Radon on:
[Wikipedia]
[Google]
[Amazon]
Radon is a
,

 High concentrations of radon in homes were discovered by chance in 1985 after the stringent radiation testing conducted at the new Limerick Generating Station nuclear power plant revealed that Stanley Watras, a construction engineer at the plant, was contaminated by radioactive substances even though the reactor had never been fueled. Typical domestic exposures are of approximately 100 Bq/m3 (2.7 pCi/L) indoors. Some level of radon will be found in all buildings. Radon mostly enters a building directly from the soil through the lowest level in the building that is in contact with the ground. High levels of radon in the water supply can also increase indoor radon air levels. Typical entry points of radon into buildings are cracks in solid foundations and walls, construction joints, gaps in suspended floors and around service pipes, cavities inside walls, and the water supply. Radon concentrations in the same place may differ by double/half over one hour. Also, the concentration in one room of a building may be significantly different from the concentration in an adjoining room. The soil characteristics of the dwellings are the most important source of radon for the ground floor and higher concentration of indoor radon observed on lower floors. Most of the high radon concentrations have been reported from places near Fault (geology), fault zones; hence the existence of a relation between the exhalation rate from faults and indoor radon concentrations is obvious.
The distribution of radon concentrations will generally differ from room to room, and the readings are averaged according to regulatory protocols. Indoor radon concentration is usually assumed to follow a log-normal distribution on a given territory. Thus, the geometric mean is generally used for estimating the "average" radon concentration in an area.
The mean concentration ranges from less than 10 Bq/m3 to over 100 Bq/m3 in some European countries. Typical geometric standard deviations found in studies range between 2 and 3, meaning (given the 68–95–99.7 rule) that the radon concentration is expected to be more than a hundred times the mean concentration for 2% to 3% of the cases.
Some of the highest radon hazard in the US is found in Iowa and in the Appalachian Mountains, Appalachian Mountain areas in southeastern Pennsylvania. Iowa has the highest average radon concentrations in the US due to significant glaciation that ground the granitic rocks from the Canadian Shield and deposited it as soils making up the rich Iowa farmland. Many cities within the state, such as Iowa City, have passed requirements for radon-resistant construction in new homes. The second highest readings in Ireland were found in office buildings in the Irish town of Mallow, County Cork, prompting local fears regarding lung cancer.
In a few places, uranium tailings have been used for landfills and were subsequently built upon, resulting in possible increased exposure to radon.
Since radon is a colorless, odorless gas, the only way to know how much is present in the air or water is to perform tests. In the US, radon test kits are available to the public at retail stores, such as hardware stores, for home use, and testing is available through licensed professionals, who are often home inspectors. Efforts to reduce indoor radon levels are called radon mitigation. In the US, the EPA recommends all houses be tested for radon. In the UK under the Housing Health & Safety Rating System (HHSRS) property owners have an obligation to evaluate potential risks and hazards to health and safety in a residential property.
High concentrations of radon in homes were discovered by chance in 1985 after the stringent radiation testing conducted at the new Limerick Generating Station nuclear power plant revealed that Stanley Watras, a construction engineer at the plant, was contaminated by radioactive substances even though the reactor had never been fueled. Typical domestic exposures are of approximately 100 Bq/m3 (2.7 pCi/L) indoors. Some level of radon will be found in all buildings. Radon mostly enters a building directly from the soil through the lowest level in the building that is in contact with the ground. High levels of radon in the water supply can also increase indoor radon air levels. Typical entry points of radon into buildings are cracks in solid foundations and walls, construction joints, gaps in suspended floors and around service pipes, cavities inside walls, and the water supply. Radon concentrations in the same place may differ by double/half over one hour. Also, the concentration in one room of a building may be significantly different from the concentration in an adjoining room. The soil characteristics of the dwellings are the most important source of radon for the ground floor and higher concentration of indoor radon observed on lower floors. Most of the high radon concentrations have been reported from places near Fault (geology), fault zones; hence the existence of a relation between the exhalation rate from faults and indoor radon concentrations is obvious.
The distribution of radon concentrations will generally differ from room to room, and the readings are averaged according to regulatory protocols. Indoor radon concentration is usually assumed to follow a log-normal distribution on a given territory. Thus, the geometric mean is generally used for estimating the "average" radon concentration in an area.
The mean concentration ranges from less than 10 Bq/m3 to over 100 Bq/m3 in some European countries. Typical geometric standard deviations found in studies range between 2 and 3, meaning (given the 68–95–99.7 rule) that the radon concentration is expected to be more than a hundred times the mean concentration for 2% to 3% of the cases.
Some of the highest radon hazard in the US is found in Iowa and in the Appalachian Mountains, Appalachian Mountain areas in southeastern Pennsylvania. Iowa has the highest average radon concentrations in the US due to significant glaciation that ground the granitic rocks from the Canadian Shield and deposited it as soils making up the rich Iowa farmland. Many cities within the state, such as Iowa City, have passed requirements for radon-resistant construction in new homes. The second highest readings in Ireland were found in office buildings in the Irish town of Mallow, County Cork, prompting local fears regarding lung cancer.
In a few places, uranium tailings have been used for landfills and were subsequently built upon, resulting in possible increased exposure to radon.
Since radon is a colorless, odorless gas, the only way to know how much is present in the air or water is to perform tests. In the US, radon test kits are available to the public at retail stores, such as hardware stores, for home use, and testing is available through licensed professionals, who are often home inspectors. Efforts to reduce indoor radon levels are called radon mitigation. In the US, the EPA recommends all houses be tested for radon. In the UK under the Housing Health & Safety Rating System (HHSRS) property owners have an obligation to evaluate potential risks and hazards to health and safety in a residential property.
 There are relatively simple tests for radon gas. In some countries these tests are methodically done in areas of known systematic hazards. Radon detection devices are commercially available. Digital radon detectors provide ongoing measurements giving both daily, weekly, short-term and long-term average readouts via a digital display. Short-term radon test devices used for initial screening purposes are inexpensive, in some cases free. There are important protocols for taking short-term radon tests and it is imperative that they be strictly followed. The kit includes a collector that the user hangs in the lowest habitable floor of the house for two to seven days. The user then sends the collector to a laboratory for analysis. Long term kits, taking collections for up to one year or more, are also available. An open-land test kit can test radon emissions from the land before construction begins. Radon concentrations can vary daily, and accurate radon exposure estimates require long-term average radon measurements in the spaces where an individual spends a significant amount of time.
Radon levels fluctuate naturally, due to factors like transient weather conditions, so an initial test might not be an accurate assessment of a home's average radon level. Radon levels are at a maximum during the coolest part of the day when pressure differentials are greatest. Therefore, a high result (over 4 pCi/L) justifies repeating the test before undertaking more expensive abatement projects. Measurements between 4 and 10 pCi/L warrant a long-term radon test. Measurements over 10 pCi/L warrant only another short-term test so that abatement measures are not unduly delayed. Purchasers of real estate are advised to delay or decline a purchase if the seller has not successfully abated radon to 4 pCi/L or less.
Because the half-life of radon is only 3.8 days, removing or isolating the source will greatly reduce the hazard within a few weeks. Another method of reducing radon levels is to modify the building's ventilation. Generally, the indoor radon concentrations increase as ventilation rates decrease. In a well-ventilated place, the radon concentration tends to align with outdoor values (typically 10 Bq/m3, ranging from 1 to 100 Bq/m3).
The four principal ways of reducing the amount of radon accumulating in a house are:
* Sub-slab depressurization (soil suction) by increasing under-floor ventilation;
* Improving the ventilation of the house and avoiding the transport of radon from the basement into living rooms;
* Installing a radon sump system in the basement;
* Installing a positive pressurization or positive supply ventilation system.
According to the EPA, the method to reduce radon "...primarily used is a vent pipe system and fan, which pulls radon from beneath the house and vents it to the outside", which is also called sub-slab depressurization, active soil depressurization, or soil suction. Generally indoor radon can be mitigated by sub-slab depressurization and exhausting such radon-laden air to the outdoors, away from windows and other building openings. "[The] EPA generally recommends methods which prevent the entry of radon. Soil suction, for example, prevents radon from entering your home by drawing the radon from below the home and venting it through a pipe, or pipes, to the air above the home where it is quickly diluted" and the "EPA does not recommend the use of sealing alone to reduce radon because, by itself, sealing has not been shown to lower radon levels significantly or consistently".
Positive pressure ventilation, Positive-pressure ventilation systems can be combined with a heat exchanger to recover energy in the process of exchanging air with the outside, and simply exhausting basement air to the outside is not necessarily a viable solution as this can actually draw radon gas into a dwelling. Homes built on a crawl space may benefit from a radon collector installed under a "radon barrier" (a sheet of plastic that covers the crawl space).
For crawl spaces, the EPA states "An effective method to reduce radon levels in crawl space homes involves covering the earth floor with a high-density plastic sheet. A vent pipe and fan are used to draw the radon from under the sheet and vent it to the outdoors. This form of soil suction is called submembrane suction, and when properly applied is the most effective way to reduce radon levels in crawl space homes."
There are relatively simple tests for radon gas. In some countries these tests are methodically done in areas of known systematic hazards. Radon detection devices are commercially available. Digital radon detectors provide ongoing measurements giving both daily, weekly, short-term and long-term average readouts via a digital display. Short-term radon test devices used for initial screening purposes are inexpensive, in some cases free. There are important protocols for taking short-term radon tests and it is imperative that they be strictly followed. The kit includes a collector that the user hangs in the lowest habitable floor of the house for two to seven days. The user then sends the collector to a laboratory for analysis. Long term kits, taking collections for up to one year or more, are also available. An open-land test kit can test radon emissions from the land before construction begins. Radon concentrations can vary daily, and accurate radon exposure estimates require long-term average radon measurements in the spaces where an individual spends a significant amount of time.
Radon levels fluctuate naturally, due to factors like transient weather conditions, so an initial test might not be an accurate assessment of a home's average radon level. Radon levels are at a maximum during the coolest part of the day when pressure differentials are greatest. Therefore, a high result (over 4 pCi/L) justifies repeating the test before undertaking more expensive abatement projects. Measurements between 4 and 10 pCi/L warrant a long-term radon test. Measurements over 10 pCi/L warrant only another short-term test so that abatement measures are not unduly delayed. Purchasers of real estate are advised to delay or decline a purchase if the seller has not successfully abated radon to 4 pCi/L or less.
Because the half-life of radon is only 3.8 days, removing or isolating the source will greatly reduce the hazard within a few weeks. Another method of reducing radon levels is to modify the building's ventilation. Generally, the indoor radon concentrations increase as ventilation rates decrease. In a well-ventilated place, the radon concentration tends to align with outdoor values (typically 10 Bq/m3, ranging from 1 to 100 Bq/m3).
The four principal ways of reducing the amount of radon accumulating in a house are:
* Sub-slab depressurization (soil suction) by increasing under-floor ventilation;
* Improving the ventilation of the house and avoiding the transport of radon from the basement into living rooms;
* Installing a radon sump system in the basement;
* Installing a positive pressurization or positive supply ventilation system.
According to the EPA, the method to reduce radon "...primarily used is a vent pipe system and fan, which pulls radon from beneath the house and vents it to the outside", which is also called sub-slab depressurization, active soil depressurization, or soil suction. Generally indoor radon can be mitigated by sub-slab depressurization and exhausting such radon-laden air to the outdoors, away from windows and other building openings. "[The] EPA generally recommends methods which prevent the entry of radon. Soil suction, for example, prevents radon from entering your home by drawing the radon from below the home and venting it through a pipe, or pipes, to the air above the home where it is quickly diluted" and the "EPA does not recommend the use of sealing alone to reduce radon because, by itself, sealing has not been shown to lower radon levels significantly or consistently".
Positive pressure ventilation, Positive-pressure ventilation systems can be combined with a heat exchanger to recover energy in the process of exchanging air with the outside, and simply exhausting basement air to the outside is not necessarily a viable solution as this can actually draw radon gas into a dwelling. Homes built on a crawl space may benefit from a radon collector installed under a "radon barrier" (a sheet of plastic that covers the crawl space).
For crawl spaces, the EPA states "An effective method to reduce radon levels in crawl space homes involves covering the earth floor with a high-density plastic sheet. A vent pipe and fan are used to draw the radon from under the sheet and vent it to the outdoors. This form of soil suction is called submembrane suction, and when properly applied is the most effective way to reduce radon levels in crawl space homes."
Radon
an
at the
National Radon Program Services
hosted by Kansas State University
UK maps of radon
Radon Information from Public Health England
Frequently Asked Questions About Radon
at National Safety Council
Radon
at ''The Periodic Table of Videos'' (University of Nottingham)
Radon and Lung Health from the American Lung Association
Radon's impact on your health – Lung Association
The Geology of Radon
James K. Otton, Linda C.S. Gundersen, and R. Randall Schumann
An article by the International Association of Certified Home Inspectors (InterNACHI)
Toxicological Profile for Radon
Draft for Public Comment, Agency for Toxic Substances and Disease Registry, September 2008 * ''Health Effects of Exposure to Radon'': BEIR VI. Committee on Health Risks of Exposure to Radon (BEIR VI), National Research Counci
available on-line
with scientific annexes: Annex B: Exposures from natural radiation sources.
Should you measure the radon concentration in your home?
Phillip N. Price, Andrew Gelman, in ''Statistics: A Guide to the Unknown'', January 2004.
Radon in the Home- An Invisible Killer
How serious can high levels of radon be in the home? Kevin Vitali {{Authority control Radon, Chemical elements Noble gases Building biology Soil contamination IARC Group 1 carcinogens Carcinogens Industrial gases
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
with the symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Rn and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
86. It is a radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
, colourless, odourless, tasteless noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemi ...
. It occurs naturally in minute quantities as an intermediate step in the normal radioactive decay chain
In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". Most radioisotopes do not decay dire ...
s through which thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high me ...
and uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
slowly decay into various short-lived radioactive elements and lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
. Radon itself is the immediate decay product of radium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rathe ...
. Its most stable isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) ...
, 222Rn, has a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of only 3.8 days, making it one of the rarest elements. Since thorium and uranium are two of the most common radioactive elements on Earth, while also having three isotopes with half-lives on the order of several billion years, radon will be present on Earth long into the future despite its short half-life. The decay of radon produces many other short-lived nuclides, known as "radon daughters", ending at stable isotopes of lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
.Toxicological profile for radon,
Agency for Toxic Substances and Disease Registry
The Agency for Toxic Substances and Disease Registry (ATSDR) is a federal public health agency within the United States Department of Health and Human Services. The agency focuses on minimizing human health risks associated with exposure to haz ...
, U.S. Public Health Service, In collaboration with U.S. Environmental Protection Agency, December 1990.
Unlike all other intermediate elements in the aforementioned decay chains, radon is, under standard conditions, gaseous and easily inhaled, and therefore a health hazard. It is often the single largest contributor to an individual's background radiation
Background radiation is a measure of the level of ionizing radiation present in the environment at a particular location which is not due to deliberate introduction of radiation sources.
Background radiation originates from a variety of source ...
dose, but due to local differences in geology, the level of exposure to radon gas differs from place to place. A common source is uranium-containing minerals in the ground, and therefore it accumulates in subterranean areas such as basements. Radon can also occur in some ground water like spring
Spring(s) may refer to:
Common uses
* Spring (season)
Spring, also known as springtime, is one of the four temperate seasons, succeeding winter and preceding summer. There are various technical definitions of spring, but local usage of ...
waters and hot springs. Climate change
In common usage, climate change describes global warming—the ongoing increase in global average temperature—and its effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes to E ...
may cause radon previously trapped underground to be released as permafrost
Permafrost is ground that continuously remains below 0 °C (32 °F) for two or more years, located on land or under the ocean. Most common in the Northern Hemisphere, around 15% of the Northern Hemisphere or 11% of the global surface ...
thaws, particularly in areas like the Arctic, Alaska, Canada, Greenland and Russia. It is possible to test for radon and use techniques such as sub slab depressurization for mitigation
Mitigation is the reduction of something harmful or the reduction of its harmful effects. It may refer to measures taken to reduce the harmful effects of hazards that remain ''in potentia'', or to manage harmful incidents that have already occur ...
.
Epidemiological
Epidemiology is the study and analysis of the distribution (who, when, and where), patterns and determinants of health and disease conditions in a defined population.
It is a cornerstone of public health, and shapes policy decisions and evidenc ...
studies have shown a clear link between breathing high concentrations of radon and incidence of lung cancer
Lung cancer, also known as lung carcinoma (since about 98–99% of all lung cancers are carcinomas), is a malignant lung tumor characterized by uncontrolled cell growth in tissue (biology), tissues of the lung. Lung carcinomas derive from tran ...
. Radon is a contaminant that affects indoor air quality
Indoor air quality (IAQ) is the air quality within and around buildings and structures. IAQ is known to affect the health, comfort, and well-being of building occupants. Poor indoor air quality has been linked to sick building syndrome, reduce ...
worldwide. According to the United States Environmental Protection Agency
The Environmental Protection Agency (EPA) is an independent executive agency of the United States federal government tasked with environmental protection matters. President Richard Nixon proposed the establishment of EPA on July 9, 1970; it be ...
(EPA), radon is the second most frequent cause of lung cancer, after cigarette smoking, causing 21,000 lung cancer deaths per year in the United States. About 2,900 of these deaths occur among people who have never smoked. While radon is the second most frequent cause of lung cancer, it is the number one cause among non-smokers, according to EPA policy-oriented estimates. Significant uncertainties exist for the health effects of low-dose exposures. Unlike the gaseous radon itself, radon daughters are solids and stick to surfaces, such as airborne dust particles, which can cause lung cancer if inhaled.
Characteristics

Physical properties
Radon is a colorless, odorless, and tasteless gas and therefore is not detectable by human senses alone. At standard temperature and pressure, it forms amonatomic gas
In physics and chemistry, "monatomic" is a combination of the words "mono" and "atomic", and means "single atom". It is usually applied to gases: a monatomic gas is a gas in which atoms are not bound to each other. Examples at standard conditions ...
with a density of 9.73 kg/m3, about 8 times the density of the Earth's atmosphere
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing for ...
at sea level, 1.217 kg/m3. It is one of the densest gases at room temperature and is the densest of the noble gases. Although colorless at standard temperature and pressure, when cooled below its freezing point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depend ...
of , it emits a brilliant radioluminescence
Radioluminescence is the phenomenon by which light is produced in a material by bombardment with ionizing radiation such as alpha particles, beta particles, or gamma rays. Radioluminescence is used as a low level light source for night illumi ...
that turns from yellow to orange-red as the temperature lowers. Upon condensation, it glows because of the intense radiation it produces. It is sparingly soluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
in water, but more soluble than lighter noble gases. It is appreciably more soluble in organic liquids than in water. Its solubility equation is as follows,
:
where is the molar fraction of radon, is the absolute temperature, and and are solvent constants.
Chemical properties
Radon is a member of the zero- valence elements that are called noble gases, and is chemically not veryreactive
Reactive may refer to:
*Generally, capable of having a reaction (disambiguation)
*An adjective abbreviation denoting a bowling ball coverstock made of reactive resin
*Reactivity (chemistry)
*Reactive mind
*Reactive programming
See also
*Reactanc ...
. The 3.8-day half-life of radon-222 makes it useful in physical sciences as a natural tracer. Because radon is a gas at standard conditions, unlike its decay-chain parents, it can readily be extracted from them for research.
It is inert to most common chemical reactions, such as combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
, because the outer valence shell
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
contains eight electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s. This produces a stable, minimum energy configuration in which the outer electrons are tightly bound. Its first ionization energy—the minimum energy required to extract one electron from it—is 1037 kJ/mol. In accordance with periodic trends
Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element. They were discovered by the Russian chemist Dmitri Mendeleev in the year 1863. Major periodic trends include atom ...
, radon has a lower electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
than the element one period before it, xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
, and is therefore more reactive. Early studies concluded that the stability of radon hydrate should be of the same order as that of the hydrates of chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
() or sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activ ...
(), and significantly higher than the stability of the hydrate of hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
().
Because of its cost and radioactivity, experimental chemical research is seldom performed with radon, and as a result there are very few reported compounds of radon, all either fluorides or oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
s. Radon can be oxidized
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
by powerful oxidizing agents such as fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
, thus forming radon difluoride
Radon difluoride () is a compound of radon, a radioactive noble gas. Radon reacts readily with fluorine to form a solid compound, but this decomposes on attempted vaporization and its exact composition is uncertain. Calculations suggest that it ...
(). It decomposes back to its elements at a temperature of above , and is reduced by water to radon gas and hydrogen fluoride: it may also be reduced back to its elements by hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
gas. It has a low volatility and was thought to be . Because of the short half-life of radon and the radioactivity of its compounds, it has not been possible to study the compound in any detail. Theoretical studies on this molecule predict that it should have a Rn–F bond distance
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of ...
of 2.08 ångström
The angstromEntry "angstrom" in the Oxford online dictionary. Retrieved on 2019-03-02 from https://en.oxforddictionaries.com/definition/angstrom.Entry "angstrom" in the Merriam-Webster online dictionary. Retrieved on 2019-03-02 from https://www.m ...
(Å), and that the compound is thermodynamically more stable and less volatile than its lighter counterpart xenon difluoride
Xenon difluoride is a powerful fluorinating agent with the chemical formula , and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherw ...
(). The octahedral molecule was predicted to have an even lower enthalpy of formation
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant p ...
than the difluoride. The nFsup>+ ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
is believed to form by the following reaction:
: Rn (g) + 2 (s) → (s) + 2 (g)
For this reason, antimony pentafluoride together with chlorine trifluoride
Chlorine trifluoride is an interhalogen compound with the formula ClF3. This colorless, poisonous, corrosive, and extremely reactive gas condenses to a pale-greenish yellow liquid, the form in which it is most often sold (pressurized at room temp ...
and have been considered for radon gas removal in uranium mines
Uranium production is carried out in about 13 countries around the world, in 2017 producing a cumulative total of 59,462 tonnes of uranium (tU). The international producers were Kazakhstan (39%), Canada (22%), Australia (10%), Namibia (7.1%), Nig ...
due to the formation of radon–fluorine compounds. Radon compounds can be formed by the decay of radium in radium halides, a reaction that has been used to reduce the amount of radon that escapes from targets during irradiation
Irradiation is the process by which an object is exposed to radiation. The exposure can originate from various sources, including natural sources. Most frequently the term refers to ionizing radiation, and to a level of radiation that will serve ...
. Additionally, salts of the nFsup>+ cation with the anions , , and are known. Radon is also oxidised by dioxygen difluoride
Dioxygen difluoride is a compound of fluorine and oxygen with the molecular formula O2F2. It can exist as an orange-colored solid which melts into a red liquid at . It is an extremely strong oxidant and decomposes into oxygen and fluorine even ...
to at .
Radon oxides are among the few other reported compounds of radon; only the trioxide () has been confirmed. The higher fluorides and have been claimed and are calculated to be stable, but their identification is unclear. They may have been observed in experiments where unknown radon-containing products distilled together with xenon hexafluoride
Xenon hexafluoride is a noble gas compound with the formula XeF6. It is one of the three binary fluorides of xenon, the other two being XeF2 and XeF4. All known are exergonic and stable at normal temperatures. XeF6 is the strongest fluorinati ...
: these may have been , , or both. Trace-scale heating of radon with xenon, fluorine, bromine pentafluoride
Bromine pentafluoride, Br F5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent.
BrF5 finds use in oxygen isotope analysis. Laser ablation of solid silicates in the presence of BrF5 releases O2 for subseq ...
, and either sodium fluoride
Sodium fluoride (NaF) is an inorganic compound with the formula . It is used in trace amounts in the fluoridation of drinking water, in toothpaste, in metallurgy, and as a flux. It is a colorless or white solid that is readily soluble in water. I ...
or nickel fluoride was claimed to produce a higher fluoride as well which hydrolysed
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
to form . While it has been suggested that these claims were really due to radon precipitating out as the solid complex nFNiF6]2−, the fact that radon Coprecipitation, coprecipitates from aqueous solution with has been taken as confirmation that was formed, which has been supported by further studies of the hydrolysed solution. That nO3Fsup>− did not form in other experiments may have been due to the high concentration of fluoride used. Electromigration studies also suggest the presence of cationic RnO3sup>+ and anionic RnO4sup>− forms of radon in weakly acidic aqueous solution (pH > 5), the procedure having previously been validated by examination of the homologous xenon trioxide.
The decay technique has also been used. Avrorin et al. reported in 1982 that 212 Fr compounds cocrystallised with their caesium analogues appeared to retain chemically bound radon after electron capture; analogies with xenon suggested the formation of RnO3, but this could not be confirmed.
It is likely that the difficulty in identifying higher fluorides of radon stems from radon being kinetically hindered from being oxidised beyond the divalent state because of the strong ionicity of radon difluoride
Radon difluoride () is a compound of radon, a radioactive noble gas. Radon reacts readily with fluorine to form a solid compound, but this decomposes on attempted vaporization and its exact composition is uncertain. Calculations suggest that it ...
() and the high positive charge on radon in RnF+; spatial separation of molecules may be necessary to clearly identify higher fluorides of radon, of which is expected to be more stable than due to spin–orbit splitting of the 6p shell of radon (RnIV would have a closed-shell 6s6p configuration). Therefore, while should have a similar stability to xenon tetrafluoride
Xenon tetrafluoride is a chemical compound with chemical formula . It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine:
: Xe + 2 →
This reaction is exothermic, rele ...
(), would likely be much less stable than xenon hexafluoride
Xenon hexafluoride is a noble gas compound with the formula XeF6. It is one of the three binary fluorides of xenon, the other two being XeF2 and XeF4. All known are exergonic and stable at normal temperatures. XeF6 is the strongest fluorinati ...
(): radon hexafluoride would also probably be a regular octahedral molecule, unlike the distorted octahedral structure of , because of the inert pair effect The inert-pair effect is the tendency of the two electrons in the outermost atomic ''s''-orbital to remain unshared in compounds of post-transition metals. The term ''inert-pair effect'' is often used in relation to the increasing stability of ox ...
. Because radon is quite electropositive for a noble gas, it is possible that radon fluorides actually take on highly fluorine-bridged structures and are not volatile. Extrapolation down the noble gas group would suggest also the possible existence of RnO, RnO2, and RnOF4, as well as the first chemically stable noble gas chlorides RnCl2 and RnCl4, but none of these have yet been found.
Radon carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
(RnCO) has been predicted to be stable and to have a linear molecular geometry. The molecules and RnXe were found to be significantly stabilized by spin-orbit coupling. Radon caged inside a fullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ...
has been proposed as a drug for tumors
A neoplasm () is a type of abnormal and excessive growth of tissue. The process that occurs to form or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists ...
. Despite the existence of Xe(VIII), no Rn(VIII) compounds have been claimed to exist; should be highly unstable chemically (XeF8 is thermodynamically unstable). It is predicted that the most stable Rn(VIII) compound would be barium perradonate (Ba2RnO6), analogous to barium perxenate In chemistry, perxenates are salts of the yellow xenon-containing anion . This anion has octahedral molecular geometry, as determined by Raman spectroscopy, having O–Xe–O bond angles varying between 87° and 93°. The Xe–O bond length was d ...
. The instability of Rn(VIII) is due to the relativistic stabilization of the 6s shell, also known as the inert pair effect The inert-pair effect is the tendency of the two electrons in the outermost atomic ''s''-orbital to remain unshared in compounds of post-transition metals. The term ''inert-pair effect'' is often used in relation to the increasing stability of ox ...
.
Radon reacts with the liquid halogen fluorides ClF, , , , , and to form . In halogen fluoride solution, radon is nonvolatile and exists as the RnF+ and Rn2+ cations; addition of fluoride anions results in the formation of the complexes and , paralleling the chemistry of beryllium
Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to form m ...
(II) and aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
(III). The standard electrode potential
In electrochemistry, standard electrode potential E^\ominus, or E^\ominus_, is a measure of the reducing power of any element or compound. The IUPAC "Gold Book" defines it as: ''"the value of the standard emf (electromotive force) of a cell in wh ...
of the Rn2+/Rn couple has been estimated as +2.0 V, although there is no evidence for the formation of stable radon ions or compounds in aqueous solution.
Isotopes
Radon has nostable isotope
The term stable isotope has a meaning similar to stable nuclide, but is preferably used when speaking of nuclides of a specific element. Hence, the plural form stable isotopes usually refers to isotopes of the same element. The relative abundanc ...
s. Thirty-nine radioactive isotopes have been characterized, with atomic mass
The atomic mass (''m''a or ''m'') is the mass of an atom. Although the SI unit of mass is the kilogram (symbol: kg), atomic mass is often expressed in the non-SI unit dalton (symbol: Da) – equivalently, unified atomic mass unit (u). 1&nb ...
es ranging from 193 to 231. The most stable isotope is 222Rn, which is a decay product of 226Ra, a decay product of 238U. A trace amount of the (highly unstable) isotope 218Rn is also among the daughters of 222Rn.
Three other radon isotopes have a half-life of over an hour: 211Rn, 210Rn and 224Rn. The 220Rn isotope is a natural decay product of the most stable thorium isotope (232Th), and is commonly referred to as thoron. It has a half-life of 55.6 seconds and also emits alpha radiation
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
. Similarly, 219Rn is derived from the most stable isotope of actinium
Actinium is a chemical element with the symbol Ac and atomic number 89. It was first isolated by Friedrich Oskar Giesel in 1902, who gave it the name ''emanium''; the element got its name by being wrongly identified with a substance An ...
(227Ac)—named "actinon"—and is an alpha emitter with a half-life of 3.96 seconds. No radon isotopes occur significantly in the neptunium
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after the planet Uranus, led to it bein ...
(237Np) decay series
In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". Most radioisotopes do not decay direc ...
, though a trace amount of the (extremely unstable) isotope 217Rn is produced.
Daughters
222Rn belongs to the radium and uranium-238 decay chain, and has a half-life of 3.8235 days. Its first four products (excluding marginaldecay scheme
The decay scheme of a radioactive substance is a graphical presentation of all the transitions occurring in a decay, and of their relationships. Examples are shown below.
It is useful to think of the decay scheme as placed in a coordinate system, ...
s) are very short-lived, meaning that the corresponding disintegrations are indicative of the initial radon distribution. Its decay goes through the following sequence:
* 222Rn, 3.82 days, alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
ing to...
* 218 Po, 3.10 minutes, alpha decaying to...
* 214 Pb, 26.8 minutes, beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
ing to...
* 214 Bi, 19.9 minutes, beta decaying to...
* 214Po, 0.1643 ms, alpha decaying to...
* 210Pb, which has a much longer half-life of 22.3 years, beta decaying to...
* 210Bi, 5.013 days, beta decaying to...
* 210Po, 138.376 days, alpha decaying to...
* 206Pb, stable.
The radon equilibrium factor is the ratio between the activity of all short-period radon progenies (which are responsible for most of radon's biological effects), and the activity that would be at equilibrium with the radon parent.
If a closed volume is constantly supplied with radon, the concentration of short-lived isotopes will increase until an equilibrium is reached where the rate of decay of each decay product will equal that of the radon itself. The equilibrium factor is 1 when both activities are equal, meaning that the decay products have stayed close to the radon parent long enough for the equilibrium to be reached, within a couple of hours. Under these conditions, each additional pCi/L of radon will increase exposure by 0.01 ''working level
Working level (WL) is a historical unit of concentration of radioactive decay products of radon, applied to uranium mining environment. One working level refers to the concentration of short-lived decay products of radon in equilibrium with 3,700 ...
'' (WL, a measure of radioactivity commonly used in mining). These conditions are not always met; in many homes, the equilibrium factor is typically 40%; that is, there will be 0.004 WL of daughters for each pCi/L of radon in the air. 210Pb takes much longer (decades) to come in equilibrium with radon, but, if the environment permits accumulation of dust over extended periods of time, 210Pb and its decay products may contribute to overall radiation levels as well.
Because of their electrostatic charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respectiv ...
, radon progenies adhere to surfaces or dust particles, whereas gaseous radon does not. Attachment removes them from the air, usually causing the equilibrium factor in the atmosphere to be less than 1. The equilibrium factor is also lowered by air circulation or air filtration devices, and is increased by airborne dust particles, including cigarette smoke. The equilibrium factor found in epidemiological studies is 0.4.
History and etymology
Radon was the fifth radioactive element to be discovered, in 1899 byErnest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson, (30 August 1871 – 19 October 1937) was a New Zealand physicist who came to be known as the father of nuclear physics.
''Encyclopædia Britannica'' considers him to be the greatest ...
and Robert B. Owens at McGill University
McGill University (french: link=no, Université McGill) is an English-language public research university located in Montreal, Quebec, Canada. Founded in 1821 by royal charter granted by King George IV,Frost, Stanley Brice. ''McGill Universit ...
in Montreal
Montreal ( ; officially Montréal, ) is the List of the largest municipalities in Canada by population, second-most populous city in Canada and List of towns in Quebec, most populous city in the Provinces and territories of Canada, Canadian ...
, after uranium, thorium, radium, and polonium. In 1899, Pierre
Pierre is a masculine given name. It is a French form of the name Peter. Pierre originally meant "rock" or "stone" in French (derived from the Greek word πέτρος (''petros'') meaning "stone, rock", via Latin "petra"). It is a translation ...
and Marie Curie
Marie Salomea Skłodowska–Curie ( , , ; born Maria Salomea Skłodowska, ; 7 November 1867 – 4 July 1934) was a Polish and naturalized-French physicist and chemist who conducted pioneering research on radioactivity. She was the first ...
observed that the gas emitted by radium remained radioactive for a month. Later that year, Rutherford and Owens noticed variations when trying to measure radiation from thorium oxide.: "The radiation from thorium oxide was not constant, but varied in a most capricious manner", whereas "All the compounds of Uranium give out a radiation which is remarkably constant." Rutherford noticed that the compounds of thorium continuously emit a radioactive gas that remains radioactive for several minutes, and called this gas "emanation" (from la, emanare, to flow out, and , expiration), and later "thorium emanation" ("Th Em"). In 1900, Friedrich Ernst Dorn reported some experiments in which he noticed that radium compounds emanate a radioactive gas he named "radium emanation" ("Ra Em"). In 1901, Rutherford and Harriet Brooks
Harriet Brooks (July 2, 1876 – April 17, 1933) was the first Canadian female nuclear physicist. She is most famous for her research on nuclear transmutations and radioactivity. Ernest Rutherford, who guided her graduate work, regarded her as ...
demonstrated that the emanations are radioactive, but credited the Curies for the discovery of the element. In 1903, similar emanations were observed from actinium by André-Louis Debierne
André-Louis Debierne (; 14 July 1874 – 31 August 1949) was a French chemist. He is often considered the discoverer of the element actinium, though H. W. Kirby disputes this and awards credit instead to German chemist Friedrich Oskar Giesel.
D ...
, and were called "actinium emanation" ("Ac Em").
Several shortened names were soon suggested for the three emanations: ''exradio'', ''exthorio'', and ''exactinio'' in 1904; ''radon'' (Ro), ''thoron'' (To), and ''akton'' or ''acton'' (Ao) in 1918; ''radeon'', ''thoreon'', and ''actineon'' in 1919, and eventually ''radon'', ''thoron'', and ''actinon'' in 1920. (The name radon is not related to that of the Austrian mathematician Johann Radon
Johann Karl August Radon (; 16 December 1887 – 25 May 1956) was an Austrian mathematician. His doctoral dissertation was on the calculus of variations (in 1910, at the University of Vienna).
Life
RadonBrigitte Bukovics: ''Biography of Johan ...
.) The likeness of the spectra of these three gases with those of argon, krypton, and xenon, and their observed chemical inertia led Sir William Ramsay
Sir William Ramsay (; 2 October 1852 – 23 July 1916) was a Scottish chemist who discovered the noble gases and received the Nobel Prize in Chemistry in 1904 "in recognition of his services in the discovery of the inert gaseous element ...
to suggest in 1904 that the "emanations" might contain a new element of the noble-gas family.
In the early 20th century in the US, gold contaminated with the radon daughter 210Pb entered the jewelry industry. This was from gold seeds that had held 222Rn that had been melted down after the radon had decayed.
In 1909, Ramsay and Robert Whytlaw-Gray isolated radon and determined its melting temperature and approximate density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematical ...
. In 1910, they determined that it was the heaviest known gas. They wrote that "" ("the expression 'radium emanation' is very awkward") and suggested the new name niton (Nt) (from la, nitens, shining) to emphasize the radioluminescence property, and in 1912 it was accepted by the Commission on Isotopic Abundances and Atomic Weights, International Commission for Atomic Weights. In 1923, the International Committee for Chemical Elements and International Union of Pure and Applied Chemistry (IUPAC) chose among the names radon (Rn), thoron (Tn), and actinon (An). Later, when isotopes were numbered instead of named, the element took the name of the most stable isotope, ''radon'', while Tn was renamed Radon-220, 220Rn and An was renamed 219Rn. This has caused some confusion in the literature regarding the element's discovery as while Dorn had discovered radon the isotope, he had not been the first to discover radon the element.
As late as the 1960s, the element was also referred to simply as ''emanation''. The first synthesized compound of radon, radon fluoride, was obtained in 1962. Even today, the word ''radon'' may refer to either the element or its isotope 222Rn, with ''thoron'' remaining in use as a short name for 220Rn to stem this ambiguity. The name ''actinon'' for 219Rn is rarely encountered today, probably due to the short half-life of that isotope.
The danger of high exposure to radon in mines, where exposures can reach 1,000,000 Becquerel, Bq/m3, has long been known. In 1530, Paracelsus described a wasting disease of miners, the ''mala metallorum'', and Georg Agricola recommended ventilation in mines to avoid this mountain sickness (''Bergsucht''). In 1879, this condition was identified as lung cancer by Harting and Hesse in their investigation of miners from Schneeberg, Germany. The first major studies with radon and health occurred in the context of uranium mining in the Jáchymov, Joachimsthal region of Bohemia. In the US, studies and mitigation only followed decades of health effects on uranium miners of the Southwestern United States, Southwestern US employed during the early Cold War; standards were not implemented until 1971.
The presence of radon in indoor air was documented as early as 1950. Beginning in the 1970s, research was initiated to address sources of indoor radon, determinants of concentration, health effects, and mitigation approaches. In the US, the problem of indoor radon received widespread publicity and intensified investigation after a widely publicized incident in 1984. During routine monitoring at a Pennsylvania nuclear power plant, a worker was found to be contaminated with radioactivity. A high concentration of radon in his home was subsequently identified as responsible.
Occurrence
Concentration units
All discussions of radon concentrations in the environment refer to 222Rn. While the average rate of production of 220Rn (from the thorium decay series) is about the same as that of 222Rn, the amount of 220Rn in the environment is much less than that of 222Rn because of the short half-life of 220Rn (55 seconds, versus 3.8 days respectively). Radon concentration in the atmosphere is usually measured in becquerel per cubic meter (Bq/m3), the SI derived unit. Another unit of measurement common in the US is Curie (unit), picocuries per liter (pCi/L); 1 pCi/L = 37 Bq/m3. Typical domestic exposures average about 48 Bq/m3 indoors, though this varies widely, and 15 Bq/m3 outdoors. In the mining industry, the exposure is traditionally measured in ''working level
Working level (WL) is a historical unit of concentration of radioactive decay products of radon, applied to uranium mining environment. One working level refers to the concentration of short-lived decay products of radon in equilibrium with 3,700 ...
'' (WL), and the cumulative exposure in ''working level month'' (WLM); 1 WL equals any combination of short-lived 222Rn daughters (218Po, 214Pb, 214Bi, and 214Po) in 1 liter of air that releases 1.3 × 105 MeV of potential alpha energy; 1 WL is equivalent to 2.08 × 10−5 joules per cubic meter of air (J/m3). The SI unit of cumulative exposure is expressed in joule-hours per cubic meter (J·h/m3). One WLM is equivalent to 3.6 × 10−3 J·h/m3. An exposure to 1 WL for 1 working-month (170 hours) equals 1 WLM cumulative exposure. A cumulative exposure of 1 WLM is roughly equivalent to living one year in an atmosphere with a radon concentration of 230 Bq/m3.
222Rn decays to 210Pb and other radioisotopes. The levels of 210Pb can be measured. The rate of deposition of this radioisotope is weather-dependent.
Radon concentrations found in natural environments are much too low to be detected by chemical means. A 1,000 Bq/m3 (relatively high) concentration corresponds to 0.17 pico-, picogram per cubic meter (pg/m3). The average concentration of radon in the atmosphere is about 6 molar percent, or about 150 atoms in each milliliter of air. The radon activity of the entire Earth's atmosphere originates from only a few tens of grams of radon, consistently replaced by decay of larger amounts of radium, thorium, and uranium.
Natural
Radon is produced by the radioactive decay of radium-226, which is found in uranium ores, phosphate rock, shales, igneous and metamorphic rocks such as granite, gneiss, and schist, and to a lesser degree, in common rocks such as limestone. Every square mile of surface soil, to a depth of 6 inches (2.6 km2 to a depth of 15 cm), contains approximately 1 gram of radium, which releases radon in small amounts to the atmosphere. On a global scale, it is estimated that 2.4 billion curies (90 EBq) of radon are released from soil annually.Harley, J. H. in This is equivalent to some . Radon concentration can differ widely from place to place. In the open air, it ranges from 1 to 100 Bq/m3, even less (0.1 Bq/m3) above the ocean. In caves or ventilated mines, or poorly ventilated houses, its concentration climbs to 20–2,000 Bq/m3. Radon concentration can be much higher in mining contexts. Ventilation regulations instruct to maintain radon concentration in uranium mines under the "working level", with 95th percentile levels ranging up to nearly 3 WL (546 pCi 222Rn per liter of air; 20.2 kBq/m3, measured from 1976 to 1985). The concentration in the air at the (unventilated) Bad Gastein, Gastein Healing Gallery averages 43 kBq/m3 (1.2 nCi/L) with maximal value of 160 kBq/m3 (4.3 nCi/L). Radon mostly appears with the decay chain of the radium anduranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
series (222Rn), and marginally with the thorium series (220Rn). The element emanates naturally from the ground, and some building materials, all over the world, wherever traces of uranium or thorium are found, and particularly in regions with soils containing granite or shale, which have a higher concentration of uranium. Not all granitic regions are prone to high emissions of radon. Being a rare gas, it usually migrates freely through faults and fragmented soils, and may accumulate in caves or water. Owing to its very short half-life (four days for 222Rn), radon concentration decreases very quickly when the distance from the production area increases. Radon concentration varies greatly with season and atmospheric conditions. For instance, it has been shown to accumulate in the air if there is a Inversion (meteorology), meteorological inversion and little wind.
High concentrations of radon can be found in some spring waters and hot springs. The towns of Boulder, Montana; Misasa, Tottori, Misasa; Bad Kreuznach, Germany; and the country of Japan have radium-rich springs that emit radon. To be classified as a radon mineral water, radon concentration must be above 2 nCi/L (74 kBq/m3). The activity of radon mineral water reaches 2,000 kBq/m3 in Merano and 4,000 kBq/m3 in Lurisia (Italy).
Natural radon concentrations in the Earth's atmosphere are so low that radon-rich water in contact with the atmosphere will continually lose radon by volatilization. Hence, ground water has a higher concentration of 222Rn than surface water, because radon is continuously produced by radioactive decay of 226Ra present in rocks. Likewise, the aquifer, saturated zone of a soil frequently has a higher radon content than the vadose zone, unsaturated zone because of diffusional losses to the atmosphere.
In 1971, Apollo 15 passed above the Aristarchus (crater), Aristarchus plateau on the Moon, and detected a significant rise in alpha particles thought to be caused by the decay of 222Rn. The presence of 222Rn has been inferred later from data obtained from the Lunar Prospector alpha particle spectrometer.
Radon is found in some petroleum. Because radon has a similar pressure and temperature curve to propane, and oil refineries separate petrochemicals based on their boiling points, the piping carrying freshly separated propane in oil refineries can become radioactive contamination, contaminated because of decaying radon and its products.
Residues from the petroleum and natural gas industry often contain radium and its daughters. The sulfate scale from an oil well can be radium rich, while the water, oil, and gas from a well often contains radon. Radon decays to form solid radioisotopes that form coatings on the inside of pipework.
Accumulation in buildings

 High concentrations of radon in homes were discovered by chance in 1985 after the stringent radiation testing conducted at the new Limerick Generating Station nuclear power plant revealed that Stanley Watras, a construction engineer at the plant, was contaminated by radioactive substances even though the reactor had never been fueled. Typical domestic exposures are of approximately 100 Bq/m3 (2.7 pCi/L) indoors. Some level of radon will be found in all buildings. Radon mostly enters a building directly from the soil through the lowest level in the building that is in contact with the ground. High levels of radon in the water supply can also increase indoor radon air levels. Typical entry points of radon into buildings are cracks in solid foundations and walls, construction joints, gaps in suspended floors and around service pipes, cavities inside walls, and the water supply. Radon concentrations in the same place may differ by double/half over one hour. Also, the concentration in one room of a building may be significantly different from the concentration in an adjoining room. The soil characteristics of the dwellings are the most important source of radon for the ground floor and higher concentration of indoor radon observed on lower floors. Most of the high radon concentrations have been reported from places near Fault (geology), fault zones; hence the existence of a relation between the exhalation rate from faults and indoor radon concentrations is obvious.
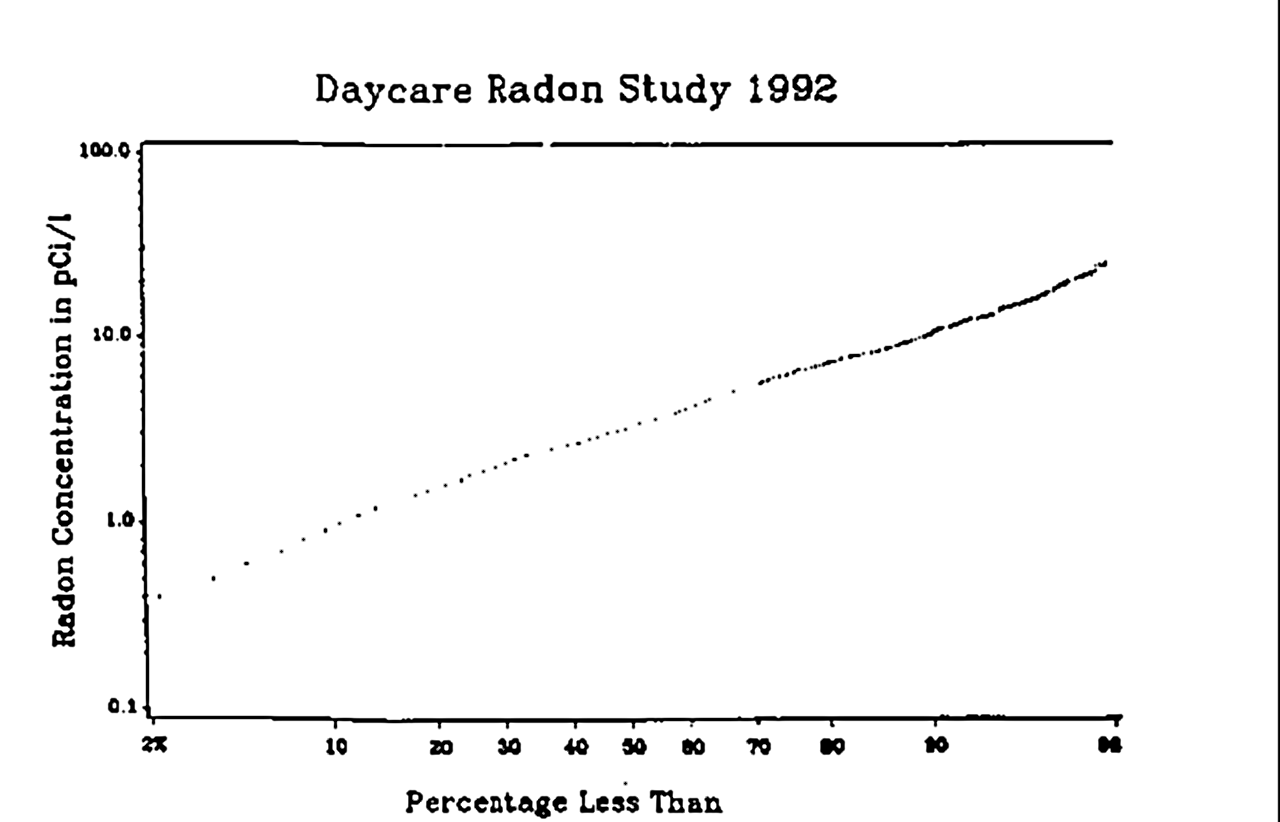
The distribution of radon concentrations will generally differ from room to room, and the readings are averaged according to regulatory protocols. Indoor radon concentration is usually assumed to follow a log-normal distribution on a given territory. Thus, the geometric mean is generally used for estimating the "average" radon concentration in an area.
The mean concentration ranges from less than 10 Bq/m3 to over 100 Bq/m3 in some European countries. Typical geometric standard deviations found in studies range between 2 and 3, meaning (given the 68–95–99.7 rule) that the radon concentration is expected to be more than a hundred times the mean concentration for 2% to 3% of the cases.
Some of the highest radon hazard in the US is found in Iowa and in the Appalachian Mountains, Appalachian Mountain areas in southeastern Pennsylvania. Iowa has the highest average radon concentrations in the US due to significant glaciation that ground the granitic rocks from the Canadian Shield and deposited it as soils making up the rich Iowa farmland. Many cities within the state, such as Iowa City, have passed requirements for radon-resistant construction in new homes. The second highest readings in Ireland were found in office buildings in the Irish town of Mallow, County Cork, prompting local fears regarding lung cancer.
In a few places, uranium tailings have been used for landfills and were subsequently built upon, resulting in possible increased exposure to radon.
Since radon is a colorless, odorless gas, the only way to know how much is present in the air or water is to perform tests. In the US, radon test kits are available to the public at retail stores, such as hardware stores, for home use, and testing is available through licensed professionals, who are often home inspectors. Efforts to reduce indoor radon levels are called radon mitigation. In the US, the EPA recommends all houses be tested for radon. In the UK under the Housing Health & Safety Rating System (HHSRS) property owners have an obligation to evaluate potential risks and hazards to health and safety in a residential property.
High concentrations of radon in homes were discovered by chance in 1985 after the stringent radiation testing conducted at the new Limerick Generating Station nuclear power plant revealed that Stanley Watras, a construction engineer at the plant, was contaminated by radioactive substances even though the reactor had never been fueled. Typical domestic exposures are of approximately 100 Bq/m3 (2.7 pCi/L) indoors. Some level of radon will be found in all buildings. Radon mostly enters a building directly from the soil through the lowest level in the building that is in contact with the ground. High levels of radon in the water supply can also increase indoor radon air levels. Typical entry points of radon into buildings are cracks in solid foundations and walls, construction joints, gaps in suspended floors and around service pipes, cavities inside walls, and the water supply. Radon concentrations in the same place may differ by double/half over one hour. Also, the concentration in one room of a building may be significantly different from the concentration in an adjoining room. The soil characteristics of the dwellings are the most important source of radon for the ground floor and higher concentration of indoor radon observed on lower floors. Most of the high radon concentrations have been reported from places near Fault (geology), fault zones; hence the existence of a relation between the exhalation rate from faults and indoor radon concentrations is obvious.
The distribution of radon concentrations will generally differ from room to room, and the readings are averaged according to regulatory protocols. Indoor radon concentration is usually assumed to follow a log-normal distribution on a given territory. Thus, the geometric mean is generally used for estimating the "average" radon concentration in an area.
The mean concentration ranges from less than 10 Bq/m3 to over 100 Bq/m3 in some European countries. Typical geometric standard deviations found in studies range between 2 and 3, meaning (given the 68–95–99.7 rule) that the radon concentration is expected to be more than a hundred times the mean concentration for 2% to 3% of the cases.
Some of the highest radon hazard in the US is found in Iowa and in the Appalachian Mountains, Appalachian Mountain areas in southeastern Pennsylvania. Iowa has the highest average radon concentrations in the US due to significant glaciation that ground the granitic rocks from the Canadian Shield and deposited it as soils making up the rich Iowa farmland. Many cities within the state, such as Iowa City, have passed requirements for radon-resistant construction in new homes. The second highest readings in Ireland were found in office buildings in the Irish town of Mallow, County Cork, prompting local fears regarding lung cancer.
In a few places, uranium tailings have been used for landfills and were subsequently built upon, resulting in possible increased exposure to radon.
Since radon is a colorless, odorless gas, the only way to know how much is present in the air or water is to perform tests. In the US, radon test kits are available to the public at retail stores, such as hardware stores, for home use, and testing is available through licensed professionals, who are often home inspectors. Efforts to reduce indoor radon levels are called radon mitigation. In the US, the EPA recommends all houses be tested for radon. In the UK under the Housing Health & Safety Rating System (HHSRS) property owners have an obligation to evaluate potential risks and hazards to health and safety in a residential property.
Industrial production
Radon is obtained as a by-product of Uranium ore deposits, uraniferous ores processing after transferring into 1% solutions of hydrochloric acid, hydrochloric or hydrobromic acids. The gas mixture extracted from the solutions contains , , He, Rn, , and hydrocarbons. The mixture is purified by passing it over copper at to remove the and the , and then potassium hydroxide, KOH and Phosphorus pentoxide, are used to remove the acids and moisture by sorption. Radon is condensed by liquid nitrogen and purified from residue gases by sublimation (phase transition), sublimation. Radon commercialization is regulated, but it is available in small quantities for the calibration of 222Rn measurement systems, at a price, in 2008, of almost per milliliter of radium solution (which only contains about 15 picograms of actual radon at any given moment). Radon is produced by a solution of radium-226 (half-life of 1,600 years). Radium-226 decays by alpha-particle emission, producing radon that collects over samples of radium-226 at a rate of about 1 mm3/day per gram of radium; equilibrium is quickly achieved and radon is produced in a steady flow, with an activity equal to that of the radium (50 Bq). Gaseous 222Rn (half-life of about four days) escapes from the capsule through diffusion.Concentration scale
Applications
Medical
An early-20th-century form of quackery was the treatment of maladies in a radiotorium. It was a small, sealed room for patients to be exposed to radon for its "medicinal effects". The carcinogenic nature of radon due to its ionizing radiation became apparent later. Radon's molecule-damaging radioactivity has been used to kill cancerous cells, but it does not increase the health of healthy cells. The ionizing radiation causes the formation of free radicals, which results in cell damage, causing increased rates of illness, including cancer. Exposure to radon has been suggested to mitigate autoimmune diseases such as arthritis in a process known as radiation hormesis. As a result, in the late 20th century and early 21st century, "health mines" established in Basin, Montana, attracted people seeking relief from health problems such as arthritis through limited exposure to radioactive mine water and radon. The practice is discouraged because of the well-documented ill effects of high doses of radiation on the body. Radioactive water baths have been applied since 1906 in Jáchymov, Czech Republic, but even before radon discovery they were used in Bad Gastein, Austria. Radium-rich springs are also used in traditional Japanese onsen in Misasa, Tottori, Misasa, Tottori Prefecture. Drinking therapy is applied in Bad Brambach, Germany, and during the early 20th century, water from springs with radon in them was bottled and sold (this water had little to no radon in it by the time it got to consumers due to radon's short half-life). Inhalation therapy is carried out in Gasteiner-Heilstollen, Austria, in Świeradów-Zdrój, Czerniawa-Zdrój, Kowary, Lądek Zdrój, Poland, in Harghita Băi, Romania, and in Boulder, Montana. In the US and Europe, there are several "radon spas", where people sit for minutes or hours in a high-radon atmosphere. Radon has been produced commercially for use in radiation therapy, but for the most part has been replaced by radionuclides made in particle accelerators and nuclear reactors. Radon has been used in implantable seeds, made of gold or glass, primarily used to treat cancers, known as brachytherapy. The gold seeds were produced by filling a long tube with radon pumped from a radium source, the tube being then divided into short sections by crimping and cutting. The gold layer keeps the radon within, and filters out the alpha and beta radiations, while allowing the gamma rays to escape (which kill the diseased tissue). The activities might range from 0.05 to 5 millicuries per seed (2 to 200 MBq). The gamma rays are produced by radon and the first short-lived elements of its decay chain (218Po, 214Pb, 214Bi, 214Po). Radon and its first decay products being very short-lived, the seed is left in place. After 11 half-lives (42 days), radon radioactivity is at 1/2,048 of its original level. At this stage, the predominant residual activity originates from the radon decay product 210Pb, whose half-life (22.3 years) is 2,000 times that of radon and its descendants 210Bi and 210Po.Scientific
Radon emanation from the soil varies with soil type and with surface uranium content, so outdoor radon concentrations can be used to track air masses to a limited degree. This fact has been put to use by some atmospheric scientists (Radon storm). Because of radon's rapid loss to air and comparatively rapid decay, radon is used in hydrology, hydrologic research that studies the interaction between groundwater and streams. Any significant concentration of radon in a stream is a good indicator that there are local inputs of groundwater. Radon soil-concentration has been used in an experimental way to map buried close-subsurface geological fault (geology), faults because concentrations are generally higher over the faults. Similarly, it has found some limited use in prospecting for geothermal gradients. Some researchers have investigated changes in groundwater radon concentrations for earthquake prediction. Increases in radon were noted before the 1966 Tashkent earthquake, 1966 Tashkent and 1994 Mindoro earthquake, 1994 Mindoro earthquakes. Radon has a half-life of approximately 3.8 days, which means that it can be found only shortly after it has been produced in the radioactive decay chain. For this reason, it has been hypothesized that increases in radon concentration is due to the generation of new cracks underground, which would allow increased groundwater circulation, flushing out radon. The generation of new cracks might not unreasonably be assumed to precede major earthquakes. In the 1970s and 1980s, scientific measurements of radon emissions near faults found that earthquakes often occurred with no radon signal, and radon was often detected with no earthquake to follow. It was then dismissed by many as an unreliable indicator. As of 2009, it was under investigation as a possible precursor by NASA. Radon is a known pollutant emitted from Geothermal power, geothermal power stations because it is present in the material pumped from deep underground. It disperses rapidly, and no radiological hazard has been demonstrated in various investigations. In addition, typical systems re-inject the material deep underground rather than releasing it at the surface, so its environmental impact is minimal. However, similar things can be said about trivial releases from operating nuclear power plants. In the 1940s and '50s, radon was used for industrial radiography. Other X-ray sources, which became available after World War II, quickly replaced radon for this application, as they were lower in cost and had less hazard ofalpha radiation
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
.
Health risks
In mines
Radon-222 decay products have been classified by the International Agency for Research on Cancer as being carcinogenic to humans, and as a gas that can be inhaled, lung cancer is a particular concern for people exposed to elevated levels of radon for sustained periods. During the 1940s and 1950s, when safety standards requiring expensive ventilation in mines were not widely implemented, radon exposure was linked to lung cancer among non-smoking miners of uranium and other hard rock materials in what is now the Czech Republic, and later among miners from the Southwestern US and South Australia. Despite these hazards being known in the early 1950s, this occupational hazard remained poorly managed in many mines until the 1970s. During this period, several entrepreneurs opened former uranium mines in the US to the general public and advertised alleged health benefits from breathing radon gas underground. Health benefits claimed included pain, sinus, asthma and arthritis relief, but these were proven to be false and the government banned such advertisements in 1975. Since that time, ventilation and other measures have been used to reduce radon levels in most affected mines that continue to operate. In recent years, the average annual exposure of uranium miners has fallen to levels similar to the concentrations inhaled in some homes. This has reduced the risk of occupationally-induced cancer from radon, although health issues may persist for those who are currently employed in affected mines and for those who have been employed in them in the past. As the relative risk for miners has decreased, so has the ability to detect excess risks among that population. Residues from processing of uranium ore can also be a source of radon. Radon resulting from the high radium content in uncovered dumps and tailing ponds can be easily released into the atmosphere and affect people living in the vicinity. In addition to lung cancer, researchers have theorized a possible increased risk of leukemia due to radon exposure. Empirical support from studies of the general population is inconsistent, and a study of uranium miners found a correlation between radon exposure and chronic lymphocytic leukemia. Miners (as well as milling and ore transportation workers) who worked in the uranium industry in the US between the 1940s and 1971 may be eligible for compensation under the Radiation Exposure Compensation Act (RECA). Surviving relatives may also apply in cases where the formerly employed person is deceased. Not only uranium mines are affected by elevated levels of radon. Coal mines in particular are affected as well since coal may contain more uranium and thorium than commercially operational uranium mines.Domestic-level exposure
Prolonged exposure to higher concentrations of radon has been linked to an increase in lung cancer. Since 1999, there has been investigations worldwide on how radon concentrations are estimated. In the United States alone averages have been recorded to be at least 40 Bq/meters cubed. Steck et al. did a study on the variation between indoor and outdoor radon in Iowa and Minnesota. Higher radiation was found in a populated region rather than in unpopulated regions in Central America as a whole. In some northwestern Iowa and southwestern Minnesota counties, the outdoor radon concentrations exceed the national average indoor radon concentrations. Despite the above average, both Minnesota and Iowa's numbers were exceptionally close, regardless of the distance. Accurate doses of radon is heavily needed to further understand the problems radon in total can have on a community. It is understood that radon poisoning does lead to bad health, and lung cancer, but with further research, controls could change results in radon emissions both inside and outside of housing units. Radon exposure (mostly radon daughters) has been linked to lung cancer in numerous case-control studies performed in the US, Europe and China. There are approximately 21,000 deaths per year in the US due to radon-induced lung cancers. In Slovenia, a country with a high concentration of radon, about 120 people yearly die because of radon. One of the most comprehensive radon studies performed in the US by Dr. R. William Field and colleagues found a 50% increased lung cancer risk even at the protracted exposures at the EPA's action level of 4 pCi/L. North American and European pooled analyses further support these findings. However, the discussion about the opposite results is still continuing, especially a 2008 retrospective case-control study of lung cancer risk which showed substantial cancer rate reduction for radon concentrations between 50 and 123 Bq/m3. Most models of residential radon exposure are based on studies of miners, and direct estimates of the risks posed to homeowners would be more desirable. Because of the difficulties of measuring the risk of radon relative to smoking, models of their effect have often made use of them. Radon has been considered the second leading cause of lung cancer and leading environmental cause of cancer mortality by the EPA, with the first one being smoking. Others have reached similar conclusions for the United Kingdom and France. Radon exposure in homes and offices may arise from certain subsurface rock formations, and also from certain building materials (e.g., some granites). The greatest risk of radon exposure arises in buildings that are airtight, insufficiently ventilated, and have foundation leaks that allow air from the soil into basements and dwelling rooms. Thoron was measured at comparatively high concentrations in buildings with earthen architecture, such as traditional Timber framing#Half-timbering, half-timbered houses and modern houses with clay wall finishes. Because of its short half-life, thoron occurs only close to the earthen surfaces as its sources whereas its progeny can be found throughout the indoor air of such buildings. Therefore, radiation exposure occurs at any location within such houses. In different dwellings with earthen architecture in Germany, a study found annual internal radiation doses due to the inhalation of thoron and its progeny of up to several milli-Sieverts.Action and reference level
WHO presented in 2009 a recommended reference level (the national reference level), 100 Bq/m3, for radon in dwellings. The recommendation also says that where this is not possible, 300 Bq/m3 should be selected as the highest level. A national reference level should not be a limit, but should represent the maximum acceptable annual average radon concentration in a dwelling. The actionable concentration of radon in a home varies depending on the organization doing the recommendation, for example, the EPA encourages that action be taken at concentrations as low as 74 Bq/m3 (2 pCi/L), and the European Union recommends action be taken when concentrations reach 400 Bq/m3 (11 pCi/L) for old houses and 200 Bq/m3 (5 pCi/L) for new ones. On 8 July 2010, the UK's Health Protection Agency issued new advice setting a "Target Level" of 100 Bq/m3 whilst retaining an "Action Level" of 200 Bq/m3. Similar levels (as in UK) are published by Norwegian Radiation and Nuclear Safety Authority (DSA) with the maximum limit for schools, kindergartens, and new dwellings set at 200 Bq/m3, where 100 Bq/m3 is set as the action level. In all new housings preventative measures should be taken against radon accumulation.Inhalation and smoking
Results from epidemiological studies indicate that the risk of lung cancer increases with exposure to residential radon. A well known example of source of error is smoking, the main risk factor for lung cancer. In the US, cigarette smoking is estimated to cause 80% to 90% of all lung cancers. According to the EPA, the risk of lung cancer for smokers is significant due to Synergy, synergistic effects of radon and smoking. For this population about 62 people in a total of 1,000 will die of lung cancer compared to 7 people in a total of 1,000 for people who have never smoked. It cannot be excluded that the risk of non-smokers should be primarily explained by an effect of radon. Radon, like other known or suspected external risk factors for lung cancer, is a threat for smokers and former smokers. This was demonstrated by the European pooling study. A commentary to the pooling study stated: "it is not appropriate to talk simply of a risk from radon in homes. The risk is from smoking, compounded by a synergistic effect of radon for smokers. Without smoking, the effect seems to be so small as to be insignificant." According to the European pooling study, there is a difference in risk for the Histology, histological subtypes of lung cancer and radon exposure. Small-cell lung carcinoma, which has a high correlation with smoking, have a higher risk after radon exposure. For other histological subtypes such as adenocarcinoma, the type that primarily affects non-smokers, the risk from radon appears to be lower. A study of radiation from post-mastectomy radiotherapy shows that the simple models previously used to assess the combined and separate risks from radiation and smoking need to be developed. This is also supported by new discussion about the calculation method, the linear no-threshold model, which routinely has been used. A study from 2001, which included 436 non-smokers and a control group of 1649 non-smokers, showed that exposure to radon increased the risk of lung cancer in non-smokers. The group that had been exposed to tobacco smoke in the home appeared to have a much higher risk, while those who were not exposed to passive smoking did not show any increased risk with increasing radon exposure.Ingestion
The effects of radon if ingested are unknown, although studies have found that its biological half-life ranges from 30 to 70 minutes, with 90% removal at 100 minutes. In 1999, the US National Research Council (United States), National Research Council investigated the issue of radon in drinking water. The risk associated with ingestion was considered almost negligible. Water from underground sources may contain significant amounts of radon depending on the surrounding rock and soil conditions, whereas surface sources generally do not.Ocean effects of radon
The ocean surface only carries about 10^-4 226 Ra, where measurements of 222 Ra concentration have been 1% over various continents. The major importance of understanding 222 Ra flux from the ocean, is to know that the increase use of radon is also circulating and increasing in the atmosphere. Ocean surface concentrations have an exchange within the atmosphere, causing 222 radon to increase through the air-sea interface. Although areas tested were very shallow, additional measurements in a wide variety of coastal regimes should help define the nature of 222 Radon observed. As well as being ingested through drinking water, radon is also released from water when temperature is increased, pressure is decreased and when water is aerated. Optimum conditions for radon release and exposure occurred during showering. Water with a radon concentration of 104 pCi/L can increase the indoor airborne radon concentration by 1 pCi/L under normal conditions.Testing and mitigation
 There are relatively simple tests for radon gas. In some countries these tests are methodically done in areas of known systematic hazards. Radon detection devices are commercially available. Digital radon detectors provide ongoing measurements giving both daily, weekly, short-term and long-term average readouts via a digital display. Short-term radon test devices used for initial screening purposes are inexpensive, in some cases free. There are important protocols for taking short-term radon tests and it is imperative that they be strictly followed. The kit includes a collector that the user hangs in the lowest habitable floor of the house for two to seven days. The user then sends the collector to a laboratory for analysis. Long term kits, taking collections for up to one year or more, are also available. An open-land test kit can test radon emissions from the land before construction begins. Radon concentrations can vary daily, and accurate radon exposure estimates require long-term average radon measurements in the spaces where an individual spends a significant amount of time.
Radon levels fluctuate naturally, due to factors like transient weather conditions, so an initial test might not be an accurate assessment of a home's average radon level. Radon levels are at a maximum during the coolest part of the day when pressure differentials are greatest. Therefore, a high result (over 4 pCi/L) justifies repeating the test before undertaking more expensive abatement projects. Measurements between 4 and 10 pCi/L warrant a long-term radon test. Measurements over 10 pCi/L warrant only another short-term test so that abatement measures are not unduly delayed. Purchasers of real estate are advised to delay or decline a purchase if the seller has not successfully abated radon to 4 pCi/L or less.
Because the half-life of radon is only 3.8 days, removing or isolating the source will greatly reduce the hazard within a few weeks. Another method of reducing radon levels is to modify the building's ventilation. Generally, the indoor radon concentrations increase as ventilation rates decrease. In a well-ventilated place, the radon concentration tends to align with outdoor values (typically 10 Bq/m3, ranging from 1 to 100 Bq/m3).
The four principal ways of reducing the amount of radon accumulating in a house are:
* Sub-slab depressurization (soil suction) by increasing under-floor ventilation;
* Improving the ventilation of the house and avoiding the transport of radon from the basement into living rooms;
* Installing a radon sump system in the basement;
* Installing a positive pressurization or positive supply ventilation system.
According to the EPA, the method to reduce radon "...primarily used is a vent pipe system and fan, which pulls radon from beneath the house and vents it to the outside", which is also called sub-slab depressurization, active soil depressurization, or soil suction. Generally indoor radon can be mitigated by sub-slab depressurization and exhausting such radon-laden air to the outdoors, away from windows and other building openings. "[The] EPA generally recommends methods which prevent the entry of radon. Soil suction, for example, prevents radon from entering your home by drawing the radon from below the home and venting it through a pipe, or pipes, to the air above the home where it is quickly diluted" and the "EPA does not recommend the use of sealing alone to reduce radon because, by itself, sealing has not been shown to lower radon levels significantly or consistently".
Positive pressure ventilation, Positive-pressure ventilation systems can be combined with a heat exchanger to recover energy in the process of exchanging air with the outside, and simply exhausting basement air to the outside is not necessarily a viable solution as this can actually draw radon gas into a dwelling. Homes built on a crawl space may benefit from a radon collector installed under a "radon barrier" (a sheet of plastic that covers the crawl space).
For crawl spaces, the EPA states "An effective method to reduce radon levels in crawl space homes involves covering the earth floor with a high-density plastic sheet. A vent pipe and fan are used to draw the radon from under the sheet and vent it to the outdoors. This form of soil suction is called submembrane suction, and when properly applied is the most effective way to reduce radon levels in crawl space homes."
There are relatively simple tests for radon gas. In some countries these tests are methodically done in areas of known systematic hazards. Radon detection devices are commercially available. Digital radon detectors provide ongoing measurements giving both daily, weekly, short-term and long-term average readouts via a digital display. Short-term radon test devices used for initial screening purposes are inexpensive, in some cases free. There are important protocols for taking short-term radon tests and it is imperative that they be strictly followed. The kit includes a collector that the user hangs in the lowest habitable floor of the house for two to seven days. The user then sends the collector to a laboratory for analysis. Long term kits, taking collections for up to one year or more, are also available. An open-land test kit can test radon emissions from the land before construction begins. Radon concentrations can vary daily, and accurate radon exposure estimates require long-term average radon measurements in the spaces where an individual spends a significant amount of time.
Radon levels fluctuate naturally, due to factors like transient weather conditions, so an initial test might not be an accurate assessment of a home's average radon level. Radon levels are at a maximum during the coolest part of the day when pressure differentials are greatest. Therefore, a high result (over 4 pCi/L) justifies repeating the test before undertaking more expensive abatement projects. Measurements between 4 and 10 pCi/L warrant a long-term radon test. Measurements over 10 pCi/L warrant only another short-term test so that abatement measures are not unduly delayed. Purchasers of real estate are advised to delay or decline a purchase if the seller has not successfully abated radon to 4 pCi/L or less.
Because the half-life of radon is only 3.8 days, removing or isolating the source will greatly reduce the hazard within a few weeks. Another method of reducing radon levels is to modify the building's ventilation. Generally, the indoor radon concentrations increase as ventilation rates decrease. In a well-ventilated place, the radon concentration tends to align with outdoor values (typically 10 Bq/m3, ranging from 1 to 100 Bq/m3).
The four principal ways of reducing the amount of radon accumulating in a house are:
* Sub-slab depressurization (soil suction) by increasing under-floor ventilation;
* Improving the ventilation of the house and avoiding the transport of radon from the basement into living rooms;
* Installing a radon sump system in the basement;
* Installing a positive pressurization or positive supply ventilation system.
According to the EPA, the method to reduce radon "...primarily used is a vent pipe system and fan, which pulls radon from beneath the house and vents it to the outside", which is also called sub-slab depressurization, active soil depressurization, or soil suction. Generally indoor radon can be mitigated by sub-slab depressurization and exhausting such radon-laden air to the outdoors, away from windows and other building openings. "[The] EPA generally recommends methods which prevent the entry of radon. Soil suction, for example, prevents radon from entering your home by drawing the radon from below the home and venting it through a pipe, or pipes, to the air above the home where it is quickly diluted" and the "EPA does not recommend the use of sealing alone to reduce radon because, by itself, sealing has not been shown to lower radon levels significantly or consistently".
Positive pressure ventilation, Positive-pressure ventilation systems can be combined with a heat exchanger to recover energy in the process of exchanging air with the outside, and simply exhausting basement air to the outside is not necessarily a viable solution as this can actually draw radon gas into a dwelling. Homes built on a crawl space may benefit from a radon collector installed under a "radon barrier" (a sheet of plastic that covers the crawl space).
For crawl spaces, the EPA states "An effective method to reduce radon levels in crawl space homes involves covering the earth floor with a high-density plastic sheet. A vent pipe and fan are used to draw the radon from under the sheet and vent it to the outdoors. This form of soil suction is called submembrane suction, and when properly applied is the most effective way to reduce radon levels in crawl space homes."
See also
* International Radon Project * Lucas cell * Pleochroic halo (aka: Radiohalo) * Radiation Exposure Compensation ActReferences
External links
Radon
an
at the
United States Environmental Protection Agency
The Environmental Protection Agency (EPA) is an independent executive agency of the United States federal government tasked with environmental protection matters. President Richard Nixon proposed the establishment of EPA on July 9, 1970; it be ...
National Radon Program Services
hosted by Kansas State University
UK maps of radon
Radon Information from Public Health England
Frequently Asked Questions About Radon
at National Safety Council
Radon
at ''The Periodic Table of Videos'' (University of Nottingham)
Radon and Lung Health from the American Lung Association
Radon's impact on your health – Lung Association
The Geology of Radon
James K. Otton, Linda C.S. Gundersen, and R. Randall Schumann
An article by the International Association of Certified Home Inspectors (InterNACHI)
Toxicological Profile for Radon
Draft for Public Comment, Agency for Toxic Substances and Disease Registry, September 2008 * ''Health Effects of Exposure to Radon'': BEIR VI. Committee on Health Risks of Exposure to Radon (BEIR VI), National Research Counci
available on-line
with scientific annexes: Annex B: Exposures from natural radiation sources.
Should you measure the radon concentration in your home?
Phillip N. Price, Andrew Gelman, in ''Statistics: A Guide to the Unknown'', January 2004.
Radon in the Home- An Invisible Killer
How serious can high levels of radon be in the home? Kevin Vitali {{Authority control Radon, Chemical elements Noble gases Building biology Soil contamination IARC Group 1 carcinogens Carcinogens Industrial gases