|
Fluorine-18
Fluorine-18 (18F) is a fluorine radioisotope which is an important source of positrons. It has a mass of 18.0009380(6) u and its half-life is 109.771(20) minutes. It decays by positron emission 96% of the time and electron capture 4% of the time. Both modes of decay yield stable oxygen-18. Natural occurrence is a natural trace radioisotope produced by cosmic ray spallation of atmospheric argon as well as by reaction of protons with natural oxygen: 18O + p → 18F + n.18O">sup>18Oater with high energy protons (typically ~18 MeV). The fluorine produced is in the form of a water solution of 18F.html" ;"title="sup>18F">sup>18F luoride, which is then used in a rapid chemical synthesis of various radio pharmaceuticals. The organic oxygen-18 pharmaceutical molecule is not made before the production of the radiopharmaceutical, as high energy protons destroy such molecules ( radiolysis). Radiopharmaceuticals using fluorine must therefore be synthesized after the fluorine-18 has been p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Fluoride
Sodium fluoride (NaF) is an inorganic compound with the formula . It is used in trace amounts in the fluoridation of drinking water, in toothpaste, in metallurgy, and as a flux. It is a colorless or white solid that is readily soluble in water. It is a common source of fluoride in the production of pharmaceuticals and is used to prevent dental cavities. In 2020, it was the 265th most commonly prescribed medication in the United States, with more than 1million prescriptions. Uses Dental caries Fluoride salts are often added to municipal drinking water (as well as to certain food products in some countries) for the purpose of maintaining dental health. The fluoride enhances the strength of teeth by the formation of fluorapatite, a naturally occurring component of tooth enamel. Although sodium fluoride is used to fluoridate water and is the standard by which other water-fluoridation compounds are gauged, hexafluorosilicic acid (H2SiF6) and its salt sodium hexafluorosilica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (''t''1/2) for t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trace Radioisotope
A trace radioisotope is a radioisotope that occurs naturally in trace amounts (i.e. extremely small). Generally speaking, trace radioisotopes have half-lives that are short in comparison with the age of the Earth, since primordial nuclides tend to occur in larger than trace amounts. Trace radioisotopes are therefore present only because they are continually produced on Earth by natural processes. Natural processes which produce trace radioisotopes include cosmic ray bombardment of stable nuclides, ordinary alpha and beta decay of the long-lived heavy nuclides, thorium-232, uranium-238, and uranium-235, spontaneous fission of uranium-238, and nuclear transmutation reactions induced by natural radioactivity, such as the production of plutonium-239 and uranium-236 from neutron capture by natural uranium. Elements The elements that occur on Earth only in traces are listed below. Isotopes of other elements (not exhaustive): *Tritium * Beryllium-7 *Beryllium-10 *Carbon-14 *Fluorin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (''t''1/2) for t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibody
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen. Each tip of the "Y" of an antibody contains a paratope (analogous to a lock) that is specific for one particular epitope (analogous to a key) on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can ''tag'' a microbe or an infected cell for attack by other parts of the immune system, or can neutralize it directly (for example, by blocking a part of a virus that is essential for its invasion). To allow the immune system to recognize millions of different antigens, the antigen-binding sites at both tips of the antibody come in an equally wide variety. In contrast, the remainder of the antibody is relatively constant. It only occurs in a few varia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen-18
Oxygen-18 (, Ω) is a natural, stable isotope of oxygen and one of the environmental isotopes. is an important precursor for the production of fluorodeoxyglucose (FDG) used in positron emission tomography (PET). Generally, in the radiopharmaceutical industry, enriched water () is bombarded with hydrogen ions in either a cyclotron or linear accelerator, creating fluorine-18. This is then synthesized into FDG and injected into a patient. It can also be used to make an extremely heavy version of water when combined with tritium (hydrogen-3): or . This compound has a density almost 30% greater than that of natural water. The accurate measurements of rely on proper procedures of analysis, sample preparation and storage. Paleoclimatology In ice cores, mainly Arctic and Antarctic, the ratio of to (known as δ) can be used to determine the temperature of precipitation through time. Assuming that atmospheric circulation and elevation has not changed significantly over the poles, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrostatic
Electrostatics is a branch of physics that studies electric charges at rest (static electricity). Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word for amber, (), was thus the source of the word 'electricity'. Electrostatic phenomena arise from the forces that electric charges exert on each other. Such forces are described by Coulomb's law. Even though electrostatically induced forces seem to be rather weak, some electrostatic forces are relatively large. The force between an electron and a proton, which together make up a hydrogen atom, is about 36 orders of magnitude stronger than the gravitational force acting between them. There are many examples of electrostatic phenomena, from those as simple as the attraction of plastic wrap to one's hand after it is removed from a package, to the apparently spontaneous explosion of grain silos, the damage of electronic components during manufacturi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Polarity
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end. Polar molecules must contain one or more polar bonds due to a difference in electronegativity between the bonded atoms. Molecules containing polar bonds have no molecular polarity if the bond dipoles cancel each other out by symmetry. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points. Polarity of bonds Not all atoms attract electrons with the same force. The amount of "pull" an atom exerts on its electrons is called its electronegativity. Atoms with high electronegativitiessuch as fluorine, oxygen, and nitrogenexert a greater pull on electrons than atoms with lower electronegativities such as alkali metals and alkaline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
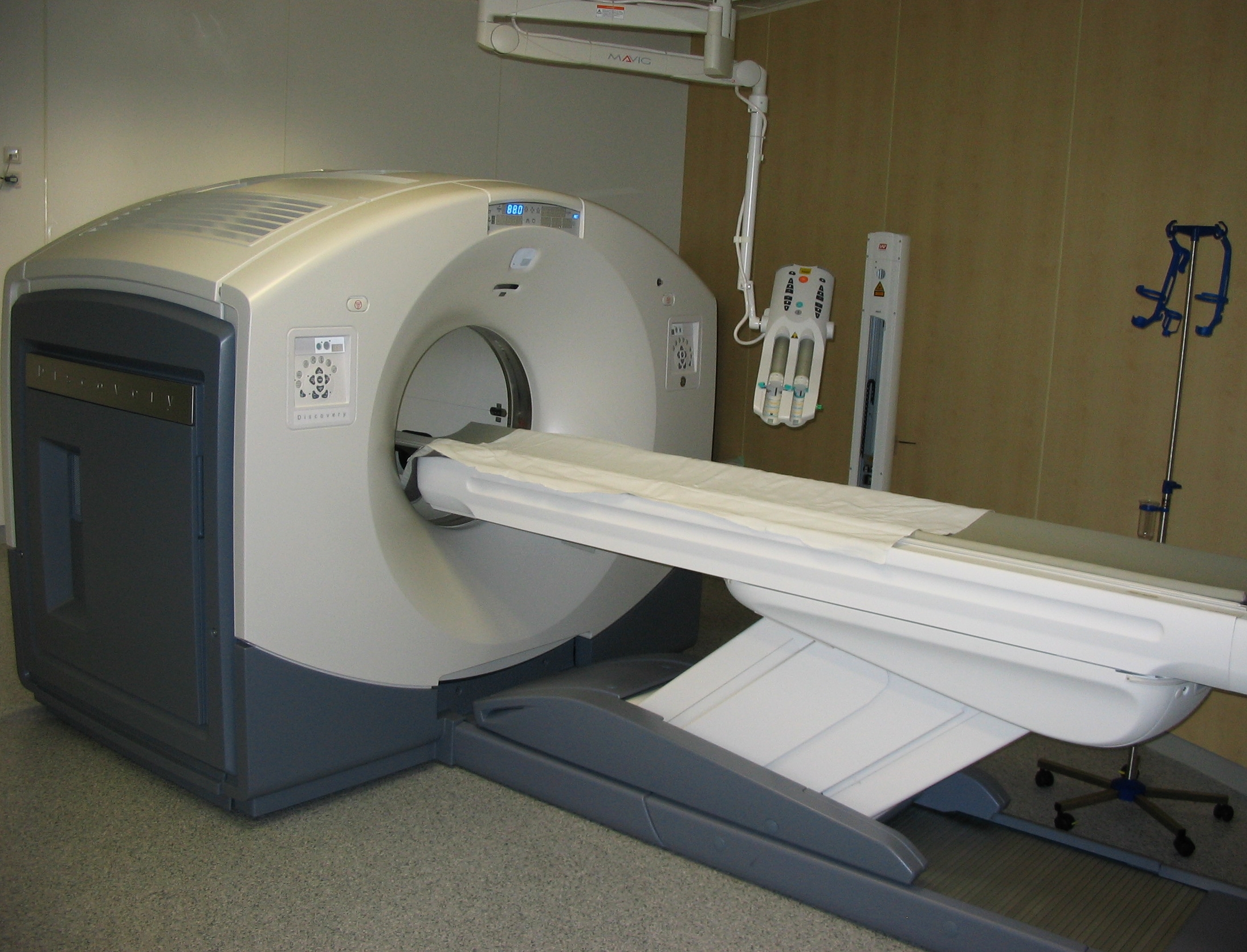
Positron Emission Tomography
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in Metabolism, metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body. For example, 18F-FDG, -FDG is commonly used to detect cancer, Sodium fluoride#Medical imaging, NaF is widely used for detecting bone formation, and Isotopes of oxygen#Oxygen-15, oxygen-15 is sometimes used to measure blood flow. PET is a common medical imaging, imaging technique, a Scintigraphy#Process, medical scintillography technique used in nuclear medicine. A radiopharmaceutical, radiopharmaceutical — a radioisotope attached to a drug — is injected into the body as a radioactive tracer, tracer. When the radiopharmaceutical undergoes beta plus decay, a positron is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy groups. Both the negatively charged anion , called hydroxide, and the neutral radical , known as the hydroxyl radical, consist of an unbonded hydroxy group. According to IUPAC definitions, the term ''hydroxyl'' refers to the hydroxyl radical () only, while the functional group is called a ''hydroxy group''. Properties Water, alcohols, carboxylic acids, and many other hydroxy-containing compounds can be readily deprotonated due to a large difference between the electronegativity of oxygen (3.5) and that of hydrogen (2.1). Hydroxy-containing compounds engage in intermolecular hydrogen bonding increasing the electrostatic attraction between molecules and thus to higher boiling and melting points than found for compounds that lack this f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorodeoxyglucose
18F.html" ;"title="sup>18F">sup>18Fluorodeoxyglucose (INN), or fluorodeoxyglucose F 18 (USAN and USP), also commonly called fluorodeoxyglucose and abbreviated 18F.html" ;"title="sup>18F">sup>18FDG, 2- 18F.html" ;"title="sup>18F">sup>18FDG or FDG, is a radiopharmaceutical, specifically a radiotracer, used in the medical imaging modality positron emission tomography (PET). Chemically, it is 2-deoxy-2- 18F.html" ;"title="sup>18F">sup>18Fluoro-D-glucose, a glucose analog, with the positron-emitting radionuclide fluorine-18 substituted for the normal hydroxyl group at the C-2 position in the glucose molecule. The uptake of 18F.html" ;"title="sup>18F">sup>18FDG by tissues is a marker for the tissue uptake of glucose, which in turn is closely correlated with certain types of tissue metabolism. After 18F.html" ;"title="sup>18F">sup>18FDG is injected into a patient, a PET scanner can form two-dimensional or three-dimensional images of the distribution of 18F.html" ;"title="sup>18F"> ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |