|
Living Polymerization
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is also much larger than the rate of chain propagation. The result is that the polymer chains grow at a more constant rate than seen in traditional chain polymerization and their lengths remain very similar (i.e. they have a very low polydispersity index). Living polymerization is a popular method for synthesizing block copolymers since the polymer can be synthesized in stages, each stage containing a different monomer. Additional advantages are predetermined molar mass and control over end-groups. Living polymerization is desirable because it offers precision and control in macromolecular synthesis. This is important since many of the novel/useful properties of polymers res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer Chemistry
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are also applicable through a wide range of other chemistry sub-disciplines like organic chemistry, analytical chemistry, and physical chemistry. Many materials have polymeric structures, from fully inorganic metals and ceramics to DNA and other biological molecules. However, polymer chemistry is typically related to synthetic and organic compositions. Synthetic polymers are ubiquitous in commercial materials and products in everyday use, such as plastics, and rubbers, and are major components of composite materials. Polymer chemistry can also be included in the broader fields of polymer science or even nanotechnology, both of which can be described as encompassing polymer physics and polymer engineering.Hans-Heinrich Moretto, Manfred Schulz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Weight
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dianion
A dianion is an anion with a net charge of −2. While there exist many stable molecular dianions, such as and , thus far no stable atomic dianion has been found: Electron shielding and other quantum mechanical effects tend to make the addition of another electron to an atomic anion unstable. The most heavily studied atomic dianion is H, usually as a short-lived resonance between an electron and a hydrogen ion. In 1976, its half-life was experimentally measured to be 23 ± 4 nanoseconds. In the field of physiology Physiology (; ) is the science, scientific study of function (biology), functions and mechanism (biology), mechanisms in a life, living system. As a branches of science, subdiscipline of biology, physiology focuses on how organisms, organ syst ..., molecular dianions play an important roles, such as the monohydrogen phosphate (), present at a concentration of around 1 mM in the blood and in cells, where it plays a role in pH buffering. See also * Dication ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical Anion
In organic chemistry, a radical anion is a free radical species that carries a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a non-carbon radical anion is the superoxide anion, formed by transfer of one electron to an oxygen molecule. Radical anions are typically indicated by M^. Polycyclic radical anions Many aromatic compounds can undergo one-electron reduction by alkali metals. The electron is transferred from the alkali metal ion to an unoccupied antibonding p-p п* orbital of the aromatic molecule. This transfer is usually only energetically favorable if the aprotic solvent efficiently solvates the alkali metal ion. Effective solvents are those that bind to the alkali metal cation: diethyl ether < THF < [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Transfer
Electron transfer (ET) occurs when an electron relocates from an atom, ion, or molecule, to another such chemical entity. ET describes the mechanism by which electrons are transferred in redox reactions. Electrochemical processes are ET reactions. ET reactions are relevant to photosynthesis and respiration and commonly involve transition metal complexes. In organic chemistry ET is a step in some industrial polymerization reactions. It is foundational to photoredox catalysis. Classes of electron transfer Inner-sphere electron transfer In inner-sphere ET, two redox centers are covalently linked during the ET. This bridge can be permanent, in which case the electron transfer event is termed intramolecular electron transfer. More commonly, however, the covalent linkage is transitory, forming just prior to the ET and then disconnecting following the ET event. In such cases, the electron transfer is termed intermolecular electron transfer. A famous example of an inner sphere ET pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent. It is an isomer of another solvent, butanone. Production About 200,000 tonnes of tetrahydrofuran are produced annually. The most widely used industrial process involves the acid-catalyzed dehydration of 1,4-Butanediol, 1,4-butanediol. Ashland Inc., Ashland/ISP is one of the biggest producers of this chemical route. The method is similar to the production of diethyl ether from ethanol. The butanediol is derived from Condensation reaction, condensation of acetylene with formaldehyde followed by hydrogenation. DuPont developed a process for producing THF by oxidizing Butane#Isomers, ''n''-butane to crude maleic anhydride, follow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white Crystal, crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 Parts-per notation, ppm by mass. As an Aromaticity, aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is the main ingredient of traditional mothballs. History In the early 1820s, two separate reports described a white solid with a pungent odor derived from the distillation of coal tar. In 1821, John Kidd (chemist), John Kidd cited these two disclosures and then described many of this substance's properties and the means of its production. He proposed the name ''naphthaline'', as it had been derived from a kind of naphtha (a broad term encompassing any volatile, flammable liquid hydrocarbon mixture, including coal tar). Naphthalene's chemical formula was determined by Michael Faraday in 1826. The structure of two f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names for the elements in some languages, such as German and Russian. rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute Group (periodic table)#Group names, group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of periodic trends, group trends in properties in the periodic table, with elements exhibiting well-characterised Homologous series, homologous behaviour. This family of elements is also known as the lithium family after its leading element. The alkali metals are all shiny, hardness, sof ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Styrene
Styrene is an organic compound with the chemical formula C6H5CH=CH2. Its structure consists of a vinyl group as substituent on benzene. Styrene is a colorless, oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concentrations have a less pleasant odor. Styrene is the precursor to polystyrene and several copolymers, and is typically made from benzene for this purpose. Approximately 25 million tonnes of styrene were produced in 2010, increasing to around 35 million tonnes by 2018. Natural occurrence Styrene is named after storax balsam (often commercially sold as ''styrax''), the resin of Liquidambar trees of the Altingiaceae plant family. Styrene occurs naturally in small quantities in some plants and foods (cinnamon, coffee beans, balsam tree (other), balsam trees and peanuts) and is also found in coal tar. History In 1839, the German apothecary Eduard Simon isolated a volatile liquid from t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michael Szwarc
Michael Szwarc (9 June 1909, Będzin, Poland – 4 August 2000, San Diego, California) was a British and American polymer chemist who discovered and studied ionic living polymerization. Biography Michael Mojżesz Szwarc was born into a Polish-Jewish family in Będzin, Poland. In 1932 he received the title of engineer in chemistry at the Warsaw University of Technology. In 1935 he emigrated to Palestine, where he joined his sister and cousin. In 1942 he defended his first Ph.D. dissertation in organic chemistry at Hebrew University in Jerusalem. In 1945 he joined Michael Polanyi's research group at the University of Manchester in the UK. In 1947 he defended his second Ph.D. thesis, this time in physical chemistry. Two years later, he was awarded D.Sc. for work on measurements of energy distribution of chemical bonds, and was promoted to senior lecturer at the University of Manchester. In 1952 Michael Szwarc moved to the United States and was a professor of physical chemistr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
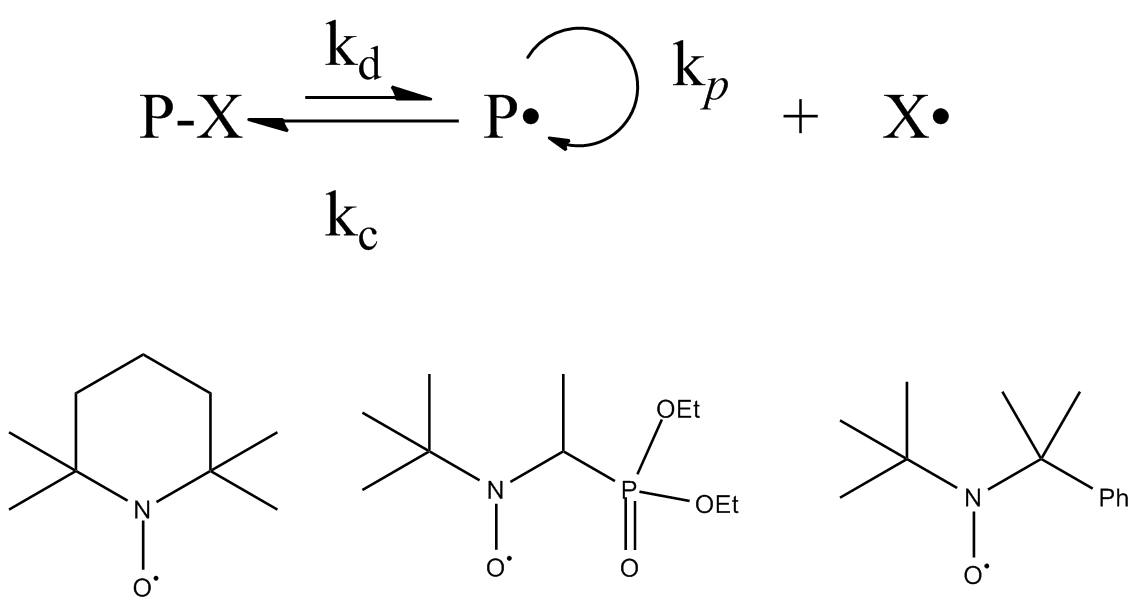
Living Free Radical Polymerization
Living free radical polymerization is a type of living polymerization where the active polymer chain end is a free radical. Several methods exist. IUPAC recommends to use the term "reversible-deactivation radical polymerization" instead of "living free radical polymerization", though the two terms are not synonymous. Reversible-deactivation polymerization There is a mode of polymerization referred to as reversible-deactivation polymerization which is distinct from living polymerization, despite some common features. Living polymerization requires a complete absence of termination reactions, whereas reversible-deactivation polymerization may contain a similar fraction of termination as conventional polymerization with the same concentration of active species. Some important aspects of these are compared in the table: Catalytic chain transfer and cobalt mediated radical polymerization Catalytic chain transfer polymerization is not a strictly living form of polymerization. Yet i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ring-opening Metathesis Polymerization
In polymer chemistry, ring-opening metathesis polymerization (ROMP) is a type of chain-growth polymerization involving olefin metathesis. The reaction is driven by relieving ring strain in cyclic olefins. A variety of heterogeneous and homogeneous catalysts have been developed for different polymers and mechanisms. Heterogeneous catalysts are typical in large-scale commercial processes, while homogeneous catalysts are used in finer laboratory chemical syntheses. Organometallic catalysts used in ROMP usually have transition metal centres, such as tungsten, ruthenium, titanium, etc., with organic ligands. Heterogeneous catalysis : Heterogeneous catalysis consists of catalysts and substrates in different physical states. The catalyst is typically in solid phase. The mechanism of heterogeneous ring-opening metathesis polymerization is still under investigation. Ring-opening metathesis polymerization of cyclic olefins has been commercialized since the 1970s. Examples of polymers pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |