|
Radon
Radon is a chemical element with the symbol Rn and atomic number 86. It is a radioactive, colourless, odourless, tasteless noble gas. It occurs naturally in minute quantities as an intermediate step in the normal radioactive decay chains through which thorium and uranium slowly decay into various short-lived radioactive elements and lead. Radon itself is the immediate decay product of radium. Its most stable isotope, 222Rn, has a half-life of only 3.8 days, making it one of the rarest elements. Since thorium and uranium are two of the most common radioactive elements on Earth, while also having three isotopes with half-lives on the order of several billion years, radon will be present on Earth long into the future despite its short half-life. The decay of radon produces many other short-lived nuclides, known as "radon daughters", ending at stable isotopes of lead.+ ion is believed to form by the following reaction: : Rn (g) + 2 (s) → (s) + 2 (g) For this reason, antimon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radon Spectrum
Radon is a chemical element with the symbol Rn and atomic number 86. It is a radioactive, colourless, odourless, tasteless noble gas. It occurs naturally in minute quantities as an intermediate step in the normal radioactive decay chains through which thorium and uranium slowly decay into various short-lived radioactive elements and lead. Radon itself is the immediate decay product of radium. Its most stable isotope, 222Rn, has a half-life of only 3.8 days, making it one of the rarest elements. Since thorium and uranium are two of the most common radioactive elements on Earth, while also having three isotopes with half-lives on the order of several billion years, radon will be present on Earth long into the future despite its short half-life. The decay of radon produces many other short-lived nuclides, known as "radon daughters", ending at stable isotopes of lead.+ ion is believed to form by the following reaction: : Rn (g) + 2 (s) → (s) + 2 (g) For this reason ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radon Mitigation
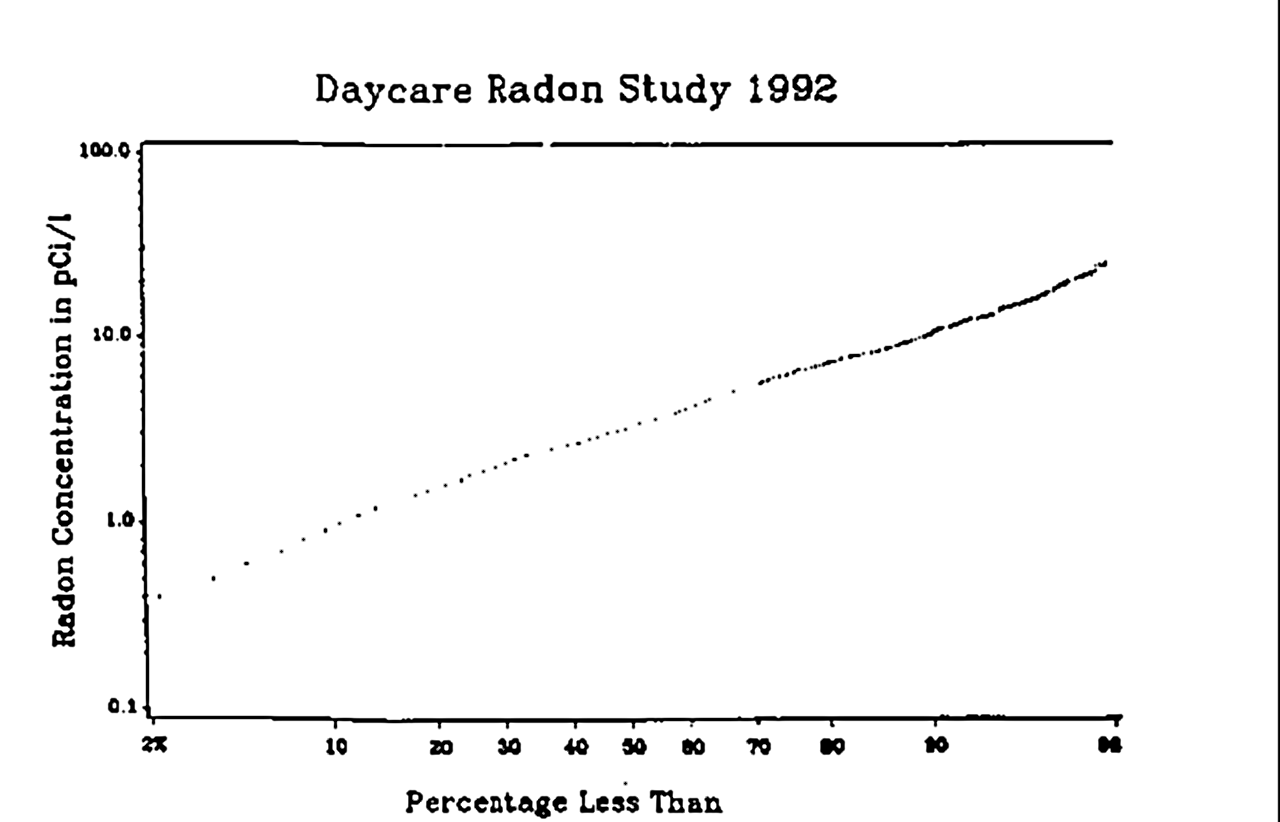
Radon mitigation is any process used to reduce radon gas concentrations in the breathing zones of occupied buildings, or radon from water supplies. Radon is a significant contributor to environmental radioactivity and can cause serious health problems such as lung cancer. Mitigation of radon in the air is accomplished through ventilation, either collected below a concrete floor slab or a membrane on the ground, or by increasing the air changes per hour in the building. Treatment systems using aeration or activated charcoal are available to remove radon from domestic water supplies. Testing The first step in mitigation is testing. No level of radiation is considered completely safe, but as it cannot be totally eliminated, governments around the world have set various ''action levels'' to provide guidance on when radon concentrations should be reduced. The World Health Organization's International Radon Project has recommended an action level of 100 Bq/m3 (2.7 pCi/L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Background Radiation
Background radiation is a measure of the level of ionizing radiation present in the environment at a particular location which is not due to deliberate introduction of radiation sources. Background radiation originates from a variety of sources, both natural and artificial. These include both cosmic radiation and environmental radioactivity from naturally occurring radioactive materials (such as radon and radium), as well as man-made medical X-rays, fallout from nuclear weapons testing and nuclear accidents. Definition Background radiation is defined by the International Atomic Energy Agency as "Dose or dose rate (or an observed measure related to the dose or dose rate) attributable to all sources other than the one(s) specified. So a distinction is made between dose which is already in a location, which is defined here as being "background", and the dose due to a deliberately introduced and specified source. This is important where radiation measurements are taken of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Noble Gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemical reactivity. The six naturally occurring noble gases are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn). Oganesson (Og) is a synthetically produced highly radioactive element. Although IUPAC has used the term "noble gas" interchangeably with "group 18" and thus included oganesson, it may not be significantly chemically noble and is predicted to break the trend and be reactive due to relativistic effects. Because of the extremely short 0.7 ms half-life of its only known isotope, its chemistry has not yet been investigated. For the first six periods of the periodic table, the noble gases are exactly the members of group 18. Noble gases are typically highly unreactive excep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indoor Air Quality
Indoor air quality (IAQ) is the air quality within and around buildings and structures. IAQ is known to affect the health, comfort, and well-being of building occupants. Poor indoor air quality has been linked to sick building syndrome, reduced productivity, and impaired learning in schools. Common pollutants of indoor air include: Secondhand tobacco smoke, air pollutants from indoor combustion, radon, molds and other allergens, carbon monoxide, volatile organic compounds, legionella and other bacteria, asbestos fibers, carbon dioxide, ozone and particulates. Source control, filtration, and the use of ventilation to dilute contaminants are the primary methods for improving indoor air quality in most buildings. Determination of IAQ involves the collection of air samples, monitoring human exposure to pollutants, collection of samples on building surfaces, and computer modelling of air flow inside buildings. IAQ is part of indoor environmental quality (IEQ), which include ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decay Chain
In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". Most radioisotopes do not decay directly to a stable state, but rather undergo a series of decays until eventually a stable isotope is reached. Decay stages are referred to by their relationship to previous or subsequent stages. A ''parent isotope'' is one that undergoes decay to form a ''daughter isotope''. One example of this is uranium (atomic number 92) decaying into thorium (atomic number 90). The daughter isotope may be stable or it may decay to form a daughter isotope of its own. The daughter of a daughter isotope is sometimes called a ''granddaughter isotope''. The time it takes for a single parent atom to decay to an atom of its daughter isotope can vary widely, not only between different parent-daughter pairs, but also randomly between identical pairings of parent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radon-222
Radon-222 (222Rn, Rn-222, historically radium emanation or radon) is the most stable isotope of radon, with a half-life of approximately 3.8 days. It is transient in the decay chain of primordial uranium-238 and is the immediate decay product of radium-226. Radon-222 was first observed in 1899, and was identified as an isotope of a new element several years later. In 1957, the name ''radon'', formerly the name of only radon-222, became the name of the element. Owing to its gaseous nature and high radioactivity, radon-222 is one of the leading causes of lung cancer. History Following the 1898 discovery of radium through chemical analysis of radioactive ore, Marie and Pierre Curie observed a new radioactive substance emanating from radium in 1899 that was strongly radioactive for several days. Around the same time, Ernest Rutherford and Robert B. Owens observed a similar (though shorter-lived) emission from thorium compounds. German physicist Friedrich Ernst Dorn extensively stud ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lung Cancer
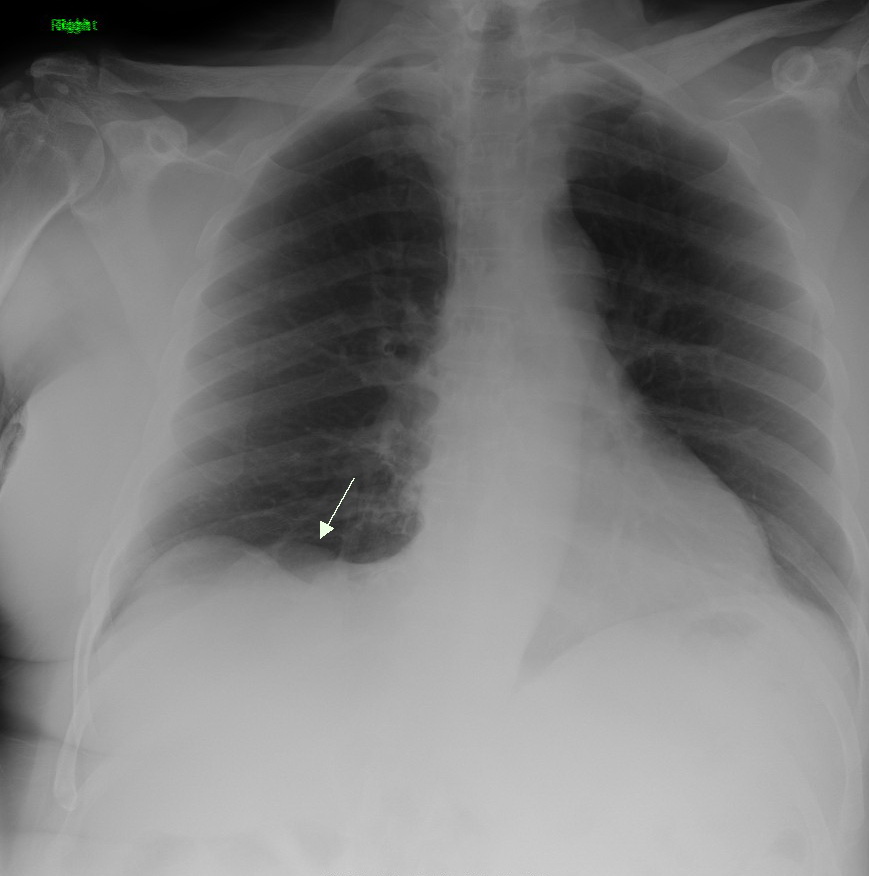
Lung cancer, also known as lung carcinoma (since about 98–99% of all lung cancers are carcinomas), is a malignant lung tumor characterized by uncontrolled cell growth in tissues of the lung. Lung carcinomas derive from transformed, malignant cells that originate as epithelial cells, or from tissues composed of epithelial cells. Other lung cancers, such as the rare sarcomas of the lung, are generated by the malignant transformation of connective tissues (i.e. nerve, fat, muscle, bone), which arise from mesenchymal cells. Lymphomas and melanomas (from lymphoid and melanocyte cell lineages) can also rarely result in lung cancer. In time, this uncontrolled growth can metastasize (spreading beyond the lung) either by direct extension, by entering the lymphatic circulation, or via hematogenous, bloodborne spread – into nearby tissue or other, more distant parts of the body. Most cancers that originate from within the lungs, known as primary lung cancers, are carcinomas. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Symbol (chemistry)
Chemical symbols are the abbreviations used in chemistry for chemical elements, functional groups and chemical compounds. Element symbols for chemical elements normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalised. History Earlier symbols for chemical elements stem from classical Latin and Greek vocabulary. For some elements, this is because the material was known in ancient times, while for others, the name is a more recent invention. For example, Pb is the symbol for lead (''plumbum'' in Latin); Hg is the symbol for mercury (''hydrargyrum'' in Greek); and He is the symbol for helium (a new Latin name) because helium was not known in ancient Roman times. Some symbols come from other sources, like W for tungsten (''Wolfram'' in German) which was not known in Roman times. A three-letter temporary symbol may be assigned to a newly synthesized (or not yet synthesized) element. For example, "Uno" was the temporary symbol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rather than oxygen) upon exposure to air, forming a black surface layer of radium nitride (Ra3N2). All isotopes of radium are radioactive, the most stable isotope being radium-226 with a half-life of 1600 years. When radium decays, it emits ionizing radiation as a by-product, which can excite fluorescent chemicals and cause radioluminescence. Radium, in the form of radium chloride, was discovered by Marie and Pierre Curie in 1898 from ore mined at Jáchymov. They extracted the radium compound from uraninite and published the discovery at the French Academy of Sciences five days later. Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne through the electrolysis of radium chloride in 1911. In nature, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Freezing Point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa. When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization point. Because of the ability of substances to supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact, the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the melting point." Examples For most substances, melting and freezing points are approximately equal. For example, the melting point ''and'' freezing point of mercury is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NASA
The National Aeronautics and Space Administration (NASA ) is an independent agency of the US federal government responsible for the civil space program, aeronautics research, and space research. NASA was established in 1958, succeeding the National Advisory Committee for Aeronautics (NACA), to give the U.S. space development effort a distinctly civilian orientation, emphasizing peaceful applications in space science. NASA has since led most American space exploration, including Project Mercury, Project Gemini, the 1968-1972 Apollo Moon landing missions, the Skylab space station, and the Space Shuttle. NASA supports the International Space Station and oversees the development of the Orion spacecraft and the Space Launch System for the crewed lunar Artemis program, Commercial Crew spacecraft, and the planned Lunar Gateway space station. The agency is also responsible for the Launch Services Program, which provides oversight of launch operations and countdown m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |