties
Perfluoro ''tert''-butylcyclohexane is a ...
, has been through initial clinical trials. These substances can aid endurance athletes and are banned from sports; one cyclist's near death in 1998 prompted an investigation into their abuse. Applications of pure perfluorocarbon liquid breathing (which uses pure perfluorocarbon liquid, not a water emulsion) include assisting burn victims and premature babies with deficient lungs. Partial and complete lung filling have been considered, though only the former has had any significant tests in humans.. An Alliance Pharmaceuticals effort reached clinical trials but was abandoned because the results were not better than normal therapies..
Biological role
 Fluorine is not essential for humans and other mammals, but small amounts are known to be beneficial for the strengthening of dental enamel (where the formation of fluorapatite makes the enamel more resistant to attack, from acids produced by bacterial fermentation of sugars). Small amounts of fluorine may be beneficial for bone strength, but the latter has not been definitively established. Both the WHO and the Institute of Medicine of the US National Academies publish recommended daily allowance (RDA) and upper tolerated intake of fluorine, which varies with age and gender.
Natural organofluorines have been found in microorganisms and plants but not animals. The most common is fluoroacetate, which is used as a defense against herbivores by at least 40 plants in Africa, Australia and Brazil. Other examples include terminally fluorinated
Fluorine is not essential for humans and other mammals, but small amounts are known to be beneficial for the strengthening of dental enamel (where the formation of fluorapatite makes the enamel more resistant to attack, from acids produced by bacterial fermentation of sugars). Small amounts of fluorine may be beneficial for bone strength, but the latter has not been definitively established. Both the WHO and the Institute of Medicine of the US National Academies publish recommended daily allowance (RDA) and upper tolerated intake of fluorine, which varies with age and gender.
Natural organofluorines have been found in microorganisms and plants but not animals. The most common is fluoroacetate, which is used as a defense against herbivores by at least 40 plants in Africa, Australia and Brazil. Other examples include terminally fluorinated fatty acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, f ...
s, fluoroacetone
Fluoroacetone is an organofluorine compound with the chemical formula . In contrast to trifluoroacetone, the compound has one fluorine atom. Under normal conditions, the substance is a colorless liquid. Fluoroacetone is also a highly toxic and fla ...
, and 2-fluorocitrate. An enzyme that binds fluorine to carbon – adenosyl-fluoride synthase – was discovered in bacteria in 2002.
Toxicity
Elemental fluorine is highly toxic to living organisms. Its effects in humans start at concentrations lower thanhydrogen cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on a ...
's 50 ppm and are similar to those of chlorine: significant irritation of the eyes and respiratory system as well as liver and kidney damage occur above 25 ppm, which is the immediately dangerous to life and health value for fluorine.. The eyes and nose are seriously damaged at 100 ppm, and inhalation of 1,000 ppm fluorine will cause death in minutes, compared to 270 ppm for hydrogen cyanide.
Hydrofluoric acid
 Hydrofluoric acid is the weakest of the hydrohalic acids, having a
Hydrofluoric acid is the weakest of the hydrohalic acids, having a pKa
PKA may refer to:
* Professionally known as:
** Pen name
** Stage persona
* p''K''a, the symbol for the acid dissociation constant at logarithmic scale
* Protein kinase A, a class of cAMP-dependent enzymes
* Pi Kappa Alpha, the North-American so ...
of 3.2 at 25 °C. It is a volatile liquid due to the presence of hydrogen bonding (while the other hydrohalic acids are gases). It is able to attack glass, concrete, metals, and organic matter.
Hydrofluoric acid is a contact poison with greater hazards than many strong acids like sulfuric acid even though it is weak: it remains neutral in aqueous solution and thus penetrates tissue faster, whether through inhalation, ingestion or the skin, and at least nine U.S. workers died in such accidents from 1984 to 1994. It reacts with calcium and magnesium in the blood leading to hypocalcemia and possible death through cardiac arrhythmia
Arrhythmias, also known as cardiac arrhythmias, heart arrhythmias, or dysrhythmias, are irregularities in the heartbeat, including when it is too fast or too slow. A resting heart rate that is too fast – above 100 beats per minute in adult ...
.. Insoluble calcium fluoride formation triggers strong pain. and burns larger than 160 cm2 (25 in2) can cause serious systemic toxicity..
Exposure may not be evident for eight hours for 50% HF, rising to 24 hours for lower concentrations, and a burn may initially be painless as hydrogen fluoride affects nerve function. If skin has been exposed to HF, damage can be reduced by rinsing it under a jet of water for 10–15 minutes and removing contaminated clothing. Calcium gluconate is often applied next, providing calcium ions to bind with fluoride; skin burns can be treated with 2.5% calcium gluconate gel or special rinsing solutions.... Hydrofluoric acid absorption requires further medical treatment; calcium gluconate may be injected or administered intravenously. Using calcium chloride – a common laboratory reagent – in lieu of calcium gluconate is contraindicated, and may lead to severe complications. Excision or amputation of affected parts may be required.
Fluoride ion
Soluble fluorides are moderately toxic: 5–10 g sodium fluoride, or 32–64 mg fluoride ions per kilogram of body mass, represents a lethal dose for adults. One-fifth of the lethal dose can cause adverse health effects, and chronic excess consumption may lead to skeletal fluorosis, which affects millions in Asia and Africa.. Ingested fluoride forms hydrofluoric acid in the stomach which is easily absorbed by the intestines, where it crosses cell membranes, binds with calcium and interferes with various enzymes, before urinaryexcretion
Excretion is a process in which metabolic waste
is eliminated from an organism. In vertebrates this is primarily carried out by the lungs, kidneys, and skin. This is in contrast with secretion, where the substance may have specific tasks after ...
. Exposure limits are determined by urine testing of the body's ability to clear fluoride ions..
Historically, most cases of fluoride poisoning have been caused by accidental ingestion of insecticides containing inorganic fluorides.. Most current calls to poison control centers for possible fluoride poisoning come from the ingestion of fluoride-containing toothpaste. Malfunctioning water fluoridation equipment is another cause: one incident in Alaska affected almost 300 people and killed one person.. Dangers from toothpaste are aggravated for small children, and the Centers for Disease Control and Prevention
The Centers for Disease Control and Prevention (CDC) is the national public health agency of the United States. It is a United States federal agency, under the Department of Health and Human Services, and is headquartered in Atlanta, Georg ...
recommends supervising children below six brushing their teeth so that they do not swallow toothpaste. One regional study examined a year of pre-teen fluoride poisoning reports totaling 87 cases, including one death from ingesting insecticide. Most had no symptoms, but about 30% had stomach pains. A larger study across the U.S. had similar findings: 80% of cases involved children under six, and there were few serious cases.
Environmental concerns
Atmosphere
 The
The Montreal Protocol
The Montreal Protocol is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances that are responsible for ozone depletion. It was agreed on 16 September 1987, and entered into force ...
, signed in 1987, set strict regulations on chlorofluorocarbons (CFCs) and bromofluorocarbons due to their ozone damaging potential (ODP). The high stability which suited them to their original applications also meant that they were not decomposing until they reached higher altitudes, where liberated chlorine and bromine atoms attacked ozone molecules.. Even with the ban, and early indications of its efficacy, predictions warned that several generations would pass before full recovery. With one-tenth the ODP of CFCs, hydrochlorofluorocarbons (HCFCs) are the current replacements,. and are themselves scheduled for substitution by 2030–2040 by hydrofluorocarbons (HFCs) with no chlorine and zero ODP.. In 2007 this date was brought forward to 2020 for developed countries;. the Environmental Protection Agency had already prohibited one HCFC's production and capped those of two others in 2003. Fluorocarbon gases are generally greenhouse gases with global-warming potentials (GWPs) of about 100 to 10,000; sulfur hexafluoride has a value of around 20,000. An outlier is HFO-1234yf which is a new type of refrigerant called a Hydrofluoroolefin (HFO) and has attracted global demand due to its GWP of less than 1 compared to 1,430 for the current refrigerant standard HFC-134a.
Biopersistence
 Organofluorines exhibit biopersistence due to the strength of the carbon–fluorine bond. Perfluoroalkyl acids (PFAAs), which are sparingly water-soluble owing to their acidic functional groups, are noted persistent organic pollutants; perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) are most often researched. PFAAs have been found in trace quantities worldwide from polar bears to humans, with PFOS and PFOA known to reside in breast milk and the blood of newborn babies. A 2013 review showed a slight correlation between groundwater and soil PFAA levels and human activity; there was no clear pattern of one chemical dominating, and higher amounts of PFOS were correlated to higher amounts of PFOA.. In the body, PFAAs bind to proteins such as serum albumin; they tend to concentrate within humans in the liver and blood before excretion through the kidneys. Dwell time in the body varies greatly by species, with half-lives of days in rodents, and years in humans.... High doses of PFOS and PFOA cause cancer and death in newborn rodents but human studies have not established an effect at current exposure levels.
Organofluorines exhibit biopersistence due to the strength of the carbon–fluorine bond. Perfluoroalkyl acids (PFAAs), which are sparingly water-soluble owing to their acidic functional groups, are noted persistent organic pollutants; perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) are most often researched. PFAAs have been found in trace quantities worldwide from polar bears to humans, with PFOS and PFOA known to reside in breast milk and the blood of newborn babies. A 2013 review showed a slight correlation between groundwater and soil PFAA levels and human activity; there was no clear pattern of one chemical dominating, and higher amounts of PFOS were correlated to higher amounts of PFOA.. In the body, PFAAs bind to proteins such as serum albumin; they tend to concentrate within humans in the liver and blood before excretion through the kidneys. Dwell time in the body varies greatly by species, with half-lives of days in rodents, and years in humans.... High doses of PFOS and PFOA cause cancer and death in newborn rodents but human studies have not established an effect at current exposure levels.
See also
* Argon fluoride laser * Electrophilic fluorination * Fluoride selective electrode, which measures fluoride concentration *Fluorine absorption dating
Fluorine absorption dating is a method used to determine the amount of time an object has been underground.
Fluorine absorption dating can be carried out based on the fact that groundwater contains fluoride ions. Items such as bone that are in t ...
* Fluorous chemistry
Fluorous chemistry involves the use of perfluorinated compounds or perfluorinated substituents to facilitate recovery of a catalyst or reaction product. Perfluorinated groups impart unique physical properties including high solubility in perfluor ...
, a process used to separate reagents from organic solvents
* Krypton fluoride laser
* Radical fluorination Radical fluorination is a type of fluorination reaction, complementary to nucleophilic and electrophilic approaches. It involves the reaction of an independently generated carbon-centered radical with an atomic fluorine source and yields an organof ...
Notes
Sources
Citations
Indexed references
* * * * * * * * * * *. * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * *< * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * *External links
* {{authority control Chemical elements Halogens Reactive nonmetals Diatomic nonmetals Fluorinating agents Oxidizing agents Industrial gases Gases with color