|
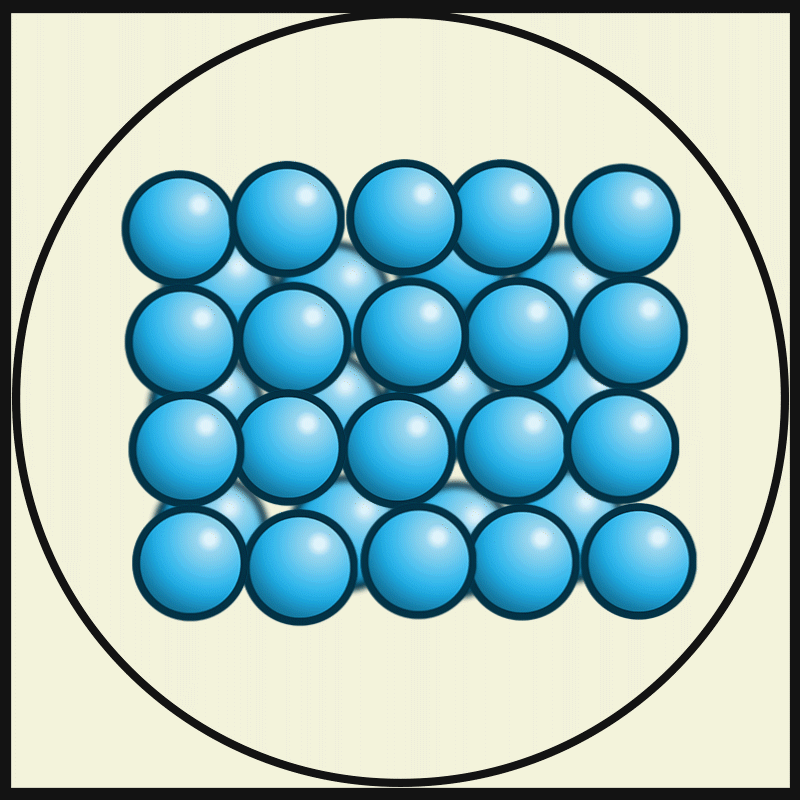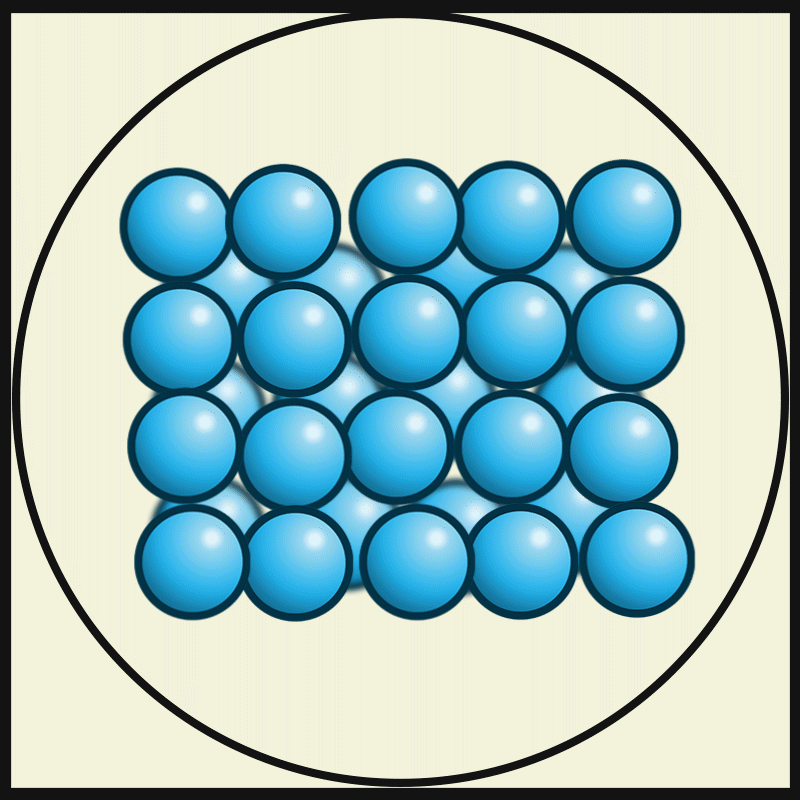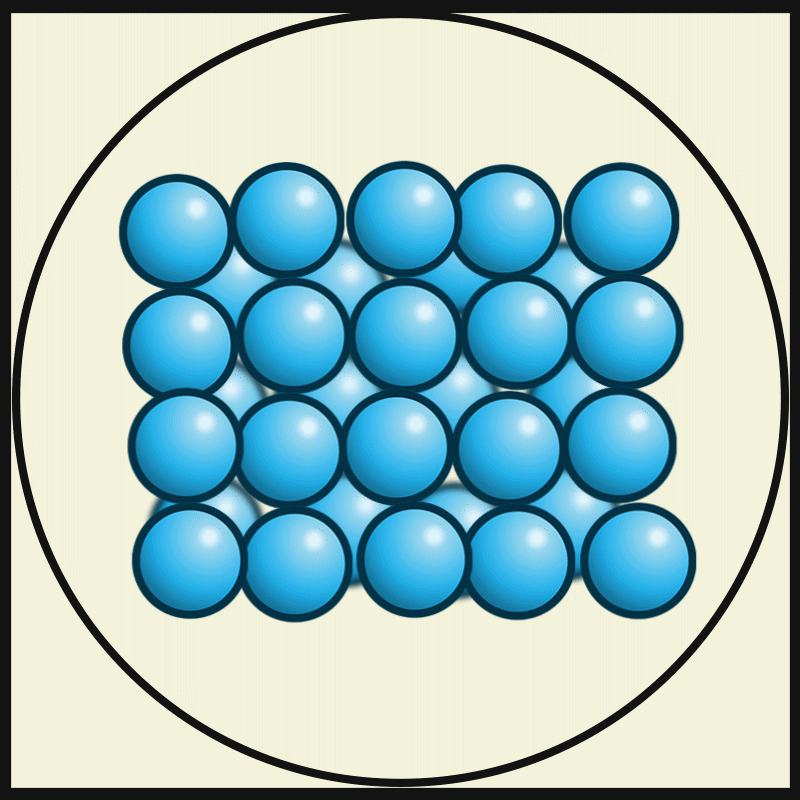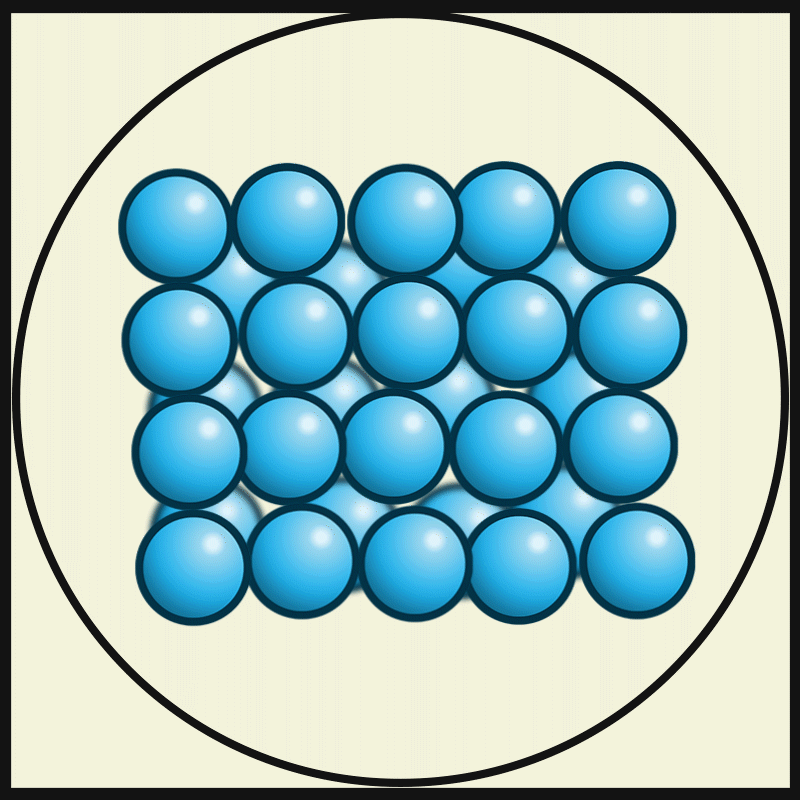
Solid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the specific material under consideration. Solids also always possess the least amount of kinetic energy per atom/molecule relative to other phases or, equivalently stated, solids are formed when matter in the liquid / gas phase is cooled below a certain temperature. This temperature is called the melting point of that substance and is an intrinsic property, i.e. independent of how much of the matter there is. All matter in solids can be arranged on a microscopic scale under certain conditions. Solids are characterized by structural rigidity and resistance to applied external forces and pressure. Unlike liquids, solids do not flow to take on the shape of their container, nor do they expand to fill the entire available volume like a gas. Much ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amorphous Solid
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymously with amorphous solid; however, these terms refer specifically to amorphous materials that undergo a glass transition. Examples of amorphous solids include glasses, metallic glasses, and certain types of plastics and polymers. Etymology The term "Amorphous" comes from the Greek language, Greek ''a'' ("without"), and ''morphé'' ("shape, form"). Structure Amorphous materials have an internal structure of molecular-scale structural blocks that can be similar to the basic structural units in the crystalline phase of the same compound. Unlike in crystalline materials, however, no long-range regularity exists: amorphous materials cannot be described by the repetition of a finite unit cell. Statistical measures, such as the atomic density ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
State Of Matter
In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and Plasma (physics), plasma. Different states are distinguished by the ways the component particles (atoms, molecules, ions and electrons) are arranged, and how they behave collectively. In a solid, the particles are tightly packed and held in fixed positions, giving the material a definite shape and volume. In a liquid, the particles remain close together but can move past one another, allowing the substance to maintain a fixed volume while adapting to the shape of its container. In a gas, the particles are far apart and move freely, allowing the substance to expand and fill both the shape and volume of its container. Plasma is similar to a gas, but it also contains charged particles (ions and free electrons) that move independently and respond to electric and magnetic fields. Beyond the classical states of matter, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
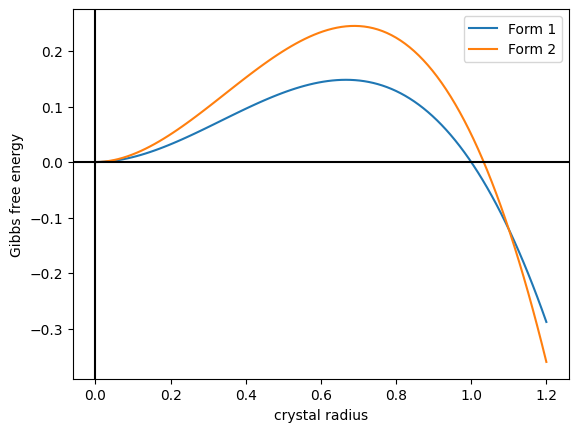
Crystal Polymorphism
In crystallography, polymorphism is the phenomenon where a compound or element can crystallize into more than one crystal structure. The preceding definition has evolved over many years and is still under discussion today. Discussion of the defining characteristics of polymorphism involves distinguishing among types of transitions and structural changes occurring in polymorphism versus those in other phenomena. Overview Phase transitions (phase changes) that help describe polymorphism include polymorphic transitions as well as melting and vaporization transitions. According to IUPAC, a polymorphic transition is "A reversible transition of a solid crystalline phase at a certain temperature and pressure (the inversion point) to another phase of the same chemical composition with a different crystal structure." Additionally, Walter McCrone described the phases in polymorphic matter as "different in crystal structure but identical in the liquid or vapor states." McCrone also defin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
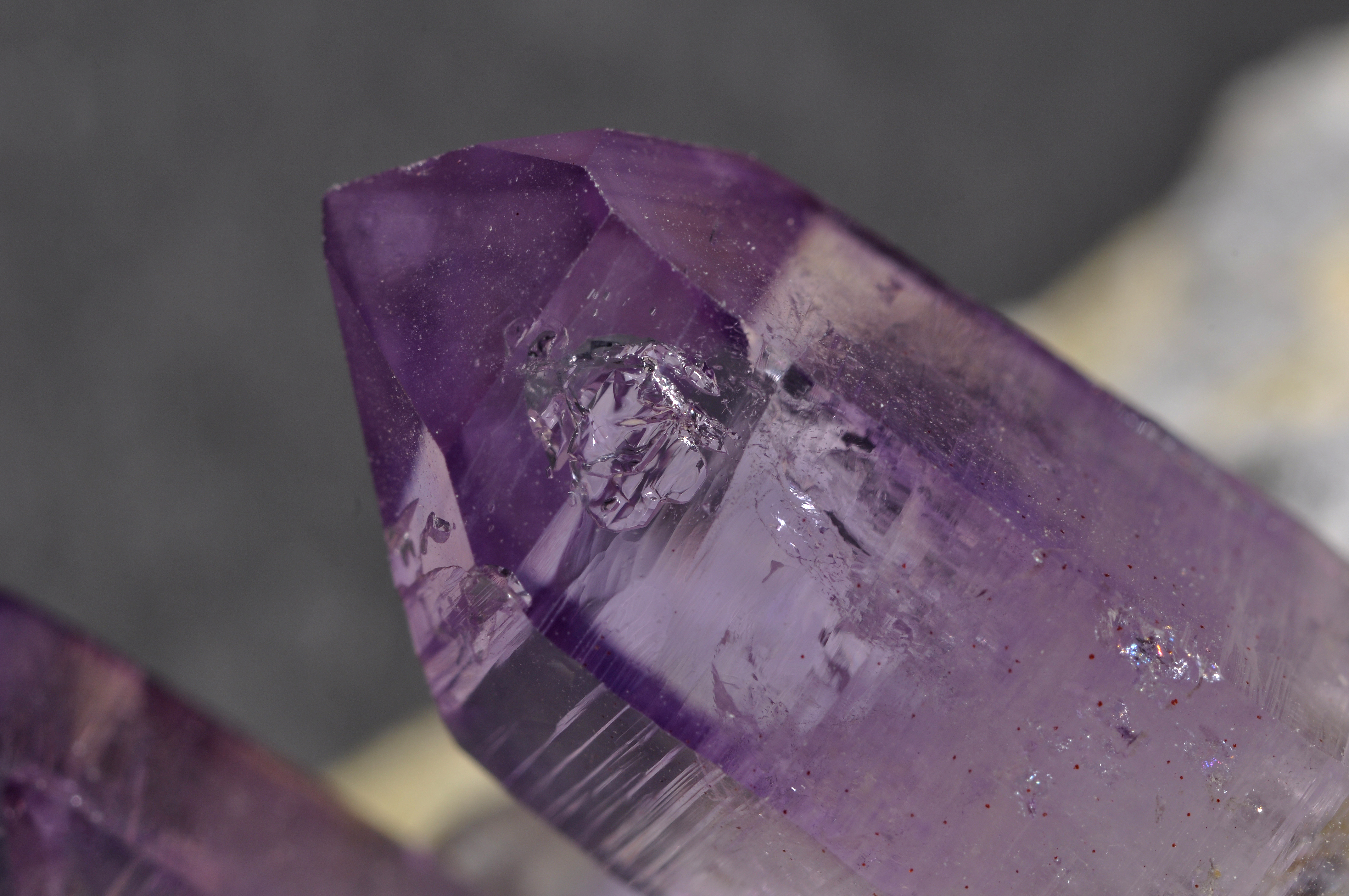
Crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both "ice" and " rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third cat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropy
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical State of matter, state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the atoms of the element are Chemical bond, bonded together in different manners. For example, the allotropes of carbon include diamond (the carbon atoms are bonded together to form a Cubic crystal system, cubic lattice of Tetrahedral molecular geometry, tetrahedra), graphite (the carbon atoms are bonded together in sheets of a hexagonal lattice), graphene (single sheets of graphite), and fullerenes (the carbon atoms are bonded together in spherical, tubular, or ellipsoidal formations). The term ''allotropy'' is used for elements only, not for Chemical compound, compounds. The more general term, used for any compound, is Polymorphism (materials science), polymorphism, although its use is usually restricted to solid materials ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sublimation (phase Transition)
Sublimation is the Phase transition, transition of a substance directly from the solid to the gas state, without passing through the liquid state. The verb form of sublimation is ''sublime'', or less preferably, ''sublimate''. ''Sublimate'' also refers to the product obtained by sublimation. The point at which sublimation occurs rapidly (for further details, see #False correspondence with vaporization, below) is called critical sublimation point, or simply sublimation point. Notable examples include sublimation of dry ice at room temperature and atmospheric pressure, and that of solid iodine with heating. The reverse process of sublimation is deposition (phase transition), ''deposition'' (also called ''desublimation''), in which a substance passes directly from a gas to a solid phase, without passing through the liquid state. Technically, all solids may sublime, though most sublime at extremely low rates that are hardly detectable under usual conditions. At standard condi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium-3
Helium-3 (3He see also helion) is a light, stable isotope of helium with two protons and one neutron. (In contrast, the most common isotope, helium-4, has two protons and two neutrons.) Helium-3 and hydrogen-1 are the only stable nuclides with more protons than neutrons. It was discovered in 1939. Helium-3 atoms are fermionic and become a superfluid at the temperature of 2.491 mK. Helium-3 occurs as a primordial nuclide, escaping from Earth's crust into its atmosphere and into outer space over millions of years. It is also thought to be a natural nucleogenic and cosmogenic nuclide, one produced when lithium is bombarded by natural neutrons, which can be released by spontaneous fission and by nuclear reactions with cosmic rays. Some found in the terrestrial atmosphere is a remnant of atmospheric and underwater nuclear weapons testing. Nuclear fusion using helium-3 has long been viewed as a desirable future energy source. The fusion of two of its atoms would be aneut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls. Materials containing much calcium carbonate or resembling it are described as calcareous. Calcium carbonate is the active ingredient in agricultural lime and is produced when calcium ions in hard water react with carbonate ions to form limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues. Chemistry Calcium carbonate shares the typical properties of other carbonates. Notably, it: *reacts with acids, releasing carbonic acid which quickly disintegrates into carbon dioxide and water: : *releases carbon dioxide upon heating, called a thermal decomposition reaction, or calcination (to above 840 °C in the case of ), t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melting Point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilibrium, equilibrium. The melting point of a substance depends on pressure and is usually specified at a Standard temperature and pressure, standard pressure such as 1 Atmosphere (unit), atmosphere or 100 Pascal (unit), kPa. When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization point. Because of the ability of substances to Supercooling, supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact, the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the #Melting point measurements, melting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of electricity, and insoluble in water. Another solid form of carbon known as graphite is the Chemical stability, chemically stable form of carbon at Standard temperature and pressure, room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest Scratch hardness, hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are boron and nitrogen). Small numbers of lattice defect, defects or impurities (about one per million of lattice atoms) can color ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, carbon-12, C and carbon-13, C being stable, while carbon-14, C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the timeline of chemical element discoveries#Pre-modern and early modern discoveries, few elements known since antiquity. Carbon is the 15th abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the abundance of the chemical elements, fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual abi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Diagram Of Water
This page provides supplementary data to the article properties of water. Further comprehensive authoritative data can be found at the ''NIST Chemistry WebBook'' page on thermophysical properties of fluids. Structure and properties Thermodynamic properties Liquid physical properties Water/steam equilibrium properties Vapor pressure formula for steam in equilibrium with liquid water: : \log_ P = A - \frac, where ''P'' is equilibrium vapor pressure in k Pa, and ''T'' is temperature in kelvins. For ''T'' = 273 K to 333 K: ''A'' = 7.2326; ''B'' = 1750.286; ''C'' = 38.1. For ''T'' = 333 K to 423 K: ''A'' = 7.0917; ''B'' = 1668.21; ''C'' = 45.1. Data in the table above is given for water–steam equilibria at various temperatures over the entire temperature range at which liquid water can exist. Pressure of the equilibrium is given in the second column in k Pa. The third column is the heat content of each gram of the liquid phase relative to water at 0 °C. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |