Homopolymerization on:
[Wikipedia]
[Google]
[Amazon]
 A polymer () is a
A polymer () is a
 A polymer () is a
A polymer () is a substance
Substance may refer to:
* Matter, anything that has mass and takes up space
Chemistry
* Chemical substance, a material with a definite chemical composition
* Drug, a chemical agent affecting an organism
Arts, entertainment, and media Music
* ' ...
or material
A material is a matter, substance or mixture of substances that constitutes an Physical object, object. Materials can be pure or impure, living or non-living matter. Materials can be classified on the basis of their physical property, physical ...
that consists of very large molecules, or macromolecule
A macromolecule is a "molecule of high relative molecular mass, the structure of which essentially comprises the multiple repetition of units derived, actually or conceptually, from molecules of low relative molecular mass." Polymers are physi ...
s, that are constituted by many repeating subunits derived from one or more species of monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
s. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastic
Plastics are a wide range of synthetic or semisynthetic materials composed primarily of polymers. Their defining characteristic, plasticity, allows them to be molded, extruded, or pressed into a diverse range of solid forms. This adapta ...
s such as polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It i ...
to natural biopolymer
Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, ...
s such as DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
and protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
of many small molecules, known as monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
s. Their consequently large molecular mass
The molecular mass () is the mass of a given molecule, often expressed in units of daltons (Da). Different molecules of the same compound may have different molecular masses because they contain different isotopes of an element. The derived quan ...
, relative to small molecule
In molecular biology and pharmacology, a small molecule or micromolecule is a low molecular weight (≤ 1000 daltons) organic compound that may regulate a biological process, with a size on the order of 1 nm. Many drugs are small molecules; ...
compounds, produces unique physical properties
A physical property is any property of a physical system that is measurable. The changes in the physical properties of a system can be used to describe its changes between momentary states. A quantifiable physical property is called ''physical ...
including toughness
In materials science and metallurgy, toughness is the ability of a material to absorb energy and plastically deform without fracturing.elasticity, 
 Polymers are of two types: naturally occurring and synthetic or ''man made''.
Polymers are of two types: naturally occurring and synthetic or ''man made''.
 Polymerization is the process of combining many
Polymerization is the process of combining many  Newer methods, such as
Newer methods, such as
 There are three main classes of biopolymers:
There are three main classes of biopolymers:
 An important microstructural feature of a polymer is its architecture and shape, which relates to the way branch points lead to a deviation from a simple linear chain. A
An important microstructural feature of a polymer is its architecture and shape, which relates to the way branch points lead to a deviation from a simple linear chain. A
 The bulk properties of a polymer are those most often of end-use interest. These are the properties that dictate how the polymer actually behaves on a macroscopic scale.
The bulk properties of a polymer are those most often of end-use interest. These are the properties that dictate how the polymer actually behaves on a macroscopic scale.
 In general, polymeric mixtures are far less
In general, polymeric mixtures are far less
Libretext in Polymer chemistry
How to Analyze Polymers Using X-ray DiffractionIntroduction to Polymers
{{Portal bar, Science, Chemistry, Numismatics, Electronics, Algae, Money Polymer chemistry Soft matter Materials science
viscoelasticity
In materials science and continuum mechanics, viscoelasticity is the property of materials that exhibit both viscous and elastic characteristics when undergoing deformation. Viscous materials, like water, resist both shear flow and strain lin ...
, and a tendency to form amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymousl ...
and semicrystalline
Crystallinity refers to the degree of structural order in a solid. In a crystal, the atoms or molecules are arranged in a regular, periodic manner. The degree of crystallinity has a large influence on hardness, density, Transparency and transluc ...
structures rather than crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
s.
Polymers are studied in the fields of polymer science
Polymer science or macromolecular science is a subfield of materials science concerned with polymers, primarily synthetic polymers such as plastics and elastomers. The field of polymer science includes researchers in multiple disciplines inclu ...
(which includes polymer chemistry
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are also applic ...
and polymer physics Polymer physics is the field of physics that studies polymers, their fluctuations, mechanical properties, as well as the kinetics of reactions involving degradation of polymers and polymerisation of monomers.P. Flory, ''Principles of Polymer Che ...
), biophysics
Biophysics is an interdisciplinary science that applies approaches and methods traditionally used in physics to study biological phenomena. Biophysics covers all scales of biological organization, from molecular to organismic and populations ...
and materials science and engineering
Materials science is an interdisciplinary field of researching and discovering materials. Materials engineering is an engineering field of finding uses for materials in other fields and industries.
The intellectual origins of materials scien ...
. Historically, products arising from the linkage of repeating units by covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
s have been the primary focus of polymer science. An emerging important area now focuses on supramolecular polymer
Supramolecular polymers are a subset of polymers where the monomeric units are connected by reversible and highly directional secondary interactions–that is, non-covalent bonds. These non-covalent interactions include van der Waals interactio ...
s formed by non-covalent
In chemistry, a non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. The ...
links. Polyisoprene
Polyisoprene is, strictly speaking, a collective name for polymers that are produced by polymerization of isoprene. In practice polyisoprene is commonly used to refer to synthetic ''cis''-1,4-polyisoprene, made by the industrial polymerisation of ...
of latex
Latex is an emulsion (stable dispersion) of polymer microparticles in water. Latices are found in nature, but synthetic latices are common as well.
In nature, latex is found as a wikt:milky, milky fluid, which is present in 10% of all floweri ...
rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds.
Types of polyisoprene ...
is an example of a natural polymer, and the polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It i ...
of styrofoam
Styrofoam is a brand of closed-cell extruded polystyrene foam (XPS), manufactured to provide continuous building insulation board used in walls, roofs, and foundations as thermal insulation and as a water barrier. This material is light blue in ...
is an example of a synthetic polymer. In biological contexts, essentially all biological macromolecule
A macromolecule is a "molecule of high relative molecular mass, the structure of which essentially comprises the multiple repetition of units derived, actually or conceptually, from molecules of low relative molecular mass." Polymers are physi ...
s—i.e., proteins (polyamides), nucleic acid
Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a pentose, 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nuclei ...
s (polynucleotides), and polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long-chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with wat ...
s—are purely polymeric, or are composed in large part of polymeric components.

Etymology
The term "polymer" derives . The term was coined in 1833 byJöns Jacob Berzelius
Baron Jöns Jacob Berzelius (; 20 August 1779 – 7 August 1848) was a Swedish chemist. Berzelius is considered, along with Robert Boyle, John Dalton, and Antoine Lavoisier, to be one of the founders of modern chemistry. Berzelius became a memb ...
, though with a definition distinct from the modern IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
definition. The modern concept of polymers as covalently bonded macromolecular structures was proposed in 1920 by Hermann Staudinger
Hermann Staudinger (; 23 March 1881 – 8 September 1965) was a German organic chemist who demonstrated the existence of macromolecules, which he characterized as polymers. For this work he received the 1953 Nobel Prize in Chemistry.
He is also ...
, who spent the next decade finding experimental evidence for this hypothesis.
Common examples
 Polymers are of two types: naturally occurring and synthetic or ''man made''.
Polymers are of two types: naturally occurring and synthetic or ''man made''.
Natural
Natural polymeric materials such ashemp
Hemp, or industrial hemp, is a plant in the botanical class of ''Cannabis sativa'' cultivars grown specifically for industrial and consumable use. It can be used to make a wide range of products. Along with bamboo, hemp is among the fastest ...
, shellac
Shellac () is a resin secreted by the female Kerria lacca, lac bug on trees in the forests of India and Thailand. Chemically, it is mainly composed of aleuritic acid, jalaric acid, shellolic acid, and other natural waxes. It is processed and s ...
, amber
Amber is fossilized tree resin. Examples of it have been appreciated for its color and natural beauty since the Neolithic times, and worked as a gemstone since antiquity."Amber" (2004). In Maxine N. Lurie and Marc Mappen (eds.) ''Encyclopedia ...
, wool
Wool is the textile fiber obtained from sheep and other mammals, especially goats, rabbits, and camelids. The term may also refer to inorganic materials, such as mineral wool and glass wool, that have some properties similar to animal w ...
, silk
Silk is a natural fiber, natural protein fiber, some forms of which can be weaving, woven into textiles. The protein fiber of silk is composed mainly of fibroin and is most commonly produced by certain insect larvae to form cocoon (silk), c ...
, and natural rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds.
Types of polyisoprene ...
have been used for centuries. A variety of other natural polymers exist, such as cellulose
Cellulose is an organic compound with the chemical formula, formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of glycosidic bond, β(1→4) linked glucose, D-glucose units. Cellulose is an important s ...
, which is the main constituent of wood and paper.
Space polymer
Hemoglycin
Hemoglycin (previously termed hemolithin) is a space polymer that is the first polymer of amino acids found in meteorites.
Structure
Structural work has determined that its 1,494- dalton core unit (glycine18 / hydroxy-glycine4 / Fe2O4) con ...
(previously termed hemolithin
Hemolithin (sometimes confused with the similar space polymer hemoglycin) is a proposed protein containing iron and lithium, of extraterrestrial origin, according to an unpublished preprint. The result has not been published in any peer-review ...
) is a space polymer that is the first polymer of amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s found in meteorite
A meteorite is a rock (geology), rock that originated in outer space and has fallen to the surface of a planet or Natural satellite, moon. When the original object enters the atmosphere, various factors such as friction, pressure, and chemical ...
s.
Synthetic
Thelist of synthetic polymers
Some familiar household synthetic polymers include: Nylons in textiles and fabrics, Teflon in non-stick pans, Bakelite for electrical switches, polyvinyl chloride (PVC) in pipes, etc. The common PET bottles are made of a synthetic polymer, polyeth ...
, roughly in order of worldwide demand, includes polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bott ...
, polypropylene
Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer Propene, propylene.
Polypropylene belongs to the group of polyolefin ...
, polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It i ...
, polyvinyl chloride
Polyvinyl chloride (alternatively: poly(vinyl chloride), colloquial: vinyl or polyvinyl; abbreviated: PVC) is the world's third-most widely produced synthetic polymer of plastic (after polyethylene and polypropylene). About 40 million tons of ...
, synthetic rubber
A synthetic rubber is an artificial elastomer. They are polymers synthesized from petroleum byproducts. About of rubber is produced annually in the United States, and of that amount two thirds are synthetic. Synthetic rubber, just like natural ru ...
, phenol formaldehyde resin
Phenol formaldehyde resins (PF), also called phenolic resins or phenoplasts, are synthetic polymers obtained by the reaction of phenol or substituted phenol with formaldehyde. Used as the basis for Bakelite, PFs were the first commercial syntheti ...
(or Bakelite
Bakelite ( ), formally , is a thermosetting polymer, thermosetting phenol formaldehyde resin, formed from a condensation reaction of phenol with formaldehyde. The first plastic made from synthetic components, it was developed by Belgian chemist ...
), neoprene
Neoprene (also polychloroprene) is a family of synthetic rubbers that are produced by polymerization of chloroprene.Werner Obrecht, Jean-Pierre Lambert, Michael Happ, Christiane Oppenheimer-Stix, John Dunn and Ralf Krüger "Rubber, 4. Emulsion Rub ...
, nylon
Nylon is a family of synthetic polymers characterised by amide linkages, typically connecting aliphatic or Polyamide#Classification, semi-aromatic groups.
Nylons are generally brownish in color and can possess a soft texture, with some varieti ...
, polyacrylonitrile
Polyacrylonitrile (PAN) is a synthetic, semicrystalline organic polymer resin, with the linear formula (CH2CHCN)n. Almost all PAN resins are copolymers with acrylonitrile as the main monomer. PAN is used to produce large variety of products in ...
, PVB, silicone
In Organosilicon chemistry, organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (, where R = Organyl group, organic group). They are typically colorless oils or elastomer, rubber ...
, and many more. More than 330 million tons of these polymers are made every year (2015).
Most commonly, the continuously linked backbone of a polymer used for the preparation of plastics consists mainly of carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atoms. A simple example is polyethylene ('polythene' in British English), whose repeat unit or monomer is ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
. Many other structures do exist; for example, elements such as silicon form familiar materials such as silicones, examples being Silly Putty
Silly Putty is a toy containing silicone polymers that have unusual physical properties. It can flow like a liquid, bounce and can be stretched or broken depending on the amount of physical stress to which it is subjected. It contains viscoelas ...
and waterproof plumbing sealant. Oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
is also commonly present in polymer backbones, such as those of polyethylene glycol
Polyethylene glycol (PEG; ) is a polyether compound derived from petroleum with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular wei ...
, polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long-chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with wat ...
s (in glycosidic bond
A glycosidic bond or glycosidic linkage is a type of ether bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.
A glycosidic bond is formed between the hemiacetal or hemiketal group o ...
s), and DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
(in phosphodiester bond
In chemistry, a phosphodiester bond occurs when exactly two of the hydroxyl groups () in phosphoric acid react with hydroxyl groups on other molecules to form two ester bonds. The "bond" involves this linkage . Discussion of phosphodiesters is d ...
s).
Synthesis
 Polymerization is the process of combining many
Polymerization is the process of combining many small molecule
In molecular biology and pharmacology, a small molecule or micromolecule is a low molecular weight (≤ 1000 daltons) organic compound that may regulate a biological process, with a size on the order of 1 nm. Many drugs are small molecules; ...
s known as monomers into a covalently bonded chain or network. During the polymerization process, some chemical groups may be lost from each monomer. This happens in the polymerization of PET polyester. The monomers are terephthalic acid
Terephthalic acid is an organic compound with formula C6H4(CO2H)2. This white solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several million tons are produced annuall ...
(HOOCC6H4COOH) and ethylene glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes: as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odo ...
(HOCH2CH2OH) but the repeating unit is OCC6H4COOCH2CH2O, which corresponds to the combination of the two monomers with the loss of two water molecules. The distinct piece of each monomer that is incorporated into the polymer is known as a repeat unit
A repeat unit or repeating unit , or mer, is a part of a polymer whose repetition would produce the complete polymer chain (except for the end groups) by linking the repeat units together successively along the chain, like the beads of a necklace ...
or monomer residue.
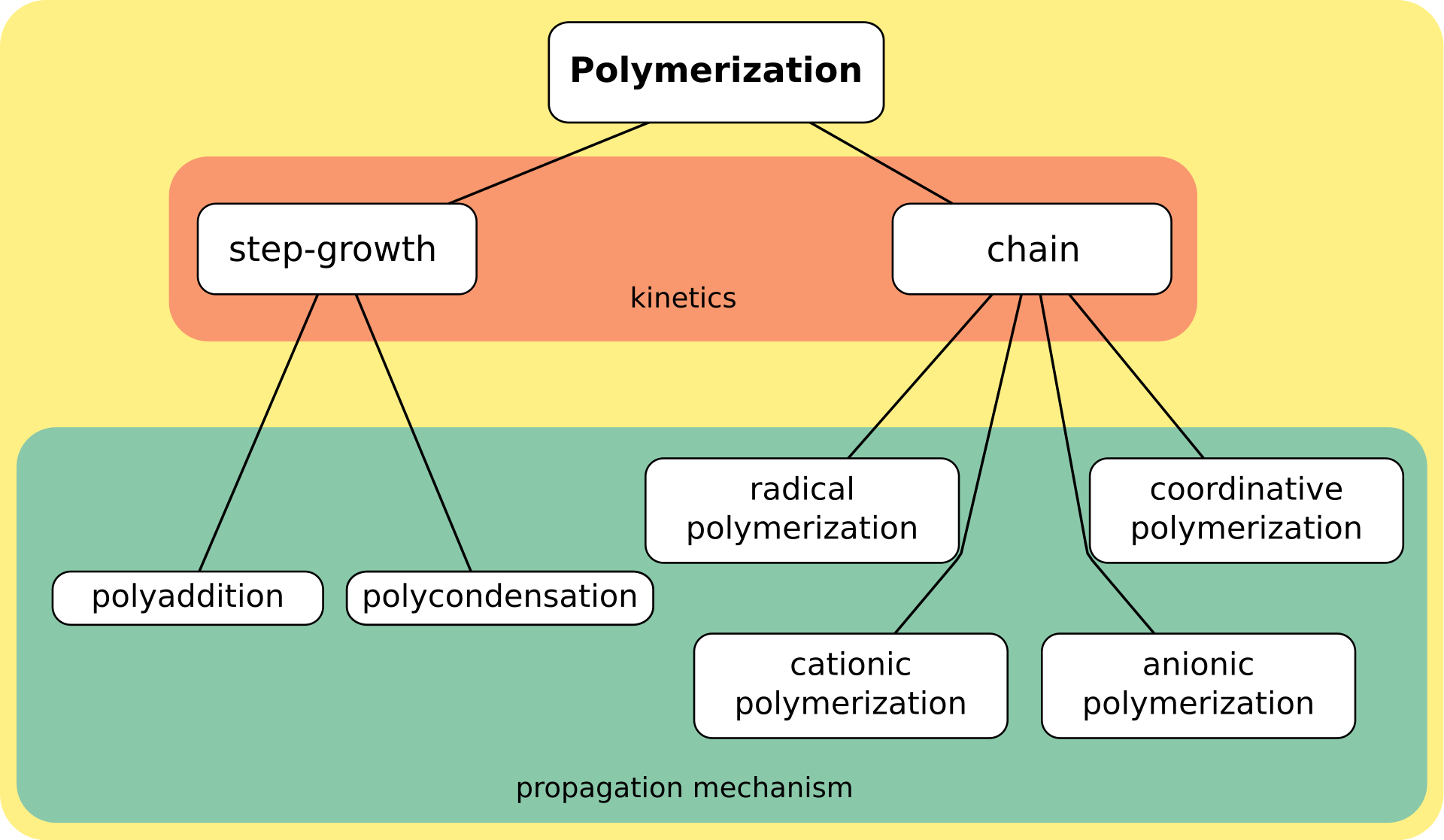
Synthetic methods are generally divided into two categories, step-growth polymerization
In polymer chemistry, step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Ma ...
and chain polymerization. The essential difference between the two is that in chain polymerization, monomers are added to the chain one at a time only, such as in polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It i ...
, whereas in step-growth polymerization chains of monomers may combine with one another directly, such as in polyester
Polyester is a category of polymers that contain one or two ester linkages in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include some natura ...
. Step-growth polymerization can be divided into polycondensation
In polymer chemistry, condensation polymers are any kind of polymers whose process of polymerization involves a condensation reaction (i.e. a small molecule, such as water or methanol, is produced as a byproduct). Natural proteins as well as s ...
, in which low-molar-mass by-product is formed in every reaction step, and polyaddition.
 Newer methods, such as
Newer methods, such as plasma polymerization
Plasma polymerization (or glow discharge polymerization) uses plasma sources to generate a gas discharge that provides energy to activate or fragmentation (chemistry), fragment gaseous or liquid monomer, often containing a vinyl group, in order to ...
do not fit neatly into either category. Synthetic polymerization reactions may be carried out with or without a catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
. Laboratory synthesis of biopolymers, especially of proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, re ...
, is an area of intensive research.
Biological synthesis
 There are three main classes of biopolymers:
There are three main classes of biopolymers: polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long-chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with wat ...
s, polypeptide
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty ...
s, and polynucleotides.
In living cells, they may be synthesized by enzyme-mediated processes, such as the formation of DNA catalyzed by DNA polymerase
A DNA polymerase is a member of a family of enzymes that catalyze the synthesis of DNA molecules from nucleoside triphosphates, the molecular precursors of DNA. These enzymes are essential for DNA replication and usually work in groups to create t ...
. The synthesis of proteins involves multiple enzyme-mediated processes to transcribe genetic information from the DNA to RNA
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins (messenger RNA). RNA and deoxyrib ...
and subsequently translate
Translation is the communication of the meaning of a source-language text by means of an equivalent target-language text. The English language draws a terminological distinction (which does not exist in every language) between ''transla ...
that information to synthesize the specified protein from amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s. The protein may be modified further following translation in order to provide appropriate structure and functioning. There are other biopolymers such as rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds.
Types of polyisoprene ...
, suberin
Suberin is a lipophilic, complex polyester biopolymer found in plants. It is composed of long-chain fatty acids (called suberin acids) and glycerol. Suberin is interconnected with cutin and lignin and forms a protective barrier in the epidermal ...
, melanin
Melanin (; ) is a family of biomolecules organized as oligomers or polymers, which among other functions provide the pigments of many organisms. Melanin pigments are produced in a specialized group of cells known as melanocytes.
There are ...
, and lignin
Lignin is a class of complex organic polymers that form key structural materials in the support tissues of most plants. Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidit ...
.
Modification of natural polymers
Naturally occurring polymers such ascotton
Cotton (), first recorded in ancient India, is a soft, fluffy staple fiber that grows in a boll, or protective case, around the seeds of the cotton plants of the genus '' Gossypium'' in the mallow family Malvaceae. The fiber is almost pure ...
, starch
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diet ...
, and rubber were familiar materials for years before synthetic polymers such as polyethene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bott ...
and perspex
Poly(methyl methacrylate) (PMMA) is a synthetic polymer derived from methyl methacrylate. It is a transparent thermoplastic, used as an engineering plastic. PMMA is also known as acrylic, acrylic glass, as well as by the trade names and bran ...
appeared on the market. Many commercially important polymers are synthesized by chemical modification of naturally occurring polymers. Prominent examples include the reaction of nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
and cellulose
Cellulose is an organic compound with the chemical formula, formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of glycosidic bond, β(1→4) linked glucose, D-glucose units. Cellulose is an important s ...
to form nitrocellulose
Nitrocellulose (also known as cellulose nitrate, flash paper, flash cotton, guncotton, pyroxylin and flash string, depending on form) is a highly flammable compound formed by nitrating cellulose through exposure to a mixture of nitric acid and ...
and the formation of vulcanized rubber
Vulcanization (British English: vulcanisation) is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to ...
by heating natural rubber in the presence of sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
. Ways in which polymers can be modified include oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
, cross-link
In chemistry and biology, a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
ing, and end-capping.
Structure
The structure of a polymeric material can be described at different length scales, from the sub-nm length scale up to the macroscopic one. There is in fact a hierarchy of structures, in which each stage provides the foundations for the next one. The starting point for the description of the structure of a polymer is the identity of its constituent monomers. Next, themicrostructure
Microstructure is the very small scale structure of a material, defined as the structure of a prepared surface of material as revealed by an optical microscope above 25× magnification. The microstructure of a material (such as metals, polymer ...
essentially describes the arrangement of these monomers within the polymer at the scale of a single chain. The microstructure determines the possibility for the polymer to form phases with different arrangements, for example through crystallization
Crystallization is a process that leads to solids with highly organized Atom, atoms or Molecule, molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regu ...
, the glass transition
The glass–liquid transition, or glass transition, is the gradual and Reversible reaction, reversible transition in amorphous solid, amorphous materials (or in amorphous regions within Crystallinity, semicrystalline materials) from a hard and rel ...
or microphase separation.
These features play a major role in determining the physical and chemical properties of a polymer.
Monomers and repeat units
The identity of the repeat units (monomer residues, also known as "mers") comprising a polymer is its first and most important attribute. Polymer nomenclature is generally based upon the type of monomer residues comprising the polymer. A polymer which contains only a single type ofrepeat unit
A repeat unit or repeating unit , or mer, is a part of a polymer whose repetition would produce the complete polymer chain (except for the end groups) by linking the repeat units together successively along the chain, like the beads of a necklace ...
is known as a homopolymer, while a polymer containing two or more types of repeat units is known as a copolymer
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are som ...
. A terpolymer is a copolymer which contains three types of repeat units.
Polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It i ...
is composed only of styrene
Styrene is an organic compound with the chemical formula C6H5CH=CH2. Its structure consists of a vinyl group as substituent on benzene. Styrene is a colorless, oily liquid, although aged samples can appear yellowish. The compound evaporates easi ...
-based repeat units, and is classified as a homopolymer. Polyethylene terephthalate
Polyethylene terephthalate (or poly(ethylene terephthalate), PET, PETE, or the obsolete PETP or PET-P), is the most common thermoplastic polymer resin of the polyester family and is used in synthetic fibre, fibres for clothing, packaging, conta ...
, even though produced from two different monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
s (ethylene glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes: as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odo ...
and terephthalic acid
Terephthalic acid is an organic compound with formula C6H4(CO2H)2. This white solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several million tons are produced annuall ...
), is usually regarded as a homopolymer because only one type of repeat unit is formed. Ethylene-vinyl acetate
Ethylene-vinyl acetate (EVA), also known as poly(ethylene-vinyl acetate) (PEVA), is a copolymer of ethylene and vinyl acetate. The weight percent of vinyl acetate usually varies from 10 to 50%, with the remainder being ethylene. There are three ...
contains more than one variety of repeat unit and is a copolymer. Some biological polymers are composed of a variety of different but structurally related monomer residues; for example, polynucleotides such as DNA are composed of four types of nucleotide
Nucleotides are Organic compound, organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both o ...
subunits.
:
A polymer containing ionizable subunits (e.g., pendant carboxylic groups) is known as a polyelectrolyte
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are t ...
or ionomer
An ionomer () ('' iono-'' + '' -mer'') is a polymer composed of repeat units of both electrically neutral repeating units and ionized units covalently bonded to the polymer backbone as pendant group moieties. Usually no more than 15 mole perce ...
, when the fraction of ionizable units is large or small respectively.
Microstructure
The microstructure of a polymer (sometimes called configuration) relates to the physical arrangement of monomer residues along the backbone of the chain. These are the elements of polymer structure that require the breaking of a covalent bond in order to change. Various polymer structures can be produced depending on the monomers and reaction conditions: A polymer may consist of linear macromolecules containing each only one unbranched chain. In the case of unbranched polyethylene, this chain is a long-chain ''n''-alkane. There are also branched macromolecules with a main chain and side chains, in the case of polyethylene the side chains would bealkyl groups
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
. In particular unbranched macromolecules can be in the solid state semi-crystalline, crystalline chain sections highlighted red in the figure below.
While branched and unbranched polymers are usually thermoplastics, many elastomer
An elastomer is a polymer with viscoelasticity (i.e. both viscosity and elasticity) and with weak intermolecular forces, generally low Young's modulus (E) and high failure strain compared with other materials. The term, a portmanteau of ''ela ...
s have a wide-meshed cross-linking between the "main chains". Close-meshed crosslinking, on the other hand, leads to thermosets
In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening (" curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and m ...
. Cross-links and branches are shown as red dots in the figures. Highly branched polymers are amorphous and the molecules in the solid interact randomly.
:
Polymer architecture
branched polymer
In polymer chemistry, branching is the regular or irregular attachment of side chains to a polymer's backbone chain. It occurs by the replacement of a substituent (e.g. a hydrogen atom) on a monomer subunit by another covalent bond, covalently ...
molecule is composed of a main chain with one or more substituent side chains or branches. Types of branched polymers include star polymer
In polymer science, star-shaped polymers are the simplest class of branched polymers with a general structure consisting of several (at least three) linear chains connected to a central core.
The core, or the center, of the polymer can be an a ...
s, comb polymers, polymer brushes, dendronized polymer
In polymer chemistry and materials science, dendronized polymers (American and British English spelling differences, British English: dendronised polymers; ) are linear polymers to every repeat unit of which dendrons are attached. Dendrons are reg ...
s, ladder polymers, and dendrimer
Dendrimers are highly ordered, Branching (polymer chemistry), branched molecules, polymeric molecules. Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a sph ...
s. There exist also two-dimensional polymers (2DP) which are composed of topologically planar repeat units. A polymer's architecture affects many of its physical properties including solution viscosity, melt viscosity, solubility in various solvents, glass-transition temperature and the size of individual polymer coils in solution. A variety of techniques may be employed for the synthesis of a polymeric material with a range of architectures, for example living polymerization
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transf ...
.
Chain length
A common means of expressing the length of a chain is thedegree of polymerization
The degree of polymerization, or DP, is the number of structural unit, monomeric units in a macromolecule or polymer or oligomer molecule.
For a homopolymer, there is only one type of monomeric unit and the ''number-average'' degree of polymeriza ...
, which quantifies the number of monomers incorporated into the chain.Rubinstein, p. 3 As with other molecules, a polymer's size may also be expressed in terms of molecular weight
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
. Since synthetic polymerization techniques typically yield a statistical distribution of chain lengths, the molecular weight is expressed in terms of weighted averages. The number-average molecular weight
In polymer chemistry, the molar mass distribution (or molecular weight distribution) describes the relationship between the number of moles of each polymer species () and the molar mass () of that species. In linear polymers, the individual polym ...
(''M''n) and weight-average molecular weight (''M''w) are most commonly reported.Rubinstein, pp. 23–24 The ratio of these two values (''M''w / ''M''n) is the dispersity
In chemistry, the dispersity is a measure of the heterogeneity of sizes of molecules or particles in a mixture. A collection of objects is called uniform if the objects have the same size, shape, or mass. A sample of objects that have an inconsi ...
(''Đ''), which is commonly used to express the width of the molecular weight distribution.
The physical properties of polymer strongly depend on the length (or equivalently, the molecular weight) of the polymer chain.Rubinstein, p. 5 One important example of the physical consequences of the molecular weight is the scaling of the viscosity
Viscosity is a measure of a fluid's rate-dependent drag (physics), resistance to a change in shape or to movement of its neighboring portions relative to one another. For liquids, it corresponds to the informal concept of ''thickness''; for e ...
(resistance to flow) in the melt. The influence of the weight-average molecular weight () on the melt viscosity () depends on whether the polymer is above or below the onset of entanglements. Below the entanglement molecular weight, , whereas above the entanglement molecular weight, . In the latter case, increasing the polymer chain length 10-fold would increase the viscosity over 1000 times. Increasing chain length furthermore tends to decrease chain mobility, increase strength and toughness, and increase the glass-transition temperature (Tg). This is a result of the increase in chain interactions such as van der Waals attractions and entanglements that come with increased chain length. These interactions tend to fix the individual chains more strongly in position and resist deformations and matrix breakup, both at higher stresses and higher temperatures.
Monomer arrangement in copolymers
Copolymers are classified either as statistical copolymers, alternating copolymers, block copolymers, graft copolymers or gradient copolymers. In the schematic figure below, Ⓐ and Ⓑ symbolize the tworepeat unit
A repeat unit or repeating unit , or mer, is a part of a polymer whose repetition would produce the complete polymer chain (except for the end groups) by linking the repeat units together successively along the chain, like the beads of a necklace ...
s.
:
*Alternating copolymers possess two regularly alternating monomer residues:Painter, p. 14 . An example is the equimolar copolymer of styrene
Styrene is an organic compound with the chemical formula C6H5CH=CH2. Its structure consists of a vinyl group as substituent on benzene. Styrene is a colorless, oily liquid, although aged samples can appear yellowish. The compound evaporates easi ...
and maleic anhydride
Maleic anhydride is an organic compound with the formula . It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor. It is produced industrially on a large scale for applications in coatings and polymers.
Str ...
formed by free-radical chain-growth polymerization.Rudin, p. 18–20 A step-growth copolymer such as Nylon 66
Nylon 66 (loosely written nylon 6-6, nylon 6/6, nylon 6,6, or nylon 6:6) is a type of polyamide or nylon. It, and nylon 6, are the two most common for textile and plastic industries. Nylon 66 is made of two monomers each containing six carbon at ...
can also be considered a strictly alternating copolymer of diamine and diacid residues, but is often described as a homopolymer with the dimeric residue of one amine and one acid as a repeat unit.Cowie, p. 104
*Periodic copolymers have more than two species of monomer units in a regular sequence.
*Statistical copolymers have monomer residues arranged according to a statistical rule. A statistical copolymer in which the probability of finding a particular type of monomer residue at a particular point in the chain is independent of the types of surrounding monomer residue may be referred to as a truly random copolymer.Painter, p. 15 For example, the chain-growth copolymer of vinyl chloride
Vinyl chloride is an organochloride with the formula H2C =CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. It is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC). Vinyl chloride is a ...
and vinyl acetate
Vinyl acetate is an organic compound with the Chemical formula, formula CH3CO2CH=CH2. This colorless liquid is the precursor to polyvinyl acetate, ethylene-vinyl acetate copolymers, polyvinyl alcohol, and other important industrial polymers.
Prod ...
is random.
*Block copolymers have long sequences of different monomer units. Polymers with two or three blocks of two distinct chemical species (e.g., A and B) are called diblock copolymers and triblock copolymers, respectively. Polymers with three blocks, each of a different chemical species (e.g., A, B, and C) are termed triblock terpolymers.
*Graft or grafted copolymers contain side chains or branches whose repeat units have a different composition or configuration than the main chain. The branches are added on to a preformed main chain macromolecule.
Monomers within a copolymer may be organized along the backbone in a variety of ways. A copolymer containing a controlled arrangement of monomers is called a sequence-controlled polymer. Alternating, periodic and block copolymers are simple examples of sequence-controlled polymers.
Tacticity
Tacticity describes the relativestereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ...
of chiral
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek language, Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is dist ...
centers in neighboring structural units within a macromolecule. There are three types of tacticity: isotactic
Tacticity (from , "relating to arrangement or order") is the relative stereochemistry of adjacent chiral centers within a macromolecule. The practical significance of tacticity rests on the effects on the physical properties of the polymer. The ...
(all substituents on the same side), atactic
Tacticity (from , "relating to arrangement or order") is the relative stereochemistry of adjacent chiral centers within a macromolecule. The practical significance of tacticity rests on the effects on the physical properties of the polymer. The ...
(random placement of substituents), and syndiotactic
Tacticity (from , "relating to arrangement or order") is the relative stereochemistry of adjacent chiral centers within a macromolecule. The practical significance of tacticity rests on the effects on the physical properties of the polymer. The ...
(alternating placement of substituents).
:
Morphology
Polymer morphology generally describes the arrangement and microscale ordering of polymer chains in space. The macroscopic physical properties of a polymer are related to the interactions between the polymer chains. * Disordered polymers: In the solid state, atactic polymers, polymers with a high degree of branching and random copolymers formamorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymousl ...
(i.e. glassy structures).Bernd Tieke: ''Makromolekulare Chemie.'' 3. Auflage, Wiley-VCH, Weinheim 2014, S. 295f (in German). In melt and solution, polymers tend to form a constantly changing "statistical cluster", see freely-jointed-chain model. In the solid state, the respective conformations of the molecules are frozen. Hooking and entanglement of chain molecules lead to a "mechanical bond" between the chains. Intermolecular
An intermolecular force (IMF; also secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles (e.g. ...
and intramolecular attractive forces only occur at sites where molecule segments are close enough to each other. The irregular structures of the molecules prevent a narrower arrangement.
* Linear polymers with periodic structure, low branching and stereoregularity (e. g. not atactic) have a semi-crystalline
Crystallinity refers to the degree of structural order in a solid. In a crystal, the atoms or molecules are arranged in a regular, periodic manner. The degree of crystallinity has a large influence on hardness, density, transparency and diffusi ...
structure in the solid state. In simple polymers (such as polyethylene), the chains are present in the crystal in zigzag conformation. Several zigzag conformations form dense chain packs, called crystallites or lamellae. The lamellae are much thinner than the polymers are long (often about 10 nm). Wolfgang Kaiser: ''Kunststoffchemie für Ingenieure.'' 3. Auflage, Carl Hanser, München 2011, S. 84. They are formed by more or less regular folding of one or more molecular chains. Amorphous structures exist between the lamellae. Individual molecules can lead to entanglements between the lamellae and can also be involved in the formation of two (or more) lamellae (chains than called tie molecules). Several lamellae form a superstructure, a spherulite
In petrology, spherulites () are small, rounded bodies that commonly occur in vitreous igneous rocks. They are often visible in specimens of obsidian, pitchstone, and rhyolite as globules about the size of millet seed or rice grain, with a du ...
, often with a diameter in the range of 0.05 to 1 mm.
:The type and arrangement of (functional) residues of the repeat units effects or determines the crystallinity and strength of the secondary valence bonds. In isotactic polypropylene, the molecules form a helix. Like the zigzag conformation, such helices allow a dense chain packing. Particularly strong intermolecular interactions occur when the residues of the repeating units allow the formation of hydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
s, as in the case of ''p''-aramid. The formation of strong intramolecular associations may produce diverse folded states of single linear chains with distinct circuit topology
The circuit topology of a folded linear polymer refers to the arrangement of its intra-molecular contacts. Examples of linear polymers with intra-molecular contacts are nucleic acids and proteins. Proteins fold via the formation of contacts of v ...
. Crystallinity and superstructure are always dependent on the conditions of their formation, see also: crystallization of polymers
Crystallization of polymers is a process associated with partial alignment of their molecular chains. These chains fold together and form ordered regions called Lamella (materials), lamellae, which compose larger spheroidal structures named Spheru ...
. Compared to amorphous structures, semi-crystalline structures lead to a higher stiffness, density, melting temperature and higher resistance of a polymer.
* Cross-linked polymers: Wide-meshed cross-linked polymers are elastomer
An elastomer is a polymer with viscoelasticity (i.e. both viscosity and elasticity) and with weak intermolecular forces, generally low Young's modulus (E) and high failure strain compared with other materials. The term, a portmanteau of ''ela ...
s and cannot be molten (unlike thermoplastic
A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
Most thermoplastics have a high molecular weight. The polymer chains as ...
s); heating cross-linked polymers only leads to decomposition
Decomposition is the process by which dead organic substances are broken down into simpler organic or inorganic matter such as carbon dioxide, water, simple sugars and mineral salts. The process is a part of the nutrient cycle and is ess ...
. Thermoplastic elastomer
Thermoplastic elastomers (TPE), sometimes referred to as thermoplastic rubbers (TPR), are a class of copolymers or a physical mix of polymers (usually a plastic and a rubber) that consist of materials with both thermoplastic and elastomeric prop ...
s, on the other hand, are reversibly "physically crosslinked" and can be molten. Block copolymers in which a hard segment of the polymer has a tendency to crystallize and a soft segment has an amorphous structure are one type of thermoplastic elastomers: the hard segments ensure wide-meshed, physical crosslinking.
Crystallinity
When applied to polymers, the term ''crystalline'' has a somewhat ambiguous usage. In some cases, the term ''crystalline'' finds identical usage to that used in conventionalcrystallography
Crystallography is the branch of science devoted to the study of molecular and crystalline structure and properties. The word ''crystallography'' is derived from the Ancient Greek word (; "clear ice, rock-crystal"), and (; "to write"). In J ...
. For example, the structure of a crystalline protein or polynucleotide, such as a sample prepared for x-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
, may be defined in terms of a conventional unit cell composed of one or more polymer molecules with cell dimensions of hundreds of angstrom
The angstrom (; ) is a unit of length equal to m; that is, one ten-billionth of a metre, a hundred-millionth of a centimetre, 0.1 nanometre, or 100 picometres. The unit is named after the Swedish physicist Anders Jonas Ångström (1814–18 ...
s or more. A synthetic polymer may be loosely described as crystalline if it contains regions of three-dimensional ordering on atomic (rather than macromolecular) length scales, usually arising from intramolecular folding or stacking of adjacent chains. Synthetic polymers may consist of both crystalline and amorphous regions; the degree of crystallinity may be expressed in terms of a weight fraction or volume fraction of crystalline material. Few synthetic polymers are entirely crystalline. The crystallinity of polymers is characterized by their degree of crystallinity, ranging from zero for a completely non-crystalline polymer to one for a theoretical completely crystalline polymer. Polymers with microcrystalline regions are generally tougher (can be bent more without breaking) and more impact-resistant than totally amorphous polymers. Polymers with a degree of crystallinity approaching zero or one will tend to be transparent, while polymers with intermediate degrees of crystallinity will tend to be opaque due to light scattering by crystalline or glassy regions. For many polymers, crystallinity may also be associated with decreased transparency.
Chain conformation
The space occupied by a polymer molecule is generally expressed in terms ofradius of gyration
The radius of gyration or gyradius of a body about the axis of rotation is defined as the radial distance to a point which would have a moment of inertia the same as the body's actual distribution of mass, if the total mass of the body were concent ...
, which is an average distance from the center of mass of the chain to the chain itself. Alternatively, it may be expressed in terms of pervaded volume, which is the volume spanned by the polymer chain and scales with the cube of the radius of gyration.
The simplest theoretical models for polymers in the molten, amorphous state are ideal chain
An ideal chain (or freely-jointed chain) is the simplest model in polymer chemistry to describe polymers, such as nucleic acids and proteins. It assumes that the monomers in a polymer are located at the steps of a hypothetical random walker that ...
s.
Properties
Polymer properties depend of their structure and they are divided into classes according to their physical bases. Many physical and chemical properties describe how a polymer behaves as a continuous macroscopic material. They are classified as bulk properties, orintensive properties
Physical or chemical properties of materials and systems can often be categorized as being either intensive or extensive, according to how the property changes when the size (or extent) of the system changes.
The terms "intensive and extensive ...
according to thermodynamics
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed b ...
.
Mechanical properties
 The bulk properties of a polymer are those most often of end-use interest. These are the properties that dictate how the polymer actually behaves on a macroscopic scale.
The bulk properties of a polymer are those most often of end-use interest. These are the properties that dictate how the polymer actually behaves on a macroscopic scale.
Tensile strength
Thetensile strength
Ultimate tensile strength (also called UTS, tensile strength, TS, ultimate strength or F_\text in notation) is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials, the ultimate ...
of a material quantifies how much elongating stress the material will endure before failure. This is very important in applications that rely upon a polymer's physical strength or durability. For example, a rubber band with a higher tensile strength will hold a greater weight before snapping. In general, tensile strength increases with polymer chain length and crosslinking Cross-linking may refer to
*Cross-link
In chemistry and biology, a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers ca ...
of polymer chains.
Young's modulus of elasticity
Young's modulus
Young's modulus (or the Young modulus) is a mechanical property of solid materials that measures the tensile or compressive stiffness when the force is applied lengthwise. It is the modulus of elasticity for tension or axial compression. Youn ...
quantifies the elasticity of the polymer. It is defined, for small strains, as the ratio of rate of change of stress to strain. Like tensile strength, this is highly relevant in polymer applications involving the physical properties of polymers, such as rubber bands. The modulus is strongly dependent on temperature. Viscoelasticity
In materials science and continuum mechanics, viscoelasticity is the property of materials that exhibit both viscous and elastic characteristics when undergoing deformation. Viscous materials, like water, resist both shear flow and strain lin ...
describes a complex time-dependent elastic response, which will exhibit hysteresis
Hysteresis is the dependence of the state of a system on its history. For example, a magnet may have more than one possible magnetic moment in a given magnetic field, depending on how the field changed in the past. Plots of a single component of ...
in the stress-strain curve when the load is removed. Dynamic mechanical analysis
Dynamic mechanical analysis (abbreviated DMA) is a technique used to study and characterize materials. It is most useful for studying the viscoelastic behavior of polymers. A sinusoidal stress is applied and the strain in the material is measured, ...
or DMA measures this complex modulus by oscillating the load and measuring the resulting strain as a function of time.
Transport properties
Transport properties such asdiffusivity
Diffusivity is a rate of diffusion, a measure of the rate at which particles or heat or fluids can spread.
It is measured differently for different mediums.
Diffusivity may refer to:
*Thermal diffusivity, diffusivity of heat
*Diffusivity of mass: ...
describe how rapidly molecules move through the polymer matrix. These are very important in many applications of polymers for films and membranes.
The movement of individual macromolecules occurs by a process called reptation
A peculiarity of thermal motion of very long linear macromolecules in ''entangled'' polymer melts or concentrated polymer solutions is reptation. Derived from the word reptile, reptation suggests the movement of entangled polymer chains as bein ...
in which each chain molecule is constrained by entanglements with neighboring chains to move within a virtual tube. The theory of reptation can explain polymer molecule dynamics and viscoelasticity
In materials science and continuum mechanics, viscoelasticity is the property of materials that exhibit both viscous and elastic characteristics when undergoing deformation. Viscous materials, like water, resist both shear flow and strain lin ...
.
Phase behavior
Crystallization and melting
Depending on their chemical structures, polymers may be either semi-crystalline or amorphous. Semi-crystalline polymers can undergo crystallization and melting transitions, whereas amorphous polymers do not. In polymers, crystallization and melting do not suggest solid-liquid phase transitions, as in the case of water or other molecular fluids. Instead, crystallization and melting refer to the phase transitions between two solid states (''i.e.'', semi-crystalline and amorphous). Crystallization occurs above the glass-transition temperature (''T''g) and below the melting temperature (''T''m).Glass transition
All polymers (amorphous or semi-crystalline) go throughglass transition
The glass–liquid transition, or glass transition, is the gradual and Reversible reaction, reversible transition in amorphous solid, amorphous materials (or in amorphous regions within Crystallinity, semicrystalline materials) from a hard and rel ...
s. The glass-transition temperature (''T''g) is a crucial physical parameter for polymer manufacturing, processing, and use. Below ''T''g, molecular motions are frozen and polymers are brittle and glassy. Above ''T''g, molecular motions are activated and polymers are rubbery and viscous. The glass-transition temperature may be engineered by altering the degree of branching or crosslinking in the polymer or by the addition of plasticizer
A plasticizer ( UK: plasticiser) is a substance that is added to a material to make it softer and more flexible, to increase its plasticity, to decrease its viscosity, and/or to decrease friction during its handling in manufacture.
Plasticizer ...
s.
Whereas crystallization and melting are first-order phase transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic Sta ...
s, the glass transition is not. The glass transition shares features of second-order phase transitions (such as discontinuity in the heat capacity, as shown in the figure), but it is generally not considered a thermodynamic transition between equilibrium states.
Mixing behavior
miscible
Miscibility () is the property of two substances to mix in all proportions (that is, to fully dissolve in each other at any concentration), forming a homogeneous mixture (a solution). Such substances are said to be miscible (etymologically ...
than mixtures of small molecule
In molecular biology and pharmacology, a small molecule or micromolecule is a low molecular weight (≤ 1000 daltons) organic compound that may regulate a biological process, with a size on the order of 1 nm. Many drugs are small molecules; ...
materials. This effect results from the fact that the driving force for mixing is usually entropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the micros ...
, not interaction energy. In other words, miscible materials usually form a solution not because their interaction with each other is more favorable than their self-interaction, but because of an increase in entropy and hence free energy associated with increasing the amount of volume available to each component. This increase in entropy scales with the number of particles (or moles) being mixed. Since polymeric molecules are much larger and hence generally have much higher specific volumes than small molecules, the number of molecules involved in a polymeric mixture is far smaller than the number in a small molecule mixture of equal volume. The energetics of mixing, on the other hand, is comparable on a per volume basis for polymeric and small molecule mixtures. This tends to increase the free energy of mixing for polymer solutions and thereby making solvation less favorable, and thereby making the availability of concentrated solutions of polymers far rarer than those of small molecules.
Furthermore, the phase behavior of polymer solutions and mixtures is more complex than that of small molecule mixtures. Whereas most small molecule solutions exhibit only an upper critical solution temperature
The upper critical solution temperature (UCST) or upper consolute temperature is the critical temperature above which the components of a mixture are miscible in all proportions. The word ''upper'' indicates that the UCST is an upper bound to a te ...
phase transition (UCST), at which phase separation occurs with cooling, polymer mixtures commonly exhibit a lower critical solution temperature
The lower critical solution temperature (LCST) or lower consolute temperature is the critical temperature below which the components of a mixture are miscible in all proportions. The word ''lower'' indicates that the LCST is a lower bound to a te ...
phase transition (LCST), at which phase separation occurs with heating.
In dilute solutions, the properties of the polymer are characterized by the interaction between the solvent and the polymer. In a good solvent, the polymer appears swollen and occupies a large volume. In this scenario, intermolecular forces between the solvent and monomer subunits dominate over intramolecular interactions. In a bad solvent or poor solvent, intramolecular forces dominate and the chain contracts. In the theta solvent, or the state of the polymer solution where the value of the second virial coefficient becomes 0, the intermolecular polymer-solvent repulsion balances exactly the intramolecular monomer-monomer attraction. Under the theta condition (also called the Flory condition), the polymer behaves like an ideal random coil
In polymer chemistry, a random coil is a conformation of polymers where the monomer subunits are oriented randomly while still being bonded to adjacent units. It is not one specific shape, but a statistical distribution of shapes for all the cha ...
. The transition between the states is known as a coil–globule transition.
Inclusion of plasticizers
Inclusion of plasticizers tends to lower Tg and increase polymer flexibility. Addition of the plasticizer will also modify dependence of the glass-transition temperature Tg on the cooling rate. The mobility of the chain can further change if the molecules of plasticizer give rise to hydrogen bonding formation. Plasticizers are generally small molecules that are chemically similar to the polymer and create gaps between polymer chains for greater mobility and fewer interchain interactions. A good example of the action of plasticizers is related to polyvinylchlorides or PVCs. A uPVC, or unplasticized polyvinylchloride, is used for things such as pipes. A pipe has no plasticizers in it, because it needs to remain strong and heat-resistant. Plasticized PVC is used in clothing for a flexible quality. Plasticizers are also put in some types of cling film to make the polymer more flexible.Chemical properties
The attractive forces between polymer chains play a large part in determining the polymer's properties. Because polymer chains are so long, they have many such interchain interactions per molecule, amplifying the effect of these interactions on the polymer properties in comparison to attractions between conventional molecules. Different side groups on the polymer can lend the polymer toionic bonding
Ionic bonding is a type of chemical bonding that involves the Coulomb's law, electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in io ...
or hydrogen bonding
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
between its own chains. These stronger forces typically result in higher tensile strength and higher crystalline melting points.
The intermolecular forces in polymers can be affected by dipole
In physics, a dipole () is an electromagnetic phenomenon which occurs in two ways:
* An electric dipole moment, electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple ...
s in the monomer units. Polymers containing amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
or carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
groups can form hydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
s between adjacent chains; the partially positively charged hydrogen atoms in N-H groups of one chain are strongly attracted to the partially negatively charged oxygen atoms in C=O groups on another. These strong hydrogen bonds, for example, result in the high tensile strength and melting point of polymers containing urethane or urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
linkages. Polyester
Polyester is a category of polymers that contain one or two ester linkages in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include some natura ...
s have dipole-dipole bonding between the oxygen atoms in C=O groups and the hydrogen atoms in H-C groups. Dipole bonding is not as strong as hydrogen bonding, so a polyester's melting point and strength are lower than Kevlar
Kevlar (para-aramid) is a strong, heat-resistant synthetic fiber, related to other aramids such as Nomex and Technora. Developed by Stephanie Kwolek at DuPont in 1965, the high-strength material was first used commercially in the early 1970s as ...
's (Twaron
Twaron (a brand name of Teijin Aramid) is a para-aramid, high-performance yarn. It is a heat-resistant fibre, helps in ballistic protection and cut protection. Twaron was developed in the early 1970s by the Dutch company Akzo Nobel's division E ...
), but polyesters have greater flexibility. Polymers with non-polar units such as polyethylene interact only through weak Van der Waals force
In molecular physics and chemistry, the van der Waals force (sometimes van der Waals' force) is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical elec ...
s. As a result, they typically have lower melting temperatures than other polymers.
When a polymer is dispersed or dissolved in a liquid, such as in commercial products like paints and glues, the chemical properties and molecular interactions influence how the solution flows and can even lead to self-assembly
Self-assembly is a process in which a disordered system of pre-existing components forms an organized structure or pattern as a consequence of specific, local interactions among the components themselves, without external direction. When the ...
of the polymer into complex structures. When a polymer is applied as a coating, the chemical properties will influence the adhesion of the coating and how it interacts with external materials, such as superhydrophobic
In chemistry and materials science, ultrahydrophobic (or superhydrophobic) surfaces are highly hydrophobic, i.e., extremely difficult to wet. The contact angles of a water droplet on an ultrahydrophobic material exceed 150°. This is also ref ...
polymer coatings leading to water resistance. Overall the chemical properties of a polymer are important elements for designing new polymeric material products.
Optical properties
Polymers such as PMMA and HEMA:MMA are used as matrices in the gain medium of solid-state dye lasers, also known as solid-state dye-doped polymer lasers. These polymers have a high surface quality and are also highly transparent so that the laser properties are dominated by thelaser dye
file:Coherent 899 dye laser.jpg, Close-up of a table-top dye laser using Rhodamine 6G as active medium.
file:rhodamine 6G.svg, Molecular structure of Rhodamine 6G, perhaps the best known laser dye.
A Laser dye is a dye used as laser medium in a dy ...
used to dope the polymer matrix. These types of lasers, that also belong to the class of organic lasers, are known to yield very narrow linewidths which is useful for spectroscopy and analytical applications. An important optical parameter in the polymer used in laser applications is the change in refractive index with temperature
also known as dn/dT. For the polymers mentioned here the (dn/dT) ~ −1.4 × 10−4 in units of K−1 in the 297 ≤ T ≤ 337 K range.
Electrical properties
Most conventional polymers such as polyethylene are electrical insulators, but the development of polymers containing π-conjugated bonds has led to a wealth of polymer-basedsemiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
s, such as polythiophene
Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocyclic compound, heterocycle. The parent PT is an insoluble colored solid with the formula (C4H2S)n.Strictly speaking, "polythiophene" is a misnomer, since the polymer consists of ...
s. This has led to many applications in the field of organic electronics
Organic electronics is a field of materials science concerning the design, Chemical synthesis, synthesis, characterization, and application of Organic compound, organic molecules or polymers that show desirable Electronics, electronic properties ...
.
Applications
Nowadays, synthetic polymers are used in almost all walks of life. Modern society would look very different without them. The spreading of polymer use is connected to their unique properties: low density, low cost, good thermal/electrical insulation properties, high resistance to corrosion, low-energy demanding polymer manufacture and facile processing into final products. For a given application, the properties of a polymer can be tuned or enhanced by combination with other materials, as in composites. Their application allows to save energy (lighter cars and planes, thermally insulated buildings), protect food and drinking water (packaging), save land and lower use of fertilizers (synthetic fibres), preserve other materials (coatings), protect and save lives (hygiene, medical applications). A representative, non-exhaustive list of applications is given below. * Clothing, sportswear and accessories:polyester
Polyester is a category of polymers that contain one or two ester linkages in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include some natura ...
and PVC clothing
PVC clothing is shiny clothing made from the plastic polyvinyl chloride (PVC). PVC plastic is often called "vinyl" and this type of clothing is commonly known as vinyl clothing. PVC is sometimes confused with the similarly shiny patent leather.
...
, spandex
Spandex, Lycra, or elastane is a synthetic fiber known for its exceptional elasticity. It is a polyether- polyurea copolymer that was invented in 1958 by chemist Joseph Shivers at DuPont.
Name
The name ''spandex'', which is an anagram of t ...
, sport shoes, wetsuit
A wetsuit is a garment worn to provide thermal protection while wet. It is usually made of foamed neoprene, and is worn by surfers, divers, windsurfers, canoeists, and others engaged in water sports and other activities in or on the water. ...
s, footballs and billiard balls
A billiard ball is a small, hard ball used in cue sports, such as carom billiards, pool, and snooker. The number, type, diameter, color, and pattern of the balls differ depending upon the specific game being played. Various particular ball pr ...
, skis and snowboard
Snowboards are boards where the user places both feet, usually secured, to the same board. The board itself is wider than most skis, with the ability to glide on snow."snowboarding." Dictionary.com Unabridged (v 1.1). Random House, Inc. 17 Mar ...
s, rackets, parachute
A parachute is a device designed to slow an object's descent through an atmosphere by creating Drag (physics), drag or aerodynamic Lift (force), lift. It is primarily used to safely support people exiting aircraft at height, but also serves va ...
s, sail
A sail is a tensile structure, which is made from fabric or other membrane materials, that uses wind power to propel sailing craft, including sailing ships, sailboats, windsurfers, ice boats, and even sail-powered land vehicles. Sails may b ...
s, tents and shelters.
* Electronic and photonic technologies: organic field effect transistors
The field-effect transistor (FET) is a type of transistor that uses an electric field to control the current through a semiconductor. It comes in two types: junction FET (JFET) and metal-oxide-semiconductor FET (MOSFET). FETs have three termin ...
(OFET), light emitting diodes
A light-emitting diode (LED) is a semiconductor device that Light#Light sources, emits light when Electric current, current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of pho ...
(OLED) and solar cells
A solar cell, also known as a photovoltaic cell (PV cell), is an electronic device that converts the energy of light directly into electricity by means of the photovoltaic effect.
, television components, compact disc
The compact disc (CD) is a Digital media, digital optical disc data storage format co-developed by Philips and Sony to store and play digital audio recordings. It employs the Compact Disc Digital Audio (CD-DA) standard and was capable of hol ...
s (CD), photoresist
A photoresist (also known simply as a resist) is a light-sensitive material used in several processes, such as photolithography and photoengraving, to form a patterned coating on a surface. This process is crucial in the electronics industry.
T ...
s, holography
Holography is a technique that allows a wavefront to be recorded and later reconstructed. It is best known as a method of generating three-dimensional images, and has a wide range of other uses, including data storage, microscopy, and interfe ...
.
* Packaging and containers: films
A film, also known as a movie or motion picture, is a work of Visual arts, visual art that simulates experiences and otherwise communicates ideas, stories, perceptions, emotions, or atmosphere through the use of moving images that are gen ...
, bottles
A bottle is a narrow-necked container made of an impermeable material (such as glass, plastic or aluminium) in various shapes and sizes that stores and transports liquids. Its mouth, at the bottling line, can be sealed with an internal st ...
, food packaging
Food packaging is a packaging system specifically designed for food and represents one of the most important aspects among the processes involved in the food industry, as it provides protection from chemical, biological and physical alterations ...
, barrel
A barrel or cask is a hollow cylindrical container with a bulging center, longer than it is wide. They are traditionally made of wooden stave (wood), staves and bound by wooden or metal hoops. The word vat is often used for large containers ...
s.
* Insulation: electrical
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by Maxwel ...
and thermal insulation
Thermal insulation is the reduction of heat transfer (i.e., the transfer of thermal energy between objects of differing temperature) between objects in thermal contact or in range of radiative influence. Thermal insulation can be achieved with s ...
, spray foam
Spray foam (expanding foam in the UK) is a chemical product used in construction and engineering primarily as Building insulation, insulation and as a filler material. It is produced as a liquid but quickly expands and hardens into a stiff, ligh ...
s.
* Construction and structural applications: garden furniture
Garden furniture, also called patio furniture or outdoor furniture, is a type of furniture specifically designed for outdoor use. It is typically made of weather-resistant materials such as aluminium which is rust-proof.
History
The oldes ...
, PVC
Polyvinyl chloride (alternatively: poly(vinyl chloride), colloquial: vinyl or polyvinyl; abbreviated: PVC) is the world's third-most widely produced synthetic polymer of plastic (after polyethylene and polypropylene). About 40 million tons o ...
windows, flooring, sealing, pipes
Pipe(s), PIPE(S) or piping may refer to:
Objects
* Pipe (fluid conveyance), a hollow cylinder following certain dimension rules
** Piping, the use of pipes in industry
* Smoking pipe
** Tobacco pipe
* Half-pipe and quarter pipe, semi-circu ...
.
* Paints, glues and lubricants: varnish
Varnish is a clear Transparency (optics), transparent hard protective coating or film. It is not to be confused with wood stain. It usually has a yellowish shade due to the manufacturing process and materials used, but it may also be pigmente ...
, adhesive
Adhesive, also known as glue, cement, mucilage, or paste, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation.
The use of adhesives offers certain advantage ...
s, dispersant
A dispersant or a dispersing agent is a substance, typically a surfactant, that is added to a suspension of solid or liquid particles in a liquid (such as a colloid or emulsion) to improve the separation of the particles and to prevent their sett ...
s, anti-graffiti coating
An anti-graffiti coating is a coating that prevents graffiti paint from bonding to surfaces.
Cleaning graffiti off buildings costs billions of dollars annually. Many cities have started anti-graffiti programs but vandalism is still a problem. Comp ...
s, antifouling coatings, non-stick surface
A non-stick surface is engineered to reduce the ability of other materials to stick to it. Non-sticking cookware is a common application, where the non-stick coating allows food to brown without sticking to the pan. Non-stick is often used to ref ...
s, lubricant
A lubricant (sometimes shortened to lube) is a substance that helps to reduce friction between surfaces in mutual contact, which ultimately reduces the heat generated when the surfaces move. It may also have the function of transmitting forces, ...
s.
* Car parts: tire
A tire (North American English) or tyre (Commonwealth English) is a ring-shaped component that surrounds a Rim (wheel), wheel's rim to transfer a vehicle's load from the axle through the wheel to the ground and to provide Traction (engineeri ...
s, bumpers, windshield
The windshield (American English and Canadian English) or windscreen (Commonwealth English) of an aircraft, car, bus, motorbike, truck, train, boat or streetcar is the front window, which provides visibility while protecting occupants from t ...
s, windscreen wiper
A windscreen wiper (Commonwealth English) or windshield wiper (American English) is a device used to remove rain, snow, ice, washer fluid, water, or other debris from a windscreen, vehicle's front window. Almost all motor vehicles, including ...
s, fuel tank
A fuel tank (also called a petrol tank or gas tank) is a safe container for Flammability, flammable fluids, often gasoline or diesel fuel. Though any storage tank for fuel may be so called, the term is typically applied to part of an engine sys ...
s, car seat
A car seat is a seat used in automobiles. Most car seats are made from inexpensive but durable material in order to withstand prolonged use. The most common material is polyester.
Bucket seat and bench seat
A bucket seat is a separate seat ...
s.
* Household items: bucket
A bucket is typically a watertight, vertical Cylinder (geometry), cylinder or Truncation (geometry), truncated Cone (geometry), cone or square, with an open top and a flat bottom that is attached to a semicircular carrying handle (grip), handle ...
s, kitchenware
:'' For a record label, see Kitchenware Records''
Kitchenware refers to the tools, utensils, appliances, dishes, and cookware used in food preparation and the serving of food. Kitchenware can also be used to hold or store food before or aft ...
, toys (e.g., construction set
A construction set is a standardized piece assortment allowing for the construction of various different models. Construction sets are most often marketed as toys. Popular construction toy brands include Lincoln Logs and ''LEGO''.
Toys
...
s and Rubik's cube).
* Medical applications: blood bag, syringe
A syringe is a simple reciprocating pump consisting of a plunger (though in modern syringes, it is actually a piston) that fits tightly within a cylindrical tube called a barrel. The plunger can be linearly pulled and pushed along the inside ...
s, rubber glove
A rubber glove is a glove made out of natural or synthetic rubber. 'Rubber' refers to durable, waterproof, and elastic material made from natural or synthetic latex. Rubber gloves can be unsupported (rubber only) or supported (rubber coating of ...
s, surgical suture
A surgical suture, also known as a stitch or stitches, is a medical device used to hold Tissue (biology), body tissues together and approximate wound edges after an injury or surgery. Application generally involves using a Sewing needle, needle w ...
, contact lens
Contact lenses, or simply contacts, are thin lenses placed directly on the surface of the eyes. Contact lenses are ocular prosthetic devices used by over 150 million people worldwide, and they can be worn to correct vision or for cosmetic ...
es, prosthesis
In medicine, a prosthesis (: prostheses; from ), or a prosthetic implant, is an artificial device that replaces a missing body part, which may be lost through physical trauma, disease, or a condition present at birth (Congenital, congenital disord ...
, controlled drug delivery and release, matrices for cell growth.
* Personal hygiene and healthcare: diaper
A diaper (, North American English) or a nappy (British English, Australian English, Hiberno-English) is a type of underwear that allows the wearer to urinate or defecate without using a toilet, by absorbing or containing waste products to p ...
s using superabsorbent polymer
A superabsorbent polymer (SAP) (also called slush powder) is a water-absorbing hydrophilic homopolymers or copolymers that can absorb and retain extremely large amounts of a liquid relative to its own mass.
Water-absorbing polymers, which are cl ...
s, toothbrush
A toothbrush is a special type of brush used to clean the Human tooth, teeth, gingiva, gums, and tongue. It consists of a head of tightly clustered bristles, atop of which toothpaste can be applied, mounted on a handle (grip), handle which facil ...
es, cosmetics
Cosmetics are substances that are intended for application to the body for cleansing, beautifying, promoting attractiveness, or altering appearance. They are mixtures of chemical compounds derived from either Natural product, natural source ...
, shampoo
Shampoo () is a hair care product, typically in the form of a viscous liquid, that is formulated to be used for cleaning (scalp) hair. Less commonly, it is available in solid bar format. (" Dry shampoo" is a separate product.) Shampoo is use ...
, condom
A condom is a sheath-shaped Barrier contraception, barrier device used during sexual intercourse to reduce the probability of pregnancy or a Sexually transmitted disease, sexually transmitted infection (STI). There are both external condo ...
s.
* Security: personal protective equipment
Personal protective equipment (PPE) is protective clothing, helmets, goggles, or other garments or equipment designed to protect the wearer's body from injury or infection. The hazards addressed by protective equipment include physical, elect ...
, bulletproof vest
A bulletproof vest, also known as a ballistic vest or bullet-resistant vest, is a type of body armor designed to absorb impact and prevent the penetration of firearm projectiles and explosion fragments to the torso. The vest can be either soft ...
s, space suit
A space suit (or spacesuit) is an environmental suit used for protection from the harsh environment of outer space, mainly from its vacuum as a highly specialized pressure suit, but also its temperature extremes, as well as radiation and ...
s, rope
A rope is a group of yarns, Plying, plies, fibres, or strands that are plying, twisted or braided together into a larger and stronger form. Ropes have high tensile strength and can be used for dragging and lifting. Rope is thicker and stronger ...
s.
* Separation technologies: synthetic membrane
Synthetic may refer to:
Science
* Synthetic biology
* Synthetic chemical or compound, produced by the process of chemical synthesis
* Synthetic elements, chemical elements that are not naturally found on Earth and therefore have to be created in ...
s, fuel cell membranes, filtration
Filtration is a physical separation process that separates solid matter and fluid from a mixture using a ''filter medium'' that has a complex structure through which only the fluid can pass. Solid particles that cannot pass through the filte ...
, ion-exchange resin
An ion-exchange resin or ion-exchange polymer is a resin or polymer that acts as a medium for ion exchange, that is also known as an ionex. It is an insoluble matrix (or support structure) normally in the form of small (0.25–1.43 mm radiu ...
s.
* Money: polymer banknote
Polymer banknotes are banknotes made from a synthetic polymer such as biaxially oriented polypropylene (BOPP). Such notes incorporate many security features not available in paper banknotes, including the use of metameric inks. Polymer banknote ...
s and payment card
Payment cards are part of a payment system issued by financial institutions, such as a bank, to a customer that enables its owner (the cardholder) to access the funds in the customer's designated bank accounts, or through a credit account and ...
s.
* 3D printing
3D printing, or additive manufacturing, is the construction of a three-dimensional object from a CAD model or a digital 3D model. It can be done in a variety of processes in which material is deposited, joined or solidified under computer ...
.
Standardized nomenclature
There are multiple conventions for naming polymer substances. Many commonly used polymers, such as those found in consumer products, are referred to by a common or trivial name. The trivial name is assigned based on historical precedent or popular usage rather than a standardized naming convention. Both theAmerican Chemical Society
The American Chemical Society (ACS) is a scientific society based in the United States that supports scientific inquiry in the field of chemistry. Founded in 1876 at New York University, the ACS currently has more than 155,000 members at all ...
(ACS) and IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
have proposed standardized naming conventions; the ACS and IUPAC conventions are similar but not identical. Examples of the differences between the various naming conventions are given in the table below:
In both standardized conventions, the polymers' names are intended to reflect the monomer(s) from which they are synthesized (source based nomenclature) rather than the precise nature of the repeating subunit. For example, the polymer synthesized from the simple alkene ethene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon double bonds).
Ethy ...
is called polyethene, retaining the ''-ene'' suffix even though the double bond is removed during the polymerization process:
:→
However, IUPAC structure based nomenclature is based on naming of the preferred constitutional repeating unit.
IUPAC has also issued guidelines for abbreviating new polymer names. 138 common polymer abbreviations are also standardized in the standard ISO
The International Organization for Standardization (ISO ; ; ) is an independent, non-governmental, international standard development organization composed of representatives from the national standards organizations of member countries.
Me ...
1043-1.
Characterization
Polymer characterization spans many techniques for determining the chemical composition, molecular weight distribution, and physical properties. Select common techniques include the following: *Size-exclusion chromatography
Size-exclusion chromatography, also known as molecular sieve chromatography, is a chromatography, chromatographic method in which molecules in Solution (chemistry), solution are separated by their Chemical structure, shape, and in some cases molec ...
(also called gel permeation chromatography
Gel permeation chromatography (GPC) is a type of size-exclusion chromatography (SEC), that separates high molecular weight or colloidal analytes on the basis of size or diameter, typically in organic solvents. The technique is often used for the an ...
), sometimes coupled with static light scattering
Static light scattering is a technique in physical chemistry that measures the intensity of the scattered light to obtain the average molecular weight ''Mw'' of a macromolecule like a polymer or a protein in solution. Measurement of the scattering ...
, can used to determine the number-average molecular weight, weight-average molecular weight, and dispersity
In chemistry, the dispersity is a measure of the heterogeneity of sizes of molecules or particles in a mixture. A collection of objects is called uniform if the objects have the same size, shape, or mass. A sample of objects that have an inconsi ...
.
*Scattering techniques, such as static light scattering
Static light scattering is a technique in physical chemistry that measures the intensity of the scattered light to obtain the average molecular weight ''Mw'' of a macromolecule like a polymer or a protein in solution. Measurement of the scattering ...
and small-angle neutron scattering
Small-angle neutron scattering (SANS) is an experimental technique that uses elastic neutron scattering at small scattering angles to investigate the structure of various substances at a mesoscopic scale of about 1–100 nm.
Small angle ...
, are used to determine the dimensions (radius of gyration
The radius of gyration or gyradius of a body about the axis of rotation is defined as the radial distance to a point which would have a moment of inertia the same as the body's actual distribution of mass, if the total mass of the body were concent ...
) of macromolecules in solution or in the melt. These techniques are also used to characterize the three-dimensional structure of microphase-separated block polymers, polymeric micelles, and other materials.
* Wide-angle X-ray scattering (also called wide-angle X-ray diffraction) is used to determine the crystalline structure of polymers (or lack thereof).
*Spectroscopy techniques, including Fourier-transform infrared spectroscopy
Fourier transform infrared spectroscopy (FTIR) is a technique used to obtain an infrared spectrum of absorption or emission of a solid, liquid, or gas. An FTIR spectrometer simultaneously collects high-resolution spectral data over a wide spectr ...
, Raman spectroscopy
Raman spectroscopy () (named after physicist C. V. Raman) is a Spectroscopy, spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Ra ...
, and nuclear magnetic resonance spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a Spectroscopy, spectroscopic technique based on re-orientation of Atomic nucleus, atomic nuclei with non-zero nuclear sp ...
, can be used to determine the chemical composition.
*Differential scanning calorimetry
Differential scanning calorimetry (DSC) is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and re ...
is used to characterize the thermal properties of polymers, such as the glass-transition temperature, crystallization temperature, and melting temperature. The glass-transition temperature can also be determined by dynamic mechanical analysis
Dynamic mechanical analysis (abbreviated DMA) is a technique used to study and characterize materials. It is most useful for studying the viscoelastic behavior of polymers. A sinusoidal stress is applied and the strain in the material is measured, ...
.
* Thermogravimetry is a useful technique to evaluate the thermal stability of the polymer.
*Rheology
Rheology (; ) is the study of the flow of matter, primarily in a fluid (liquid or gas) state but also as "soft solids" or solids under conditions in which they respond with plastic flow rather than deforming elastically in response to an applie ...
is used to characterize the flow and deformation behavior. It can be used to determine the viscosity
Viscosity is a measure of a fluid's rate-dependent drag (physics), resistance to a change in shape or to movement of its neighboring portions relative to one another. For liquids, it corresponds to the informal concept of ''thickness''; for e ...
, modulus, and other rheological properties. Rheology is also often used to determine the molecular architecture (molecular weight, molecular weight distribution, branching) and to understand how the polymer can be processed.
Degradation
Polymer degradation is a change in the properties—tensile strength,color
Color (or colour in English in the Commonwealth of Nations, Commonwealth English; American and British English spelling differences#-our, -or, see spelling differences) is the visual perception based on the electromagnetic spectrum. Though co ...
, shape, or molecular weight—of a polymer or polymer-based product under the influence of one or more environmental factors, such as heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
, light
Light, visible light, or visible radiation is electromagnetic radiation that can be visual perception, perceived by the human eye. Visible light spans the visible spectrum and is usually defined as having wavelengths in the range of 400– ...
, and the presence of certain chemical
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be combin ...
s, oxygen, and enzymes
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as pro ...
. This change in properties is often the result of bond breaking in the polymer backbone (chain scission
Chain scission is a term used in polymer chemistry describing the degradation of a polymer main chain. It is often caused by thermal stress (heat) or ionizing radiation (e.g. light, UV radiation or gamma radiation), often involving oxygen. Durin ...
) which may occur at the chain ends or at random positions in the chain.
Although such changes are frequently undesirable, in some cases, such as biodegradation
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegrada ...
and recycling
Recycling is the process of converting waste materials into new materials and objects. This concept often includes the recovery of energy from waste materials. The recyclability of a material depends on its ability to reacquire the propert ...
, they may be intended to prevent environmental pollution
Pollution is the introduction of contaminants into the natural environment that cause harm. Pollution can take the form of any substance (solid, liquid, or gas) or energy (such as radioactivity, heat, sound, or light). Pollutants, the component ...
. Degradation can also be useful in biomedical settings. For example, a copolymer of polylactic acid
Polylactic acid, also known as poly(lactic acid) or polylactide (PLA), is a plastic material. As a thermoplastic polyester (or polyhydroxyalkanoate) it has the backbone formula or . PLA is formally obtained by condensation of lactic acid with ...
and polyglycolic acid is employed in hydrolysable stitches that slowly degrade after they are applied to a wound.
The susceptibility of a polymer to degradation depends on its structure. Epoxies and chains containing aromatic functionalities are especially susceptible to UV degradation
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of th ...
while polyesters are susceptible to degradation by hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
. Polymers containing an unsaturated backbone degrade via ozone cracking
Cracks can be formed in many different elastomers by ozone attack, and the characteristic form of attack of vulnerable rubbers is known as ozone cracking. The problem was formerly very common, especially in tires, but is now rarely seen in those ...
. Carbon based polymers are more susceptible to thermal degradation than inorganic polymer
In polymer chemistry, an inorganic polymer is a polymer with a skeletal structure that does not include carbon atoms in the Polymer backbone, backbone. Polymers containing Inorganic compound, inorganic and Organic compound, organic components are ...
s such as polydimethylsiloxane
Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, is a silicone polymer with a wide variety of uses, from cosmetics to industrial lubrication and passive daytime radiative cooling.
PDMS is particularly known for its ...
and are therefore not ideal for most high-temperature applications.
The degradation of polyethylene occurs by random scission—a random breakage of the bonds that hold the atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s of the polymer together. When heated above 450 °C, polyethylene degrades to form a mixture of hydrocarbons. In the case of chain-end scission, monomers are released and this process is referred to as unzipping or depolymerization Depolymerization (or depolymerisation) is the process of converting a polymer into a monomer or a mixture of monomers. This process is driven by an increase in entropy.
Ceiling temperature
The tendency of polymers to depolymerize is indicated by ...
. Which mechanism dominates will depend on the type of polymer and temperature; in general, polymers with no or a single small substituent in the repeat unit will decompose via random-chain scission.
The sorting of polymer waste for recycling purposes may be facilitated by the use of the resin identification code
The Resin Identification Code (RIC) is a technical standard with a set of symbols appearing on plastic products that identify the Synthetic resin, plastic resin out of which the product is made. It was developed in 1988 by the Society of the P ...
s developed by the Society of the Plastics Industry
Founded in 1937, the Society of the Plastics Industry, Inc. was a professional society representing individuals in the plastics industry. In 2010, the organization began doing business as SPI: The Plastics Industry Trade Association, before changi ...
to identify the type of plastic.
Product failure
Failure ofsafety-critical
A safety-critical system or life-critical system is a system whose failure or malfunction may result in one (or more) of the following outcomes:
* death or serious injury to people
* loss or severe damage to equipment/property
* environmental h ...
polymer components can cause serious accidents, such as fire in the case of cracked and degraded polymer fuel line
A fuel line is a hose or pipe used to transfer fuel from one point in a vehicle to another. The United States Environmental Protection Agency defines a fuel line as "all hoses or tubing designed to contain liquid fuel or fuel vapor. This incl ...
s. Chlorine-induced cracking of acetal resin plumbing joints and polybutylene
Polybutylene (polybutene-1, poly(1-butene), PB-1) is a polyolefin or saturated polymer with the chemical formula (CH2CH(Et))n. Not be confused with polybutene, PB-1 is mainly used in piping.
Production
Polybutylene is produced by polymerisation ...
pipes has caused many serious floods in domestic properties, especially in the US in the 1990s. Traces of chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
in the water supply attacked polymers present in the plumbing, a problem which occurs faster if any of the parts have been poorly extruded
Extrusion is a process used to create objects of a fixed cross-sectional profile by pushing material through a die of the desired cross-section. Its two main advantages over other manufacturing processes are its ability to create very complex ...
or injection molded
Injection or injected may refer to:
Science and technology
* Injective function, a mathematical function mapping distinct arguments to distinct values
* Injection (medicine), insertion of liquid into the body with a syringe
* Injection, in broadca ...
. Attack of the acetal joint occurred because of faulty molding, leading to cracking along the threads of the fitting where there is stress concentration
In solid mechanics, a stress concentration (also called a stress raiser or a stress riser or notch sensitivity) is a location in an object where the stress (mechanics), stress is significantly greater than the surrounding region. Stress concentra ...
.
Polymer oxidation has caused accidents involving medical device
A medical device is any device intended to be used for medical purposes. Significant potential for hazards are inherent when using a device for medical purposes and thus medical devices must be proved safe and effective with reasonable assura ...
s. One of the oldest known failure modes is ozone cracking caused by chain scission when ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
gas attacks susceptible elastomer
An elastomer is a polymer with viscoelasticity (i.e. both viscosity and elasticity) and with weak intermolecular forces, generally low Young's modulus (E) and high failure strain compared with other materials. The term, a portmanteau of ''ela ...
s, such as natural rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds.
Types of polyisoprene ...
and nitrile rubber
Nitrile rubber, also known as nitrile butadiene rubber, NBR, Buna-N, and acrylonitrile butadiene rubber, is a synthetic rubber derived from acrylonitrile (ACN) and butadiene. Trade names include Perbunan, Nipol, Krynac and Europrene. This rubber is ...
. They possess double bonds in their repeat units which are cleaved during ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the Saturated and unsaturated compounds, unsaturated bonds are Bond cleavage, cleaved with ozone (). Multiple carbon–carbon bond are replaced by carbonyl () groups, such as aldehydes ...
. Cracks in fuel lines can penetrate the bore of the tube and cause fuel leakage. If cracking occurs in the engine compartment, electric sparks can ignite the gasoline
Gasoline ( North American English) or petrol ( Commonwealth English) is a petrochemical product characterized as a transparent, yellowish, and flammable liquid normally used as a fuel for spark-ignited internal combustion engines. When for ...
and can cause a serious fire. In medical use degradation of polymers can lead to changes of physical and chemical characteristics of implantable devices.
Nylon 66
Nylon 66 (loosely written nylon 6-6, nylon 6/6, nylon 6,6, or nylon 6:6) is a type of polyamide or nylon. It, and nylon 6, are the two most common for textile and plastic industries. Nylon 66 is made of two monomers each containing six carbon at ...
is susceptible to acid hydrolysis, and in one accident, a fractured fuel line led to a spillage of diesel into the road. If diesel fuel
Diesel fuel, also called diesel oil, heavy oil (historically) or simply diesel, is any liquid fuel specifically designed for use in a diesel engine, a type of internal combustion engine in which fuel ignition takes place without a spark as a re ...
leaks onto the road, accidents to following cars can be caused by the slippery nature of the deposit, which is like black ice
Black ice, sometimes called clear ice, is a coating of glaze ice on a surface, for example on streets or on lakes. The ice itself is not black, but visually transparent, allowing the often black road below to be seen through it and light to be ...
. Furthermore, the asphalt concrete
Asphalt concrete (commonly called asphalt, blacktop, or pavement in North America, and Tarmacadam, tarmac or bitumen macadam in the United Kingdom and the Republic of Ireland) is a composite material commonly used to surface road surface, roads ...
road surface will suffer damage as a result of the diesel fuel dissolving the asphaltene
Asphaltenes are molecular substances that are found in crude oil, along with resins, aromatic hydrocarbons, and saturates (i.e. saturated hydrocarbons such as alkanes). The word "asphaltene" was coined by Jean-Baptiste Boussingault in 1837 whe ...
s from the composite material, this resulting in the degradation of the asphalt surface and structural integrity of the road.
History
Polymers have been essential components of commodities since the early days of humankind. The use ofwool
Wool is the textile fiber obtained from sheep and other mammals, especially goats, rabbits, and camelids. The term may also refer to inorganic materials, such as mineral wool and glass wool, that have some properties similar to animal w ...
(keratin
Keratin () is one of a family of structural fibrous proteins also known as ''scleroproteins''. It is the key structural material making up Scale (anatomy), scales, hair, Nail (anatomy), nails, feathers, horn (anatomy), horns, claws, Hoof, hoove ...
), cotton
Cotton (), first recorded in ancient India, is a soft, fluffy staple fiber that grows in a boll, or protective case, around the seeds of the cotton plants of the genus '' Gossypium'' in the mallow family Malvaceae. The fiber is almost pure ...
and linen
Linen () is a textile made from the fibers of the flax plant.
Linen is very strong and absorbent, and it dries faster than cotton. Because of these properties, linen is comfortable to wear in hot weather and is valued for use in garments. Lin ...
fibres (cellulose
Cellulose is an organic compound with the chemical formula, formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of glycosidic bond, β(1→4) linked glucose, D-glucose units. Cellulose is an important s ...
) for garments, paper reed (cellulose
Cellulose is an organic compound with the chemical formula, formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of glycosidic bond, β(1→4) linked glucose, D-glucose units. Cellulose is an important s ...
) for paper
Paper is a thin sheet material produced by mechanically or chemically processing cellulose fibres derived from wood, Textile, rags, poaceae, grasses, Feces#Other uses, herbivore dung, or other vegetable sources in water. Once the water is dra ...
are just a few examples of how ancient societies exploited polymer-containing raw materials to obtain artefacts. The latex sap of "caoutchouc" trees (natural rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds.
Types of polyisoprene ...
) reached Europe in the 16th century from South America long after the Olmec
The Olmecs () or Olmec were an early known major Mesoamerican civilization, flourishing in the modern-day Mexican states of Veracruz and Tabasco from roughly 1200 to 400 Before the Common Era, BCE during Mesoamerica's Mesoamerican chronolog ...
, Maya
Maya may refer to:
Ethnic groups
* Maya peoples, of southern Mexico and northern Central America
** Maya civilization, the historical civilization of the Maya peoples
** Mayan languages, the languages of the Maya peoples
* Maya (East Africa), a p ...
and Aztec
The Aztecs ( ) were a Mesoamerican civilization that flourished in central Mexico in the Post-Classic stage, post-classic period from 1300 to 1521. The Aztec people included different Indigenous peoples of Mexico, ethnic groups of central ...
had started using it as a material to make balls, waterproof textiles and containers.
The chemical manipulation of polymers dates back to the 19th century, although at the time the nature of these species was not understood. The behaviour of polymers was initially rationalised according to the theory proposed by Thomas Graham Thomas Graham may refer to:
Politicians and diplomats
*Thomas Graham, 1st Baron Lynedoch (1748–1843), British politician and soldier
* Thomas Graham Jr. (diplomat) (born 1933), nuclear expert and senior U.S. diplomat
*Sir Thomas Graham (barriste ...
which considered them as colloidal aggregates of small molecules held together by unknown forces.
Notwithstanding the lack of theoretical knowledge, the potential of polymers to provide innovative, accessible and cheap materials was immediately grasped. The work carried out by Braconnot, Parkes Parkes may refer to:
* Sir Henry Parkes (1815–1896), Australian politician, one of the earliest and most prominent advocates for Australian federation
Named for Henry Parkes
* Parkes, New South Wales, a regional town
* Parkes Observatory, a radi ...
, Ludersdorf, Hayward and many others on the modification of natural polymers determined many significant advances in the field. Their contributions led to the discovery of materials such as celluloid
Celluloids are a class of materials produced by mixing nitrocellulose and camphor, often with added dyes and other agents. Once much more common for its use as photographic film before the advent of safer methods, celluloid's common present-day ...
, galalith
Galalith (Erinoid in the United Kingdom) is a synthetic plastic material manufactured by the interaction of casein and formaldehyde. The commercial name is derived from the Ancient Greek words (, "milk") and (, "stone"). It is odourless, ...
, parkesine
Celluloids are a class of materials produced by mixing nitrocellulose and camphor, often with added dyes and other agents. Once much more common for its use as photographic film before the advent of safer methods, celluloid's common present-day ...
, rayon
Rayon, also called viscose and commercialised in some countries as sabra silk or cactus silk, is a semi-synthetic fiber made from natural sources of regenerated cellulose fiber, cellulose, such as wood and related agricultural products. It has t ...
, vulcanised rubber and, later, Bakelite
Bakelite ( ), formally , is a thermosetting polymer, thermosetting phenol formaldehyde resin, formed from a condensation reaction of phenol with formaldehyde. The first plastic made from synthetic components, it was developed by Belgian chemist ...
: all materials that quickly entered industrial manufacturing processes and reached households as garments components (''e.g.'', fabrics, buttons), crockery and decorative items.
In 1920, Hermann Staudinger
Hermann Staudinger (; 23 March 1881 – 8 September 1965) was a German organic chemist who demonstrated the existence of macromolecules, which he characterized as polymers. For this work he received the 1953 Nobel Prize in Chemistry.
He is also ...
published his seminal work "Über Polymerisation", in which he proposed that polymers were in fact long chains of atoms linked by covalent bonds. His work was debated at length, but eventually it was accepted by the scientific community. Because of this work, Staudinger was awarded the Nobel Prize in 1953.
After the 1930s polymers entered a golden age during which new types were discovered and quickly given commercial applications, replacing naturally-sourced materials. This development was fuelled by an industrial sector with a strong economic drive and it was supported by a broad academic community that contributed innovative syntheses of monomers from cheaper raw material, more efficient polymerisation processes, improved techniques for polymer characterisation and advanced, theoretical understanding of polymers.
Since 1953, six Nobel prizes have been awarded in the area of polymer science, excluding those for research on biological macromolecules. This further testifies to its impact on modern science and technology. As Lord Todd summarised in 1980, "I am inclined to think that the development of polymerization is perhaps the biggest thing that chemistry has done, where it has had the biggest effect on everyday life".
See also
*Ideal chain
An ideal chain (or freely-jointed chain) is the simplest model in polymer chemistry to describe polymers, such as nucleic acids and proteins. It assumes that the monomers in a polymer are located at the steps of a hypothetical random walker that ...
*Catenation
In chemistry, catenation is the chemical bond, bonding of atoms of the same Chemical element, element into a series, called a ''chain''. A chain or a Ring (chemistry), ring may be ''open'' if its ends are not bonded to each other (an open-chain c ...
*Inorganic polymer
In polymer chemistry, an inorganic polymer is a polymer with a skeletal structure that does not include carbon atoms in the Polymer backbone, backbone. Polymers containing Inorganic compound, inorganic and Organic compound, organic components are ...
* Important publications in polymer chemistry
*Oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
*Polymer adsorption Adsorption is the adhesion of ions or molecules onto the surface of another phase. Adsorption may occur via physisorption and chemisorption. Ions and molecules can adsorb to many types of surfaces including polymer surfaces. A polymer is a large mol ...
* Polymer classes
*Polymer engineering
Polymer engineering is generally an engineering field that designs, analyses, and modifies polymer materials. Polymer engineering covers aspects of the petrochemical industry, polymerization, structure and characterization of polymers, properties o ...
*Polymery (botany) Merosity (from the greek "méros," which means "having parts")) refers to the number of component parts in a distinct whorl of a plant structure. The term is most commonly used in the context of a flower where it refers to the number of sepals in a ...
* Reactive compatibilization
* Sequence-controlled polymer
* Shape-memory polymer
* Sol–gel process
*Supramolecular polymer
Supramolecular polymers are a subset of polymers where the monomeric units are connected by reversible and highly directional secondary interactions–that is, non-covalent bonds. These non-covalent interactions include van der Waals interactio ...
*Thermoplastic
A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
Most thermoplastics have a high molecular weight. The polymer chains as ...
*Thermosetting polymer
In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening (" curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and ...
References
Bibliography
* * * *External links
Libretext in Polymer chemistry
How to Analyze Polymers Using X-ray Diffraction
{{Portal bar, Science, Chemistry, Numismatics, Electronics, Algae, Money Polymer chemistry Soft matter Materials science