Ozone (), or trioxygen, is an inorganic
molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
with the
chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
. It is a pale blue gas with a distinctively pungent smell. It is an
allotrope
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the ...
of
oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
that is much less stable than the
diatomic
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear. Ot ...
allotrope
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the ...
, breaking down in the lower atmosphere to (
dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (O2), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (O3). Others are:
*A ...
). Ozone is formed from dioxygen by the action of
ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nanometer, nm (with a corresponding frequency around 30 Hertz, PHz) to 400 nm (750 Hertz, THz), shorter than that of visible light, but longer than ...
(UV) light and electrical discharges within the
Earth's atmosphere
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing for ...
. It is present in very low concentrations throughout the latter, with its highest concentration high in the
ozone layer
The ozone layer or ozone shield is a region of Earth's stratosphere that absorbs most of the Sun's ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the atmosphere, although still small in rela ...
of the
stratosphere
The stratosphere () is the second layer of the atmosphere of the Earth, located above the troposphere and below the mesosphere. The stratosphere is an atmospheric layer composed of stratified temperature layers, with the warm layers of air ...
, which absorbs most of the
Sun
The Sun is the star at the center of the Solar System. It is a nearly perfect ball of hot plasma, heated to incandescence by nuclear fusion reactions in its core. The Sun radiates this energy mainly as light, ultraviolet, and infrared radi ...
's ultraviolet (UV) radiation.
Ozone's odour is reminiscent of
chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
, and detectable by many people at concentrations of as little as in air. Ozone's O
3 structure was determined in 1865. The molecule was later proven to have a bent structure and to be weakly
diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted ...
. In
standard conditions
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union ...
, ozone is a pale blue gas that condenses at
cryogenic
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of ŌĆ£cryogenicsŌĆØ and ŌĆ£cr ...
temperatures to a dark blue
liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, a ...
and finally a violet-black
solid
Solid is one of the State of matter#Four fundamental states, four fundamental states of matter (the others being liquid, gas, and Plasma (physics), plasma). The molecules in a solid are closely packed together and contain the least amount o ...
. Ozone's instability with regard to more common dioxygen is such that both concentrated gas and liquid ozone may decompose explosively at elevated temperatures, physical shock, or fast warming to the boiling point.
It is therefore used commercially only in low concentrations.
Ozone is a powerful
oxidant
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxid ...
(far more so than
dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (O2), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (O3). Others are:
*A ...
) and has many industrial and consumer applications related to oxidation. This same high oxidizing potential, however, causes ozone to damage mucous and respiratory tissues in animals, and also tissues in plants, above concentrations of about . While this makes ozone a potent respiratory hazard and pollutant near
ground level Ground level may refer to:
* Earth's surface
* Storey of a building/structure on (level with) the ground; also called the "ground floor"
* Ground Level, Australian band
* "Ground Level", a song by Stereo MCs from the album ''Connected''
See als ...
, a higher concentration in the ozone layer (from two to eight ppm) is beneficial, preventing damaging UV light from reaching the Earth's surface.
Nomenclature
The
trivial name
In chemistry, a trivial name is a nonsystematic name for a chemical substance. That is, the name is not recognized according to the rules of any formal system of chemical nomenclature such as IUPAC nomenclature of inorganic chemistry, IUPAC inor ...
''ozone'' is the most commonly used and
preferred IUPAC name
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among the possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choo ...
. The systematic names ''2╬╗
4-trioxidiene'' and ''catena-trioxygen'', valid
IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
names, are constructed according to the substitutive and additive nomenclatures, respectively. The name ''ozone'' derives from ''ozein'' (ßĮä╬Č╬Ą╬╣╬Į), the
Greek
Greek may refer to:
Greece
Anything of, from, or related to Greece, a country in Southern Europe:
*Greeks, an ethnic group.
*Greek language, a branch of the Indo-European language family.
**Proto-Greek language, the assumed last common ancestor ...
verb for smell, referring to ozone's distinctive smell.
In appropriate contexts, ozone can be viewed as
trioxidane
Trioxidane (systematically named ╬╝-trioxidanediidodihydrogen), also called dihydrogen trioxide, is an inorganic compound with the chemical formula (can be written as or ). It is one of the unstable hydrogen polyoxides. In aqueous solutions, ...
with two hydrogen atoms removed, and as such, ''trioxidanylidene'' may be used as a systematic name, according to substitutive nomenclature. By default, these names pay no regard to the radicality of the ozone molecule. In an even more specific context, this can also name the non-radical singlet ground state, whereas the diradical state is named ''trioxidanediyl''.
''Trioxidanediyl'' (or ''ozonide'') is used, non-systematically, to refer to the substituent group (-OOO-). Care should be taken to avoid confusing the name of the group for the context-specific name for the ozone given above.
History


In 1785, Dutch chemist
Martinus van Marum
Martin(us) van Marum (20 March 1750, Delft ŌĆō 26 December 1837, Haarlem) was a Dutch physician, inventor, scientist and teacher, who studied medicine and philosophy in Groningen. Van Marum introduced modern chemistry in the Netherlands after ...
was conducting experiments involving electrical sparking above water when he noticed an unusual smell, which he attributed to the electrical reactions, failing to realize that he had in fact created ozone.
A half century later,
Christian Friedrich Sch├Čnbein
Christian Friedrich Sch├Čnbein H FRSE(18 October 1799 ŌĆō 29 August 1868) was a German-Swiss chemist who is best known for inventing the fuel cell (1838) at the same time as William Robert Grove and his discoveries of guncotton and ozone.
Lif ...
noticed the same pungent odour and recognized it as the smell often following a bolt of
lightning
Lightning is a naturally occurring electrostatic discharge during which two electric charge, electrically charged regions, both in the atmosphere or with one on the land, ground, temporarily neutralize themselves, causing the instantaneous ...
. In 1839, he succeeded in isolating the gaseous chemical and named it "ozone", from the Greek word ' () meaning "to smell".
For this reason, Sch├Čnbein is generally credited with the discovery of ozone.
The formula for ozone, O
3, was not determined until 1865 by
Jacques-Louis Soret
Jacques-Louis Soret (30 June 1827 – 13 May 1890) was a Swiss chemist and spectroscopist. He studied both spectroscopy and electrolysis. He held the chairs of chemistry (1873-1887) and medical physics (1887-1890) at the University of Genev ...
and confirmed by Sch├Čnbein in 1867.
For much of the second half of the 19th century and well into the 20th, ozone was considered a healthy component of the environment by naturalists and health-seekers.
Beaumont
Beaumont may refer to:
Places Canada
* Beaumont, Alberta
* Beaumont, Quebec
England
* Beaumont, Cumbria
* Beaumont, Essex
** Beaumont Cut, a canal closed in the 1930s
* Beaumont Street, Oxford
France (communes)
* Beaumont, Ard├©che
* ...
,
California
California is a U.S. state, state in the Western United States, located along the West Coast of the United States, Pacific Coast. With nearly 39.2million residents across a total area of approximately , it is the List of states and territori ...
had as its official slogan "Beaumont: Zone of Ozone", as evidenced on postcards and Chamber of Commerce letterhead. Naturalists working outdoors often considered the higher elevations beneficial because of their ozone content. "There is quite a different atmosphere
t higher elevationwith enough ozone to sustain the necessary energy
o work, wrote naturalist
Henry Henshaw
Henry Wetherbee Henshaw (March 3, 1850 ŌĆō August 1, 1930) was an American ornithologist and ethnologist. He worked at the U.S. Bureau of Ethnology from 1888 to 1892 and was editor of the journal ''American Anthropologist''.
Biography
Early li ...
, working in Hawaii. Seaside air was considered to be healthy because of its believed ozone content. The smell giving rise to this belief is in fact that of
halogenated
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polyme ...
seaweed metabolites
and
dimethyl sulfide.
Much of ozone's appeal seems to have resulted from its "fresh" smell, which evoked associations with purifying properties. Scientists noted its harmful effects. In 1873
James Dewar
Sir James Dewar (20 September 1842 ŌĆō 27 March 1923) was a British chemist and physicist. He is best known for his invention of the vacuum flask, which he used in conjunction with research into the liquefaction of gases. He also studied a ...
and
John Gray McKendrick
John Gray McKendrick FRS FRSE FRCPE LLD (12 August 1841 ŌĆō 2 January 1926) was a distinguished Scottish physiologist. He was born and studied in Aberdeen, Scotland, and served as Regius Professor of Physiology at the University of Glasgow fr ...
documented that frogs grew sluggish, birds gasped for breath, and rabbits' blood showed decreased levels of oxygen after exposure to "ozonized air", which "exercised a destructive action".
Sch├Čnbein himself reported that chest pains, irritation of the
mucous membranes
A mucous membrane or mucosa is a membrane that lines various cavities in the body of an organism and covers the surface of internal organs. It consists of one or more layers of epithelial cells overlying a layer of loose connective tissue. It is ...
and difficulty breathing occurred as a result of inhaling ozone, and small mammals died.
In 1911,
Leonard Hill and
Martin Flack
Martin William Flack (20 March 1882 ŌĆō 16 August 1931) was a British physiologist who co-discovered the sinoatrial node with Sir Arthur Keith in 1907.
Flack later became demonstrator of physiology at the London Hospital and later a lecturer. H ...
stated in the ''
Proceedings of the Royal Society
''Proceedings of the Royal Society'' is the main research journal of the Royal Society. The journal began in 1831 and was split into two series in 1905:
* Series A: for papers in physical sciences and mathematics.
* Series B: for papers in life s ...
B'' that ozone's healthful effects "have, by mere iteration, become part and parcel of common belief; and yet exact physiological evidence in favour of its good effects has been hitherto almost entirely wanting ... The only thoroughly well-ascertained knowledge concerning the physiological effect of ozone, so far attained, is that it causes irritation and ┼ōdema of the lungs, and death if inhaled in relatively strong concentration for any time."
During
World War I
World War I (28 July 1914 11 November 1918), often abbreviated as WWI, was one of the deadliest global conflicts in history. Belligerents included much of Europe, the Russian Empire, the United States, and the Ottoman Empire, with fightin ...
, ozone was tested at
Queen Alexandra Military Hospital
The Queen Alexandra Military Hospital (QAMH) opened in July 1905. It was constructed immediately to the north of the Tate Britain (across a side-street) adjacent to the River Thames on the borders of the neighbourhoods of Millbank and Pimlico, W ...
in London as a possible
disinfectant
A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than st ...
for wounds. The gas was applied directly to wounds for as long as 15 minutes. This resulted in damage to both bacterial cells and human tissue. Other sanitizing techniques, such as irrigation with
antiseptics
An antiseptic (from Greek ß╝Ć╬ĮŽä╬» ''anti'', "against" and Žā╬ĘŽĆŽä╬╣╬║ŽīŽé ''s─ōptikos'', "putrefactive") is an antimicrobial substance or compound that is applied to living tissue/skin to reduce the possibility of infection, sepsis, or putre ...
were found preferable.
Until the 1920s, it was not certain whether small amounts of
oxozone, , were also present in ozone samples due to the difficulty of applying analytical chemistry techniques to the explosive concentrated chemical.
In 1923,
Georg-Maria Schwab (working for his doctoral thesis under
Ernst Hermann Riesenfeld) was the first to successfully solidify ozone and perform accurate analysis which conclusively refuted the oxozone hypothesis.
Further hitherto unmeasured physical properties of pure concentrated ozone were determined by the Riesenfeld group in the 1920s.
Physical properties
Ozone is a colourless or pale blue gas, slightly soluble in water and much more soluble in inert non-polar solvents such as
carbon tetrachloride
Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVAC ...
or fluorocarbons, in which it forms a blue solution. At , it condenses to form a dark blue
liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, a ...
. It is dangerous to allow this liquid to warm to its boiling point, because both concentrated gaseous ozone and liquid ozone can detonate. At temperatures below , it forms a violet-black
solid
Solid is one of the State of matter#Four fundamental states, four fundamental states of matter (the others being liquid, gas, and Plasma (physics), plasma). The molecules in a solid are closely packed together and contain the least amount o ...
.
Most people can detect about 0.01 ╬╝mol/mol of ozone in air where it has a very specific sharp odour somewhat resembling
chlorine bleach
Bleach is the generic name for any chemical product that is used industrially or domestically to remove color (whitening) from a fabric or fiber or to clean or to remove stains in a process called bleaching. It often refers specifically, to ...
. Exposure of 0.1 to 1 ╬╝mol/mol produces headaches, burning eyes and irritation to the respiratory passages.
Even low concentrations of ozone in air are very destructive to organic materials such as latex, plastics and animal lung tissue.
Ozone is weakly diamagnetic.
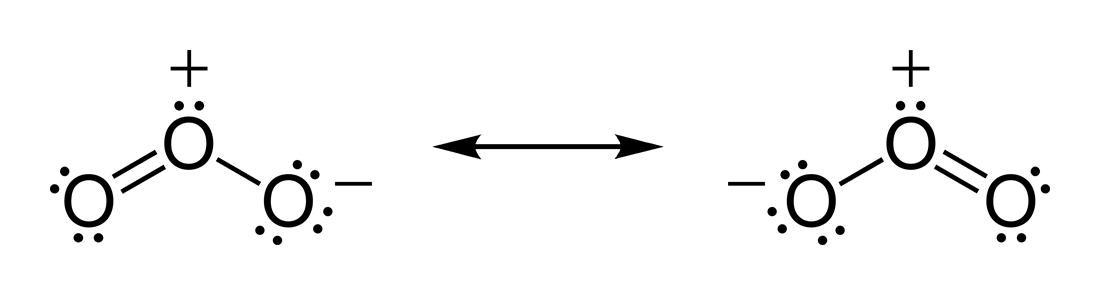
Structure
According to experimental evidence from
microwave spectroscopy
Microwave spectroscopy is the spectroscopy method that employs microwaves, i.e. electromagnetic radiation at GHz frequencies, for the study of matter.
History
The ammonia molecule NH3 is shaped like a pyramid 0.38 ├ģ in height, with an equilatera ...
, ozone is a bent molecule, with C
2v symmetry
Symmetry (from grc, ŽāŽģ╬╝╬╝╬ĄŽäŽü╬»╬▒ "agreement in dimensions, due proportion, arrangement") in everyday language refers to a sense of harmonious and beautiful proportion and balance. In mathematics, "symmetry" has a more precise definit ...
(similar to the
water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
molecule). The O ŌĆō O distances are . The O ŌĆō O ŌĆō O angle is 116.78┬░. The central atom is ''sp''┬▓ hybridized with one lone pair. Ozone is a polar molecule with a
dipole moment of 0.53
D. The molecule can be represented as a
resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillatin ...
hybrid with two contributing structures, each with a
single bond
In chemistry, a single bond is a chemical bond between two atoms involving two valence electrons. That is, the atoms share one pair of electrons where the bond forms. Therefore, a single bond is a type of covalent bond. When shared, each of th ...
on one side and
double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
on the other. The arrangement possesses an overall
bond order
In chemistry, bond order, as introduced by Linus Pauling, is defined as the difference between the number of bonds and anti-bonds.
The bond order itself is the number of electron pairs (covalent bonds) between two atoms. For example, in diat ...
of 1.5 for both sides. It is
isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in the ...
with
the nitrite anion. Naturally occurring ozone can be composed of substituted isotopes (
16O,
17O,
18O). A
cyclic form
Cyclic form is a technique of musical construction, involving multiple sections or movements, in which a theme, melody, or thematic material occurs in more than one movement as a unifying device. Sometimes a theme may occur at the beginning and ...
has been predicted but not observed.
Reactions
Ozone is among the most powerful
oxidizing
Redox (reductionŌĆōoxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
agents known, far stronger than O
2. It is also unstable at high concentrations, decaying into ordinary diatomic oxygen. Its
half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ato ...
varies with atmospheric conditions such as temperature, humidity, and air movement. Under laboratory conditions, Half-Life Time (HLT) will average ~1500 minutes (25 hours) in ''still'' air at room temperature (24 ┬░C), ''zero'' humidity with ''zero'' air changes per hour (ACH).
:2 ŌåÆ 3
This reaction proceeds more rapidly with increasing temperature.
Deflagration
Deflagration (Lat: ''de + flagrare'', "to burn down") is subsonic combustion in which a pre-mixed flame propagates through a mixture of fuel and oxidizer. Deflagrations can only occur in pre-mixed fuels. Most fires found in daily life are diffu ...
of ozone can be triggered by a spark and can occur in ozone concentrations of 10
wt% or higher.
Ozone can also be produced from oxygen at the anode of an electrochemical cell. This reaction can create smaller quantities of ozone for research purposes.
:(g) + 2H
+ + 2e
ŌłÆ (g) + ''E''┬░= 2.075V
This can be observed as an unwanted reaction in a Hoffman gas apparatus during the electrolysis of water when the voltage is set above the necessary voltage.
With metals
Ozone will oxidize most
metal
A metal (from Greek ╬╝╬ŁŽä╬▒╬╗╬╗╬┐╬Į ''m├®tallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
s (except
gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile met ...
,
platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
, and
iridium
Iridium is a chemical element with the symbol Ir and atomic number 77. A very hard, brittle, silvery-white transition metal of the platinum group, it is considered the second-densest naturally occurring metal (after osmium) with a density of ...
) to
oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2ŌĆō (molecular) ion. with oxygen in the oxidation state of ŌłÆ2. Most of the E ...
s of the metals in their highest
oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
. For example:
: + ŌåÆ +
: + ŌåÆ +
With nitrogen and carbon compounds
Ozone also oxidizes
nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its che ...
to
nitrogen dioxide
Nitrogen dioxide is a chemical compound with the formula . It is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the producti ...
:
: NO + ŌåÆ +
This reaction is accompanied by
chemiluminescence
Chemiluminescence (also chemoluminescence) is the emission of light (luminescence) as the result of a chemical reaction. There may also be limited emission of heat. Given reactants A and B, with an excited intermediate ŌŚŖ,
: + -> lozenge -> ...
. The can be further oxidized to
nitrate radical
Trioxidonitrogen(ŌĆó) or nitrate radical is an oxide of nitrogen with formula , consisting of three oxygen atoms covalently bound to a nitrogen atom. This highly unstable blue compound has not been isolated in pure form, but can be generated and ...
:
: + ŌåÆ +
The formed can react with to form .
Solid
nitronium perchlorate
Nitronium perchlorate, NO2ClO4, also known as nitryl perchlorate and nitroxyl perchlorate, is an inorganic chemical, the salt of the perchlorate anion and the nitronium cation. It forms colorless monoclinic crystals. It is hygroscopic, and is a ...
can be made from NO
2, ClO
2, and gases:
: + + 2 ŌåÆ + 2
Ozone does not react with ammonium
salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
s, but it oxidizes
ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
to
ammonium nitrate
Ammonium nitrate is a chemical compound with the chemical formula . It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is ...
:
: 2 + 4 ŌåÆ + 4 +
Ozone reacts with
carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
to form
carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
, even at room temperature:
: C + 2 ŌåÆ + 2
With sulfur compounds
Ozone oxidizes
sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2ŌłÆ or a compound containing one or more S2ŌłÆ ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds lar ...
s to
sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ar ...
s. For example,
lead(II) sulfide
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, le ...
is oxidized to
lead(II) sulfate
Lead(II) sulfate (PbSO4) is a white solid, which appears white in microcrystalline form. It is also known as ''fast white'', ''milk white'', ''sulfuric acid lead salt'' or ''anglesite''.
It is often seen in the plates/electrodes of car batteries ...
:
: PbS + 4 O
3 ŌåÆ PbSO
4 + 4 O
2
Sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
can be produced from ozone, water and either elemental
sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
or
sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activ ...
:
: S + H
2O + O
3 ŌåÆ H
2SO
4
: 3 SO
2 + 3 H
2O + O
3 ŌåÆ 3 H
2SO
4
In the
gas phase
In the outline of physical science, physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, ref ...
, ozone reacts with
hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
to form sulfur dioxide:
: H
2S + O
3 ŌåÆ SO
2 + H
2O
In an
aqueous
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be rep ...
solution, however, two competing simultaneous reactions occur, one to produce elemental sulfur, and one to produce
sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
:
: H
2S + O
3 ŌåÆ S + O
2 + H
2O
: 3 H
2S + 4 O
3 ŌåÆ 3 H
2SO
4
With alkenes and alkynes
Alkenes can be oxidatively cleaved by ozone, in a process called
ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds of alkenes (), alkynes (), or azo compounds () are cleaved with ozone (). Alkenes and alkynes form organic compounds in which the multiple carbonŌĆōcarbon b ...
, giving alcohols, aldehydes, ketones, and carboxylic acids, depending on the second step of the workup.

Ozone can also cleave alkynes to form an
acid anhydride An acid anhydride is a type of chemical compound derived by the removal of water molecules from an acid.
In organic chemistry, organic acid anhydrides contain the functional group R(CO)O(CO)R'. Organic acid anhydrides often form when one equivalent ...
or
diketone
In organic chemistry, a dicarbonyl is a molecule containing two carbonyl () groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls ...
product. If the reaction is performed in the presence of water, the anhydride hydrolyzes to give two
carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
s.
:

Usually ozonolysis is carried out in a solution of
dichloromethane
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with ...
, at a temperature of ŌłÆ78 ┬░C. After a sequence of cleavage and rearrangement, an organic ozonide is formed. With reductive workup (e.g.
zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
in
acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
or
dimethyl sulfide), ketones and aldehydes will be formed, with oxidative workup (e.g. aqueous or alcoholic
hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%ŌĆ ...
), carboxylic acids will be formed.
Other substrates
All three
atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s of ozone may also react, as in the reaction of
tin(II) chloride
Tin(II) chloride, also known as stannous chloride, is a white crystalline solid with the formula . It forms a stable dihydrate, but aqueous solutions tend to undergo hydrolysis, particularly if hot. SnCl2 is widely used as a reducing agent (in aci ...
with
hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
and ozone:
:
3SnCl_2 + 6 HCl + O3 -> 3 SnCl4 + 3 H2O
Iodine perchlorate can be made by treating
iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
dissolved in cold
anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achie ...
perchloric acid
Perchloric acid is a mineral acid with the formula H Cl O4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueous sol ...
with ozone:
:
I2 + 6HClO4 + O3 -> 2I(ClO4)3 + 3H2O
Ozone could also react with potassium iodide to give oxygen and iodine gas that can be titrated for quantitative determination :
:
2KI + O3 + H2O -> 2KOH + O2 + I2
Combustion
Ozone can be used for
combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
reactions and combustible gases; ozone provides higher temperatures than burning in
dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (O2), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (O3). Others are:
*A ...
(O
2). The following is a reaction for the combustion of
carbon subnitride
Dicyanoacetylene, also called carbon subnitride or but-2-ynedinitrile (IUPAC), is a compound of carbon and nitrogen with chemical formula . It has a linear molecular structure, (often abbreviated as ), with alternating triple and single covalen ...
which can also cause higher temperatures:
: 3 + 4 ŌåÆ 12 CO + 3
Ozone can react at cryogenic temperatures. At , atomic
hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
reacts with liquid ozone to form a hydrogen
superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of the ...
radical
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
, which
dimerizes
A dimer () ('' di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic ch ...
:
: H + ŌåÆ HO
2 + O
: 2 HO
2 ŌåÆ
Ozone decomposition
Types of ozone decomposition
Ozone is a toxic substance,
commonly found or generated in human environments (aircraft cabins, offices with photocopiers, laser printers, sterilizers...) and its catalytic decomposition is very important to reduce pollution. This type of decomposition is the most widely used, especially with solid catalysts, and it has many advantages such as a higher conversion with a lower temperature. Furthermore, the product and the catalyst can be instantaneously separated, and this way the catalyst can be easily recovered without using any separation operation. Moreover, the most used materials in the catalytic decomposition of ozone in the gas phase are noble metals like Pt, Rh or Pd and transition metals such as Mn, Co, Cu, Fe, Ni or Ag.
There are two other possibilities for the ozone decomposition in gas phase:
The first one is a thermal decomposition where the ozone can be decomposed using only the action of heat. The problem is that this type of decomposition is very slow with temperatures below 250 ┬░C. However, the decomposition rate can be increased working with higher temperatures but this would involve a high energy cost.
The second one is a photochemical decomposition, which consists of radiating ozone with ultraviolet radiation (UV) and it gives rise to oxygen and radical peroxide.
Kinetics of ozone decomposition into molecular oxygen
The process of ozone decomposition is a complex reaction involving two elementary reactions that finally lead to molecular oxygen, and this means that the reaction order and the rate law cannot be determined by the stoichiometry of the fitted equation.
Overall reaction: 2 O
3 ŌåÆ 3 O
2
Rate law (observed): V = K ┬Ę
3sup>2 ┬Ę
2sup>ŌłÆ1
It has been determined that the ozone decomposition follows a first order kinetics, and from the rate law above it can be determined that the partial order respect to molecular oxygen is -1 and respect to ozone is 2, therefore the global reaction order is 1.
The ozone decomposition consists of two elementary steps: The first one corresponds to a unimolecular reaction because one only molecule of ozone decomposes into two products (molecular oxygen and oxygen). Then, the oxygen from the first step is an intermediate because it participates as a reactant in the second step, which is a bimolecular reaction because there are two different reactants (ozone and oxygen) that give rise to one product, that corresponds to molecular oxygen in the gas phase.
Step 1: Unimolecular reaction O
3 ŌåÆ O
2 + O
Step 2: Bimolecular reaction O
3 + O ŌåÆ 2 O
2
These two steps have different reaction rates, the first one is reversible and faster than the second reaction, which is slower, so this means that the determining step is the second reaction and this is used to determine the observed reaction rate. The reaction rate laws for every step are the ones that follow:
V
1 = K
1 ┬Ę
3 V
2 = K
2 ┬Ę
┬Ę
3
The following mechanism allows to explain the rate law of the ozone decomposition observed experimentally, and also it allows to determine the reaction orders with respect to ozone and oxygen, with which the overall reaction order will be determined. The slower step, the bimolecular reaction, is the one that determines the rate of product formation, and considering that this step gives rise to two oxygen molecules the rate law has this form:
V = 2 K
2 ┬Ę
┬Ę
3
However, this equation depends on the concentration of oxygen (intermediate), which can be determined considering the first step. Since the first step is faster and reversible and the second step is slower, the reactants and products from the first step are in equilibrium, so the concentration of the intermediate can be determined as follows:
Then using these equations, the formation rate of molecular oxygen is as shown below:
Finally, the mechanism presented allows to establish the rate observed experimentally, with a rate constant (K
obs) and corresponding to a first order kinetics, as follows:
where
Reduction to ozonides
Reduction of ozone gives the
ozonide
Ozonide is the polyatomic anion . Cyclic organic compounds formed by the addition of ozone () to an alkene are also called ozonides.
Ionic ozonides
Inorganic ozonides are dark red salts. The anion has the bent shape of the ozone molecule.
Inor ...
anion, O. Derivatives of this anion are explosive and must be stored at cryogenic temperatures. Ozonides for all the
alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
s are known. KO
3, RbO
3, and CsO
3 can be prepared from their respective superoxides:
: KO
2 + O
3 ŌåÆ KO
3 + O
2
Although KO
3 can be formed as above, it can also be formed from
potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which exp ...
and ozone:
: 2 KOH + 5 O
3 ŌåÆ 2 KO
3 + 5 O
2 + H
2O
NaO
3 and LiO
3 must be prepared by action of CsO
3 in liquid NH
3 on an
ion-exchange resin
An ion-exchange resin or ion-exchange polymer is a resin or polymer that acts as a medium for ion exchange. It is an insoluble matrix (or support structure) normally in the form of small (0.25ŌĆō1.43 mm radius) microbeads, usually white or ye ...
containing Na
+ or Li
+ ions:
: CsO
3 + Na
+ ŌåÆ Cs
+ + NaO
3
A solution of
calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to ...
in ammonia reacts with ozone to give
ammonium ozonide
Ammonium ozonide is an oxygen rich molecule containing an ammonium cation (NH4+) and an ozonide anion (O3ŌłÆ). Ammonium ozonide, like alkali ozonides, is a red solid. Ammonium ozonide is stable at low temperatures, but it decomposes to ammonium n ...
and not calcium ozonide:
: 3 Ca + 10 NH
3 + 6 ŌåÆ Ca┬Ę6NH
3 + Ca(OH)
2 + Ca(NO
3)
2 + 2 NH
4O
3 + 2 O
2 + H
2
Applications
Ozone can be used to remove
iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
and
manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy use ...
from
water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
, forming a
precipitate
In an aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a super-saturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading ...
which can be filtered:
: 2 Fe
2+ + O
3 + 5 H
2O ŌåÆ 2 Fe(OH)
3(s) + O
2 + 4 H
+
: 2 Mn
2+ + 2 O
3 + 4 H
2O ŌåÆ 2 MnO(OH)
2(s) + 2 O
2 + 4 H
+
Ozone will also oxidize dissolved
hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
in water to
sulfurous acid
Sulfurous acid (also sulfuric(IV) acid, sulphurous acid (UK), sulphuric(IV) acid (UK)) is the chemical compound with the formula . There is no evidence that sulfurous acid exists in solution, but the molecule has been detected in the gas phase. ...
:
: 3 + H
2S ŌåÆ H
2SO
3 + 3 O
2
These three reactions are central in the use of ozone-based well water treatment.
Ozone will also detoxify
cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of ...
s by converting them to
cyanate
Cyanate is an anion with the structural formula , usually written . It also refers to any salt containing it, such as ammonium cyanate.
It is an isomer of the much less stable fulminate anion .William R. Martin and David W. Ball (2019): "Small ...
s.
: CN
ŌłÆ + O
3 ŌåÆ + O
2
Ozone will also completely decompose
urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (ŌĆō) joined by a carbonyl functional group (ŌĆōC(=O)ŌĆō). It is thus the simplest amide of carbamic acid.
Urea serves an important r ...
:
:(NH
2)
2CO + O
3 ŌåÆ N
2 + CO
2 + 2 H
2O
Spectroscopic properties
Ozone is a bent
triatomic molecule
Triatomic molecules are molecules composed of three atoms, of either the same or different chemical elements. Examples include H2O, CO2 (pictured), HCN and O3 (ozone)
Molecular vibrations
The vibrational modes of a triatomic molecule can be ...
with three vibrational modes: the symmetric stretch (1103.157 cm
ŌłÆ1), bend (701.42 cm
ŌłÆ1) and antisymmetric stretch (1042.096 cm
ŌłÆ1). The symmetric stretch and bend are weak absorbers, but the antisymmetric stretch is strong and responsible for ozone being an important minor
greenhouse gas
A greenhouse gas (GHG or GhG) is a gas that Absorption (electromagnetic radiation), absorbs and Emission (electromagnetic radiation), emits radiant energy within the thermal infrared range, causing the greenhouse effect. The primary greenhouse ...
. This IR band is also used to detect ambient and atmospheric ozone although UV-based measurements are more common.
The electromagnetic spectrum of ozone is quite complex. An overview can be seen at the MPI Mainz UV/VIS Spectral Atlas of Gaseous Molecules of Atmospheric Interest.
All of the bands are dissociative, meaning that the molecule falls apart to after absorbing a photon. The most important absorption is the Hartley band, extending from slightly above 300 nm down to slightly above 200 nm. It is this band that is responsible for absorbing UV C in the stratosphere.
On the high wavelength side, the Hartley band transitions to the so-called Huggins band, which falls off rapidly until disappearing by ~360 nm. Above 400 nm, extending well out into the NIR, are the Chappius and Wulf bands. There, unstructured absorption bands are useful for detecting high ambient concentrations of ozone, but are so weak that they do not have much practical effect.
There are additional absorption bands in the far UV, which increase slowly from 200 nm down to reaching a maximum at ~120 nm.
Ozone in Earth's atmosphere

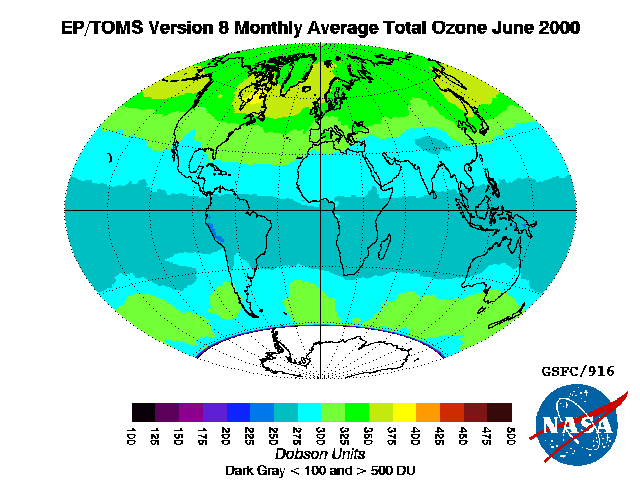
The standard way to express total ozone levels (the amount of ozone in a given vertical column) in the atmosphere is by using
Dobson unit The Dobson unit (DU) is a unit of measurement of the amount of a trace gas in a vertical column through the Earth's atmosphere. It originated, and continues to be primarily used in respect to, atmospheric ozone, whose total column amount, usually te ...
s. Point measurements are reported as
mole fraction
In chemistry, the mole fraction or molar fraction (''xi'' or ) is defined as unit of the amount of a constituent (expressed in moles), ''ni'', divided by the total amount of all constituents in a mixture (also expressed in moles), ''n''tot. This ex ...
s in nmol/mol (parts per billion, ppb) or as
concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', ''molar concentration'', ''number concentration'', an ...
s in ╬╝g/m
3. The study of ozone concentration in the atmosphere started in the 1920s.
Ozone layer
Location and production
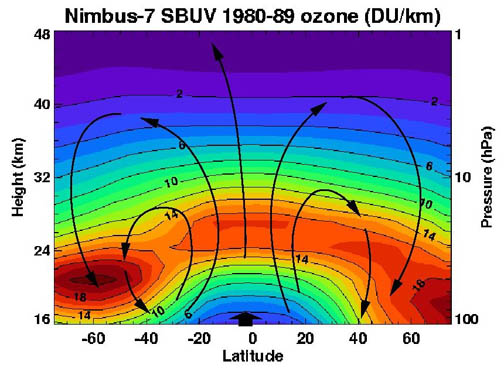
The highest levels of ozone in the atmosphere are in the
stratosphere
The stratosphere () is the second layer of the atmosphere of the Earth, located above the troposphere and below the mesosphere. The stratosphere is an atmospheric layer composed of stratified temperature layers, with the warm layers of air ...
, in a region also known as the
ozone layer
The ozone layer or ozone shield is a region of Earth's stratosphere that absorbs most of the Sun's ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the atmosphere, although still small in rela ...
between about 10 and 50 km above the surface (or between about 6 and 31 miles). However, even in this "layer", the ozone concentrations are only two to eight parts per million, so most of the oxygen there is dioxygen, O
2, at about 210,000 parts per million by volume.
Ozone in the stratosphere is mostly produced from short-wave ultraviolet rays between 240 and 160 nm. Oxygen starts to absorb weakly at 240 nm in the Herzberg bands, but most of the oxygen is dissociated by absorption in the strong
SchumannŌĆōRunge bands between 200 and 160 nm where ozone does not absorb. While shorter wavelength light, extending to even the X-Ray limit, is energetic enough to dissociate molecular oxygen, there is relatively little of it, and, the strong solar emission at Lyman-alpha, 121 nm, falls at a point where molecular oxygen absorption is a minimum.
The process of ozone creation and destruction is called the
Chapman cycle and starts with the photolysis of molecular oxygen
:
O2 -> ce \ce\ \lambda\ <\ 240\ \ce)2O
followed by reaction of the oxygen atom with another molecule of oxygen to form ozone.
:O + + M ŌåÆ + M
where "M" denotes the third body that carries off the excess energy of the reaction. The ozone molecule can then absorb a UV-C photon and dissociate
: ŌåÆ O + + kinetic energy
The excess kinetic energy heats the stratosphere when the O atoms and the molecular oxygen fly apart and collide with other molecules. This conversion of UV light into kinetic energy warms the stratosphere. The oxygen atoms produced in the photolysis of ozone then react back with other oxygen molecule as in the previous step to form more ozone. In the clear atmosphere, with only nitrogen and oxygen, ozone can react with the atomic oxygen to form two molecules of O
2
: + O ŌåÆ 2
An estimate of the rate of this termination step to the cycling of atomic oxygen back to ozone can be found simply by taking the ratios of the concentration of O
2 to O
3. The termination reaction is
catalysed
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
by the presence of certain free radicals, of which the most important are hydroxyl (OH), nitric oxide (NO) and atomic chlorine (Cl) and bromine (Br). In the second half of the 20th century, the amount of ozone in the stratosphere was discovered to be declining, mostly because of increasing concentrations of
chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and prop ...
s (CFC) and similar
chlorinated and brominated organic molecules. The concern over the health effects of the decline led to the 1987
Montreal Protocol
The Montreal Protocol is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances that are responsible for ozone depletion
Ozone depletion consists of two related events observed sinc ...
, the ban on the production of many
ozone depleting
Ozone depletion consists of two related events observed since the late 1970s: a steady lowering of about four percent in the total amount of ozone in Earth's atmosphere, and a much larger springtime decrease in stratospheric ozone (the ozone l ...
chemicals and in the first and second decade of the 21st century the beginning of the recovery of stratospheric ozone concentrations.
Importance to surface-dwelling life on Earth
Ozone in the ozone layer filters out sunlight wavelengths from about 200 nm UV rays to 315 nm, with ozone peak absorption at about 250 nm. This ozone UV absorption is important to life, since it extends the absorption of UV by ordinary oxygen and nitrogen in air (which absorb all wavelengths < 200 nm) through the lower UV-C (200ŌĆō280 nm) and the entire UV-B band (280ŌĆō315 nm). The small unabsorbed part that remains of UV-B after passage through ozone causes sunburn in humans, and direct DNA damage in living tissues in both plants and animals. Ozone's effect on mid-range UV-B rays is illustrated by its effect on UV-B at 290 nm, which has a radiation intensity 350 million times as powerful at the top of the atmosphere as at the surface. Nevertheless, enough of UV-B radiation at similar frequency reaches the ground to cause some sunburn, and these same wavelengths are also among those responsible for the production of
vitamin D
Vitamin D is a group of fat-soluble secosteroids responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, and many other biological effects. In humans, the most important compounds in this group are vitamin D3 (c ...
in humans.
The ozone layer has little effect on the longer UV wavelengths called UV-A (315ŌĆō400 nm), but this radiation does not cause sunburn or direct DNA damage, and while it probably does cause long-term skin damage in certain humans, it is not as dangerous to plants and to the health of surface-dwelling organisms on Earth in general (see
ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nanometer, nm (with a corresponding frequency around 30 Hertz, PHz) to 400 nm (750 Hertz, THz), shorter than that of visible light, but longer than ...
for more information on near ultraviolet).
Low level ozone
Low level ozone (or tropospheric ozone) is an atmospheric pollutant.
[Health Aspects of Air Pollution with Particulate Matter, Ozone and Nitrogen Dioxide]
WHO-Europe report 13ŌĆō15 January 2003 (PDF) It is not emitted directly by
car engines or by industrial operations, but formed by the reaction of sunlight on air containing
hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ex ...
s and
nitrogen oxide Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:
Charge-neutral
*Nitric oxide (NO), nitrogen(II) oxide, or nitrogen monoxide
*Nitrogen dioxide (), nitrogen(IV) oxide
* Nitrogen trioxide (), or n ...
s that react to form ozone directly at the source of the pollution or many kilometers downwind.
Ozone reacts directly with some hydrocarbons such as
aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s and thus begins their removal from the air, but the products are themselves key components of
smog
Smog, or smoke fog, is a type of intense air pollution. The word "smog" was coined in the early 20th century, and is a portmanteau of the words ''smoke'' and '' fog'' to refer to smoky fog due to its opacity, and odor. The word was then inte ...
. Ozone
photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. ...
by UV light leads to production of the
hydroxyl radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the ...
HOŌĆó and this plays a part in the removal of hydrocarbons from the air, but is also the first step in the creation of components of smog such as
peroxyacyl nitrates
In organic chemistry, peroxyacyl nitrates (also known as Acyl peroxy nitrates, APN or PANs) are powerful respiratory and eye irritants present in photochemical smog. They are nitrates produced in the thermal equilibrium between organic peroxy ...
, which can be powerful eye irritants. The atmospheric lifetime of tropospheric ozone is about 22 days; its main removal mechanisms are being deposited to the ground, the above-mentioned reaction giving HOŌĆó, and by reactions with OH and the peroxy radical HO
2ŌĆó.
There is evidence of significant reduction in agricultural yields because of increased ground-level ozone and pollution which interferes with
photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
and stunts overall growth of some plant species.
The
United States Environmental Protection Agency
The Environmental Protection Agency (EPA) is an independent executive agency of the United States federal government tasked with environmental protection matters. President Richard Nixon proposed the establishment of EPA on July 9, 1970; it be ...
is proposing a secondary regulation to reduce crop damage, in addition to the primary regulation designed for the protection of human health.
Low level ozone in urban areas
Certain examples of cities with elevated ozone readings are
Denver, Colorado
Denver () is a consolidated city and county, the capital, and most populous city of the U.S. state of Colorado. Its population was 715,522 at the 2020 census, a 19.22% increase since 2010. It is the 19th-most populous city in the Unit ...
;
Houston, Texas
Houston (; ) is the most populous city in Texas, the most populous city in the Southern United States, the fourth-most populous city in the United States, and the sixth-most populous city in North America, with a population of 2,304,580 in ...
; and
Mexico City
Mexico City ( es, link=no, Ciudad de M├®xico, ; abbr.: CDMX; Nahuatl: ''Altepetl Mexico'') is the capital and largest city of Mexico, and the most populous city in North America. One of the world's alpha cities, it is located in the Valley o ...
,
Mexico
Mexico (Spanish: M├®xico), officially the United Mexican States, is a country in the southern portion of North America. It is bordered to the north by the United States; to the south and west by the Pacific Ocean; to the southeast by Guatema ...
. Houston has a reading of around 41 nmol/mol, while Mexico City is far more hazardous, with a reading of about 125 nmol/mol.
Low level ozone, or tropospheric ozone, is the most concerning type of ozone pollution in urban areas and is increasing in general. Ozone pollution in urban areas affects denser populations, and is worsened by high populations of vehicles, which emit pollutants NO
2 and
VOC
VOC, VoC or voc may refer to:
Science and technology
* Open-circuit voltage (VOC), the voltage between two terminals when there is no external load connected
* Variant of concern, a category used during the assessment of a new variant of a virus
...
s, the main contributors to problematic ozone levels.
Ozone pollution in urban areas is especially concerning with increasing temperatures, raising heat-related mortality during
heat wave
A heat wave, or heatwave, is a period of excessively hot weather, which may be accompanied by high humidity, especially in oceanic climate countries. While definitions vary, a heat wave is usually measured relative to the usual climate in the ...
s. During heat waves in urban areas,
ground level ozone
Ground-level ozone (O3), also known as surface-level ozone and tropospheric ozone, is a trace gas in the troposphere (the lowest level of the atmosphere of Earth, Earth's atmosphere), with an average concentration of 20ŌĆō30 parts per billion by v ...
pollution can be 20% higher than usual. Ozone pollution in urban areas reaches higher levels of exceedance in the summer and autumn, which may be explained by weather patterns and traffic patterns.
People experiencing poverty are more affected by pollution in general, even though these populations are less likely to be contributing to pollution levels.
As mentioned above, Denver, Colorado, is one of the many cities in the United States that have high amounts of ozone. According to the American Lung Association, the Denver-Aurora area is the 14th most ozone-polluted area in the United States. The problem of high ozone levels is not new to this area. In 2004, "the US Environmental Protection Agency designated the Denver Metro/North Front Range (Adams, Arapahoe, Boulder, Broomfield, Denver, Douglas, Jefferson, and parts of Larimer and Weld counties) as nonattainment for the 1997 8-hour ozone standard", but later deferred this nonattainment status until 2007. The nonattainment standard indicates that an area does not meet the EPA's air quality standards. The Colorado Ozone Action Plan was created in response, and numerous changes were implemented from this plan. The first major change was that car emission testing was expanded across the state to more counties that did not previously mandate emissions testing, like areas of Larimer and Weld County. There have also been changes made to decrease Nitrogen Oxides (NOx) and
Volatile Organic Compound
Volatile organic compounds (VOCs) are organic compounds that have a high vapour pressure at room temperature. High vapor pressure correlates with a low boiling point, which relates to the number of the sample's molecules in the surrounding air, a ...
(VOC) emissions, which should help lower ozone levels.
One large contributor to high ozone levels in the area is the oil and
natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbo ...
industry situated in the Denver-Julesburg Basin (DJB) which overlaps with a majority of Colorado's metropolitan areas. Ozone is created naturally in the Earth's stratosphere, but is also created in the troposphere from human efforts. Briefly mentioned above, NOx and VOCs react with sunlight to create ozone through a process called photochemistry. One hour elevated ozone events (<75 ppb) "occur during JuneŌĆōAugust indicating that elevated ozone levels are driven by regional photochemistry".
According to an article from the University of Colorado-Boulder, "Oil and natural gas VOC emission have a major role in ozone production and bear the potential to contribute to elevated O
3 levels in the Northern Colorado Front Range (NCFR)".
Using complex analyses to research wind patterns and emissions from large oil and natural gas operations, the authors concluded that "elevated O
3 levels in the NCFR are predominantly correlated with air transport from NŌĆō ESE, which are the upwind sectors where the O&NG operations in the Wattenberg Field area of the DJB are located".
Contained in the Colorado Ozone Action Plan, created in 2008, plans exist to evaluate "emission controls for large industrial sources of NOx" and "statewide control requirements for new oil and gas condensate tanks and pneumatic valves". In 2011, the Regional Haze Plan was released that included a more specific plan to help decrease NOx emissions. These efforts are increasingly difficult to implement and take many years to come to pass. Of course there are also other reasons that ozone levels remain high. These include: a growing population meaning more car emissions, and the mountains along the NCFR that can trap emissions. If interested, daily air quality readings can be found at the Colorado Department of Public Health and Environment's website. As noted earlier, Denver continues to experience high levels of ozone to this day. It will take many years and a systems-thinking approach to combat this issue of high ozone levels in the Front Range of Colorado.
Ozone cracking
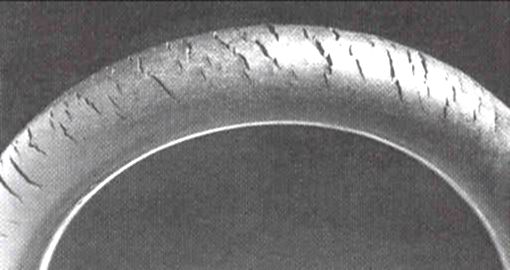
Ozone gas attacks any
polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
possessing olefinic or
double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
s within its chain structure, such as
natural rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, and ...
,
nitrile rubber
Nitrile rubber, also known as nitrile butadiene rubber, NBR, Buna-N, and acrylonitrile butadiene rubber, is a synthetic rubber derived from acrylonitrile (ACN) and butadiene. Trade names include Perbunan, Nipol, Krynac and Europrene. This rubber is ...
, and
styrene-butadiene
Styrene-butadiene or styrene-butadiene rubber (SBR) describe families of synthetic rubbers derived from styrene and butadiene (the version developed by Goodyear is called Neolite). These materials have good abrasion resistance and good aging st ...
rubber. Products made using these polymers are especially susceptible to attack, which causes cracks to grow longer and deeper with time, the rate of crack growth depending on the load carried by the rubber component and the concentration of ozone in the atmosphere. Such materials can be protected by adding
antiozonant
An antiozonant, also known as anti-ozonant, is an organic compound that prevents or retards damage caused by ozone. The most important antiozonants are those which prevent degradation of elastomers like rubber. A number of research projects study ...
s, such as waxes, which bond to the surface to create a protective film or blend with the material and provide long term protection.
Ozone cracking
Cracks can be formed in many different elastomers by ozone attack, and the characteristic form of attack of vulnerable rubbers is known as ozone cracking. The problem was formerly very common, especially in tires, but is now rarely seen in those ...
used to be a serious problem in car tires, for example, but it is not an issue with modern tires. On the other hand, many critical products, like
gasket
Some seals and gaskets
A gasket is a mechanical seal which fills the space between two or more mating surfaces, generally to prevent leakage from or into the joined objects while under compression. It is a deformable material that is used to c ...
s and
O-ring
An O-ring, also known as a packing or a toric joint, is a mechanical gasket in the shape of a torus; it is a loop of elastomer with a round cross-section, designed to be seated in a groove and compressed during assembly between two or more par ...
s, may be attacked by ozone produced within compressed air systems.
Fuel line
A fuel line is a hose or pipe used to transfer fuel from one point in a vehicle to another. The United States Environmental Protection Agency defines a fuel line as "all hoses or tubing designed to contain liquid fuel or fuel vapor. This includes ...
s made of reinforced rubber are also susceptible to attack, especially within the engine compartment, where some ozone is produced by electrical components. Storing rubber products in close proximity to a
DC electric motor
An electric motor is an Electric machine, electrical machine that converts electrical energy into mechanical energy. Most electric motors operate through the interaction between the motor's magnetic field and electric current in a Electromagneti ...
can accelerate ozone cracking. The
commutator
In mathematics, the commutator gives an indication of the extent to which a certain binary operation fails to be commutative. There are different definitions used in group theory and ring theory.
Group theory
The commutator of two elements, a ...
of the motor generates sparks which in turn produce ozone.
Ozone as a greenhouse gas
Although ozone was present at ground level before the
Industrial Revolution
The Industrial Revolution was the transition to new manufacturing processes in Great Britain, continental Europe, and the United States, that occurred during the period from around 1760 to about 1820ŌĆō1840. This transition included going f ...
, peak concentrations are now far higher than the pre-industrial levels, and even background concentrations well away from sources of pollution are substantially higher. Ozone acts as a
greenhouse gas
A greenhouse gas (GHG or GhG) is a gas that Absorption (electromagnetic radiation), absorbs and Emission (electromagnetic radiation), emits radiant energy within the thermal infrared range, causing the greenhouse effect. The primary greenhouse ...
, absorbing some of the
infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around ...
energy emitted by the earth. Quantifying the greenhouse gas potency of ozone is difficult because it is not present in uniform concentrations across the globe. However, the most widely accepted scientific assessments relating to
climate change
In common usage, climate change describes global warmingŌĆöthe ongoing increase in global average temperatureŌĆöand its effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes to E ...
(e.g. the
Intergovernmental Panel on Climate Change
The Intergovernmental Panel on Climate Change (IPCC) is an intergovernmental body of the United Nations. Its job is to advance scientific knowledge about climate change caused by human activities. The World Meteorological Organization (WMO) a ...
Third Assessment Report
The IPCC Third Assessment Report (TAR), ''Climate Change 2001'', is an assessment of available scientific and socio-economic information on climate change by the IPCC. Statements of the IPCC or information from the TAR are often used as a referenc ...
) suggest that the
radiative forcing
Radiative forcing (or climate forcing) is the change in energy flux in the atmosphere caused by natural or anthropogenic factors of climate change as measured by watts / metre2. It is a scientific concept used to quantify and compare the external ...
of tropospheric ozone is about 25% that of
carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
.
The annual
global warming potential
Global warming potential (GWP) is the heat absorbed by any greenhouse gas in the atmosphere, as a multiple of the heat that would be absorbed by the same mass of carbon dioxide (). GWP is 1 for . For other gases it depends on the gas and the time f ...
of tropospheric ozone is between 918 and 1022 tons
carbon dioxide equivalent
Global warming potential (GWP) is the heat absorbed by any greenhouse gas in the atmosphere, as a multiple of the heat that would be absorbed by the same mass of carbon dioxide (). GWP is 1 for . For other gases it depends on the gas and the time ...
/tons tropospheric ozone. This means on a per-molecule basis, ozone in the troposphere has a
radiative forcing
Radiative forcing (or climate forcing) is the change in energy flux in the atmosphere caused by natural or anthropogenic factors of climate change as measured by watts / metre2. It is a scientific concept used to quantify and compare the external ...
effect roughly 1,000 times as strong as
carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
. However, tropospheric ozone is a short-lived
greenhouse gas
A greenhouse gas (GHG or GhG) is a gas that Absorption (electromagnetic radiation), absorbs and Emission (electromagnetic radiation), emits radiant energy within the thermal infrared range, causing the greenhouse effect. The primary greenhouse ...
, which decays in the atmosphere much more quickly than
carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
. This means that over a 20-year span, the
global warming potential
Global warming potential (GWP) is the heat absorbed by any greenhouse gas in the atmosphere, as a multiple of the heat that would be absorbed by the same mass of carbon dioxide (). GWP is 1 for . For other gases it depends on the gas and the time f ...
of tropospheric ozone is much less, roughly 62 to 69 tons
carbon dioxide equivalent
Global warming potential (GWP) is the heat absorbed by any greenhouse gas in the atmosphere, as a multiple of the heat that would be absorbed by the same mass of carbon dioxide (). GWP is 1 for . For other gases it depends on the gas and the time ...
/ ton tropospheric ozone.
Because of its short-lived nature, tropospheric ozone does not have strong global effects, but has very strong radiative forcing effects on regional scales. In fact, there are regions of the world where tropospheric ozone has a
radiative forcing
Radiative forcing (or climate forcing) is the change in energy flux in the atmosphere caused by natural or anthropogenic factors of climate change as measured by watts / metre2. It is a scientific concept used to quantify and compare the external ...
up to 150% of
carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
. For example, ozone increase in the
troposphere
The troposphere is the first and lowest layer of the atmosphere of the Earth, and contains 75% of the total mass of the planetary atmosphere, 99% of the total mass of water vapour and aerosols, and is where most weather phenomena occur. From ...
is shown to be responsible for ~30% of upper
Southern Ocean
The Southern Ocean, also known as the Antarctic Ocean, comprises the southernmost waters of the World Ocean, generally taken to be south of 60┬░ S latitude and encircling Antarctica. With a size of , it is regarded as the second-small ...
interior warming between 1955 and 2000.
Health effects
For the last few decades, scientists studied the effects of acute and chronic ozone exposure on human health. Hundreds of studies suggest that ozone is harmful to people at levels currently found in urban areas. Ozone has been shown to affect the respiratory, cardiovascular and central nervous system. Early death and problems in reproductive health and development are also shown to be associated with ozone exposure.
Vulnerable populations
The
American Lung Association
The American Lung Association is a voluntary health organization whose mission is to save lives by improving lung health and preventing lung disease through education, advocacy and research.
History
The organization was founded in 1904 to figh ...
has identified five populations who are especially vulnerable to the effects of breathing ozone:
# Children and teens
# People 65 years old and older
# People who work or exercise outdoors
# People with existing lung diseases, such as asthma and chronic obstructive pulmonary disease (also known as COPD, which includes emphysema and chronic bronchitis)
# People with
cardiovascular disease
Cardiovascular disease (CVD) is a class of diseases that involve the heart or blood vessels. CVD includes coronary artery diseases (CAD) such as angina and myocardial infarction (commonly known as a heart attack). Other CVDs include stroke, h ...
Additional evidence suggests that women, those with obesity and low-income populations may also face higher risk from ozone, although more research is needed.
Acute ozone exposure
Acute ozone exposure ranges from hours to a few days. Because ozone is a gas, it directly affects the lungs and the entire respiratory system. Inhaled ozone causes inflammation and acuteŌĆöbut reversibleŌĆöchanges in lung function, as well as airway hyperresponsiveness. These changes lead to shortness of breath, wheezing, and coughing which may exacerbate lung diseases, like asthma or chronic obstructive pulmonary disease (COPD) resulting in the need to receive medical treatment. Acute and chronic exposure to ozone has been shown to cause an increased risk of respiratory infections, due to the following mechanism.
Multiple studies have been conducted to determine the mechanism behind ozone's harmful effects, particularly in the lungs. These studies have shown that exposure to ozone causes changes in the immune response within the lung tissue, resulting in disruption of both the innate and adaptive immune response, as well as altering the protective function of lung epithelial cells.
It is thought that these changes in immune response and the related inflammatory response are factors that likely contribute to the increased risk of lung infections, and worsening or triggering of asthma and reactive airways after exposure to ground-level ozone pollution.
[Informed Health Online nternet Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-. The innate and adaptive immune systems. 2010 Dec 7 pdated 2016 Aug 4 Available from https://www.ncbi.nlm.nih.gov/books/NBK279396/]
The innate (cellular) immune system consists of various chemical signals and cell types that work broadly and against multiple pathogen types, typically bacteria or foreign bodies/substances in the host.
[Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. The components of the immune system. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27092/] The cells of the innate system include phagocytes, neutrophils,
both thought to contribute to the mechanism of ozone pathology in the lungs, as the functioning of these cell types have been shown to change after exposure to ozone.
Macrophages, cells that serve the purpose of eliminating pathogens or foreign material through the process of "phagocytosis",
have been shown to change the level of inflammatory signals they release in response to ozone, either up-regulating and resulting in an inflammatory response in the lung, or down-regulating and reducing immune protection.
Neutrophils, another important cell type of the innate immune system that primarily targets bacterial pathogens,
are found to be present in the airways within 6 hours of exposure to high ozone levels. Despite high levels in the lung tissues, however, their ability to clear bacteria appears impaired by exposure to ozone.
The adaptive immune system is the branch of immunity that provides long-term protection via the development of antibodies targeting specific pathogens and is also impacted by high ozone exposure.
Lymphocytes, a cellular component of the adaptive immune response, produce an increased amount of inflammatory chemicals called "cytokines" after exposure to ozone, which may contribute to airway hyperreactivity and worsening asthma symptoms.
The airway epithelial cells also play an important role in protecting individuals from pathogens. In normal tissue, the epithelial layer forms a protective barrier, and also contains specialized ciliary structures that work to clear foreign bodies, mucus and pathogens from the lungs. When exposed to ozone, the cilia become damaged and mucociliary clearance of pathogens is reduced. Furthermore, the epithelial barrier becomes weakened, allowing pathogens to cross the barrier, proliferate and spread into deeper tissues. Together, these changes in the epithelial barrier help make individuals more susceptible to pulmonary infections.
Inhaling ozone not only affects the immune system and lungs, but it may also affect the heart as well. Ozone causes short-term autonomic imbalance leading to changes in heart rate and reduction in heart rate variability; and high levels exposure for as little as one-hour results in a supraventricular arrhythmia in the elderly, both increase the risk of premature death and stroke. Ozone may also lead to vasoconstriction resulting in increased systemic arterial pressure contributing to increased risk of cardiac morbidity and mortality in patients with pre-existing cardiac diseases.
Chronic ozone exposure
Breathing ozone for periods longer than eight hours at a time for weeks, months or years defines chronic exposure. Numerous studies suggest a serious impact on the health of various populations from this exposure.
One study finds significant positive associations between chronic ozone and all-cause, circulatory, and respiratory mortality with 2%, 3%, and 12% increases in risk per 10 ppb and report an association (95% CI) of annual ozone and all-cause mortality with a hazard ratio of 1.02 (1.01ŌĆō1.04), and with cardiovascular mortality of 1.03 (1.01ŌĆō1.05). A similar study finds similar associations with all-cause mortality and even larger effects for cardiovascular mortality. An increased risk of mortality from respiratory causes is associated with long-term chronic exposure to ozone.
Chronic ozone has detrimental effects on children, especially those with asthma. The risk for hospitalization in children with asthma increases with chronic exposure to ozone; younger children and those with low-income status are even at greater risk.
Adults suffering from respiratory diseases (asthma, COPD, lung cancer) are at a higher risk of mortality and morbidity and critically ill patients have an increased risk of developing acute respiratory distress syndrome with chronic ozone exposure as well.
Ozone produced by air cleaners
Ozone generators sold as air cleaners intentionally produce the gas ozone.
These are often marketed to control indoor air pollution, and use misleading terms to describe ozone. Some examples are describing it as "energized oxygen" or "pure air", suggesting that ozone is a healthy or "better" kind of oxygen.
However, according to the
EPA
The Environmental Protection Agency (EPA) is an independent executive agency of the United States federal government tasked with environmental protection matters. President Richard Nixon proposed the establishment of EPA on July 9, 1970; it be ...
, "''ozone is not effective at removing many odor-causing chemicals"'' and "''does not effectively remove viruses, bacteria, mold, or other biological pollutants".''
Furthermore, another report states that "results of some controlled studies show that concentrations of ozone considerably higher than these
uman safety
Uman ( uk, ąŻą╝ą░ąĮčī, ; pl, Huma┼ä; yi, ūÉūĢū×ūÉųĘū¤) is a city located in Cherkasy Oblast in central Ukraine, to the east of Vinnytsia. Located in the historical region of the eastern Podolia, the city rests on the banks of the Umanka River ...
standards are possible even when a user follows the manufacturer's operating instructions".
The
California Air Resources Board
The California Air Resources Board (CARB or ARB) is the "clean air agency" of the government of California. Established in 1967 when then-governor Ronald Reagan signed the Mulford-Carrell Act, combining the Bureau of Air Sanitation and the Motor ...
has a page listing air cleaners (many with
ionizers) meeting their indoor ozone limit of 0.050 parts per million.
[California Certified Air Cleaning Devices]
From California Air Resources Board
The California Air Resources Board (CARB or ARB) is the "clean air agency" of the government of California. Established in 1967 when then-governor Ronald Reagan signed the Mulford-Carrell Act, combining the Bureau of Air Sanitation and the Motor ...
. From that article:
Ozone air pollution


Ozone precursors
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic Allotropy, allotrope , breaking down i ...
are a group of pollutants, predominantly those emitted during the combustion of
fossil fuels
A fossil fuel is a hydrocarbon-containing material formed naturally in the Earth's crust from the remains of dead plants and animals that is extracted and burned as a fuel. The main fossil fuels are coal, oil, and natural gas. Fossil fuels ...
. Ground-level ozone pollution (
tropospheric ozone
Ground-level ozone (O3), also known as surface-level ozone and tropospheric ozone, is a trace gas in the troposphere (the lowest level of the Earth's atmosphere), with an average concentration of 20ŌĆō30 parts per billion by volume (ppbv), with c ...
) is created near the Earth's surface by the action of daylight
UV rays on these precursors. The
ozone at ground level is primarily from fossil fuel precursors, but
methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Eart ...
is a natural precursor, and the very low natural background level of ozone at ground level is considered safe. This section examines the health impacts of fossil fuel burning, which raises ground level ozone far above background levels.
There is a great deal of evidence to show that ground-level ozone can harm lung function and irritate the
respiratory system
The respiratory system (also respiratory apparatus, ventilatory system) is a biological system consisting of specific organs and structures used for gas exchange in animals and plants. The anatomy and physiology that make this happen varies grea ...
.
Exposure to ozone (and the pollutants that produce it) is linked to premature
death
Death is the irreversible cessation of all biological functions that sustain an organism. For organisms with a brain, death can also be defined as the irreversible cessation of functioning of the whole brain, including brainstem, and brain ...
,
asthma
Asthma is a long-term inflammatory disease of the airways of the lungs. It is characterized by variable and recurring symptoms, reversible airflow obstruction, and easily triggered bronchospasms. Symptoms include episodes of wheezing, cou ...
,
bronchitis
Bronchitis is inflammation of the bronchi (large and medium-sized airways) in the lungs that causes coughing. Bronchitis usually begins as an infection in the nose, ears, throat, or sinuses. The infection then makes its way down to the bronchi. ...
,
heart attack
A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow decreases or stops to the coronary artery of the heart, causing damage to the heart muscle. The most common symptom is chest pain or discomfort which may tr ...
, and other cardiopulmonary problems.
Long-term exposure to ozone has been shown to increase risk of death from
respiratory illness
Respiratory diseases, or lung diseases, are pathological conditions affecting the organs and tissues that make gas exchange difficult in air-breathing animals. They include conditions of the respiratory tract including the trachea, bronchi, bron ...
.
A study of 450,000 people living in United States cities saw a significant correlation between ozone levels and respiratory illness over the 18-year follow-up period. The study revealed that people living in cities with high ozone levels, such as Houston or Los Angeles, had an over 30% increased risk of dying from lung disease.
Air quality guidelines such as those from the
World Health Organization
The World Health Organization (WHO) is a specialized agency of the United Nations responsible for international public health. The WHO Constitution states its main objective as "the attainment by all peoples of the highest possible level of h ...
, the
United States Environmental Protection Agency
The Environmental Protection Agency (EPA) is an independent executive agency of the United States federal government tasked with environmental protection matters. President Richard Nixon proposed the establishment of EPA on July 9, 1970; it be ...
(EPA) and the
European Union
The European Union (EU) is a supranational political and economic union of member states that are located primarily in Europe. The union has a total area of and an estimated total population of about 447million. The EU has often been des ...
are based on detailed studies designed to identify the levels that can cause measurable ill
health effect
Health effects (or health impacts) are changes in health resulting from exposure to a source. Health effects are an important consideration in many areas, such as hygiene, pollution studies, occupational safety and health, ( utrition and health s ...
s.
According to scientists with the US EPA, susceptible people can be adversely affected by ozone levels as low as 40 nmol/mol.
In the EU, the current target value for ozone concentrations is 120 ┬Ąg/m
3 which is about 60 nmol/mol. This target applies to all member states in accordance with Directive 2008/50/EC. Ozone concentration is measured as a maximum daily mean of 8 hour averages and the target should not be exceeded on more than 25 calendar days per year, starting from January 2010. Whilst the directive requires in the future a strict compliance with 120 ┬Ąg/m
3 limit (i.e. mean ozone concentration not to be exceeded on any day of the year), there is no date set for this requirement and this is treated as a long-term objective.
In the US, the
Clean Air Act directs the EPA to set
National Ambient Air Quality Standards
The U.S. National Ambient Air Quality Standards (NAAQS, pronounced ) are limits on atmospheric concentration of six pollutants that cause smog, acid rain, and other health hazards. Established by the United States Environmental Protection Agency ...
for several pollutants, including ground-level ozone, and counties out of compliance with these standards are required to take steps to reduce their levels. In May 2008, under a court order, the EPA lowered its ozone standard from 80 nmol/mol to 75 nmol/mol. The move proved controversial, since the Agency's own scientists and advisory board had recommended lowering the standard to 60 nmol/mol.
Many public health and environmental groups also supported the 60 nmol/mol standard, and the
World Health Organization
The World Health Organization (WHO) is a specialized agency of the United Nations responsible for international public health. The WHO Constitution states its main objective as "the attainment by all peoples of the highest possible level of h ...
recommends 100 ┬Ąg/m
3 (51 nmol/mol).
On January 7, 2010, the U.S. Environmental Protection Agency (EPA) announced proposed revisions to the National Ambient Air Quality Standard (NAAQS) for the pollutant ozone, the principal component of smog:
... EPA proposes that the level of the 8-hour primary standard, which was set at 0.075 ╬╝mol/mol in the 2008 final rule, should instead be set at a lower level within the range of 0.060 to 0.070 ╬╝mol/mol, to provide increased protection for children and other ''at risk'' populations against an array of ŌĆō related adverse health effects that range from decreased lung function and increased respiratory symptoms to serious indicators of respiratory morbidity including emergency department visits and hospital admissions for respiratory causes, and possibly cardiovascular-related morbidity as well as total non- accidental and cardiopulmonary mortality ...
On October 26, 2015, the EPA published a final rule with an effective date of December 28, 2015 that revised the 8-hour primary NAAQS from 0.075 ppm to 0.070 ppm.
The EPA has developed an
air quality index
An air quality index (AQI) is used by government agencies to communicate to the public how polluted the air currently is or how polluted it is forecast to become. AQI information is obtained by averaging readings from an air quality sensor, whi ...
(AQI) to help explain air pollution levels to the general public. Under the current standards, eight-hour average ozone mole fractions of 85 to 104 nmol/mol are described as "unhealthy for sensitive groups", 105 nmol/mol to 124 nmol/mol as "unhealthy", and 125 nmol/mol to 404 nmol/mol as "very unhealthy".
Ozone can also be present in
indoor air pollution
Indoor air quality (IAQ) is the air quality within and around buildings and structures. IAQ is known to affect the health, comfort, and well-being of building occupants. Poor indoor air quality has been linked to sick building syndrome, reduce ...
, partly as a result of electronic equipment such as photocopiers. A connection has also been known to exist between the increased pollen, fungal spores, and ozone caused by thunderstorms and hospital admissions of
asthma
Asthma is a long-term inflammatory disease of the airways of the lungs. It is characterized by variable and recurring symptoms, reversible airflow obstruction, and easily triggered bronchospasms. Symptoms include episodes of wheezing, cou ...
sufferers.
In the
Victorian era
In the history of the United Kingdom and the British Empire, the Victorian era was the period of Queen Victoria's reign, from 20 June 1837 until her death on 22 January 1901. The era followed the Georgian period and preceded the Edwardia ...
, one British folk myth held that the smell of the sea was caused by ozone. In fact, the characteristic "smell of the sea" is caused by
dimethyl sulfide, a chemical generated by
phytoplankton
Phytoplankton () are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater ecosystems. The name comes from the Greek words (), meaning 'plant', and (), meaning 'wanderer' or 'drifter'.
Ph ...
. Victorian Britons considered the resulting smell "bracing".
Heat waves
An investigation to assess the joint mortality effects of ozone and heat during the European
heat wave
A heat wave, or heatwave, is a period of excessively hot weather, which may be accompanied by high humidity, especially in oceanic climate countries. While definitions vary, a heat wave is usually measured relative to the usual climate in the ...
s in 2003, concluded that these appear to be additive.
Physiology
Ozone, along with reactive forms of oxygen such as
superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of the ...
,
singlet oxygen
Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemical with the formula O=O (also written as or ), which is in a quantum state where all electrons are spin paired. It is kinetically unstable at ambie ...
,
hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%ŌĆ ...
, and
hypochlorite
In chemistry, hypochlorite is an anion with the chemical formula ClOŌłÆ. It combines with a number of cations to form hypochlorite salts. Common examples include sodium hypochlorite (household bleach) and calcium hypochlorite (a component of ble ...
ions, is produced by
white blood cell
White blood cells, also called leukocytes or leucocytes, are the cell (biology), cells of the immune system that are involved in protecting the body against both infectious disease and foreign invaders. All white blood cells are produced and de ...
s and other biological systems (such as the roots of
marigolds) as a means of destroying foreign bodies. Ozone reacts directly with organic double bonds. Also, when ozone breaks down to dioxygen it gives rise to oxygen
free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
s, which are highly reactive and capable of damaging many
organic molecules
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The s ...
. Moreover, it is believed that the powerful oxidizing properties of ozone may be a contributing factor of
inflammation
Inflammation (from la, wikt:en:inflammatio#Latin, inflammatio) is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or Irritation, irritants, and is a protective response involving im ...
. The cause-and-effect relationship of how the ozone is created in the body and what it does is still under consideration and still subject to various interpretations, since other body chemical processes can trigger some of the same reactions. There is evidence linking the antibody-catalyzed water-oxidation pathway of the human
immune response
An immune response is a reaction which occurs within an organism for the purpose of defending against foreign invaders. These invaders include a wide variety of different microorganisms including viruses, bacteria, parasites, and fungi which could ...
to the production of ozone. In this system, ozone is produced by antibody-catalyzed production of
trioxidane
Trioxidane (systematically named ╬╝-trioxidanediidodihydrogen), also called dihydrogen trioxide, is an inorganic compound with the chemical formula (can be written as or ). It is one of the unstable hydrogen polyoxides. In aqueous solutions, ...
from water and neutrophil-produced singlet oxygen.
When inhaled, ozone reacts with compounds lining the lungs to form specific, cholesterol-derived metabolites that are thought to facilitate the build-up and pathogenesis of
atherosclerotic plaques
Atherosclerosis is a pattern of the disease arteriosclerosis in which the wall of the artery develops abnormalities, called lesions. These lesions may lead to narrowing due to the buildup of atheromatous plaque. At onset there are usually no sy ...
(a form of
heart disease
Cardiovascular disease (CVD) is a class of diseases that involve the heart or blood vessels. CVD includes coronary artery diseases (CAD) such as angina and myocardial infarction (commonly known as a heart attack). Other CVDs include stroke, hea ...
). These metabolites have been confirmed as naturally occurring in human atherosclerotic arteries and are categorized into a class of secosterols termed ''
atheronals
Atheronals are biologically relevant oxysterols formed in the reaction of cholesterol with ozone. Atheronal A (secosterol A) is the major product of ozonolysis which is 3╬▓-hydroxy-5-oxo-5,6-secocholestan-6-al. Atheronal B (secosterol B) is forme ...
'', generated by
ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds of alkenes (), alkynes (), or azo compounds () are cleaved with ozone (). Alkenes and alkynes form organic compounds in which the multiple carbonŌĆōcarbon b ...
of cholesterol's double bond to form a
5,6 secosterol as well as a secondary condensation product via aldolization.
Impact on plant growth and crop yields
Ozone has been implicated to have an adverse effect on plant growth: "... ozone reduced total chlorophylls, carotenoid and carbohydrate concentration, and increased 1-aminocyclopropane-1-carboxylic acid (ACC) content and ethylene production. In treated plants, the ascorbate leaf pool was decreased, while lipid peroxidation and solute leakage were significantly higher than in ozone-free controls. The data indicated that ozone triggered protective mechanisms against oxidative stress in citrus." Studies that have used pepper plants as a model have shown that ozone decreased fruit yield and changed fruit quality.
Furthermore, it was also observed a decrease in chlorophylls levels and antioxidant defences on the leaves, as well as increased the reactive oxygen species (ROS) levels and lipid and protein damages.
A 2022 study concludes that East Asia loses 63 billion Dollars in crops per year due to ozone pollution, a by-product of fossile fuel combustion. China loses about one third of its potential wheat production and one fourth of its rice production.
Safety regulations
Because of the strongly oxidizing properties of ozone, ozone is a primary irritant, affecting especially the eyes and respiratory systems and can be hazardous at even low concentrations. The Canadian Centre for Occupation Safety and Health reports that:
Even very low concentrations of ozone can be harmful to the upper respiratory tract and the lungs. The severity of injury depends on both the concentration of ozone and the duration of exposure. Severe and permanent lung injury or death could result from even a very short-term exposure to relatively low concentrations."
To protect workers potentially exposed to ozone,
U.S. Occupational Safety and Health Administration
The Occupational Safety and Health Administration'' (OSHA ) is a large regulatory agency of the United States Department of Labor that originally had federal visitorial powers to inspect and examine workplaces. Congress established the agenc ...
has established a permissible exposure limit (PEL) of 0.1 ╬╝mol/mol (29 CFR 1910.1000 table Z-1), calculated as an 8-hour time weighted average. Higher concentrations are especially hazardous and
NIOSH
The National Institute for Occupational Safety and Health (NIOSH, ) is the United States federal agency responsible for conducting research and making recommendations for the prevention of work-related injury and illness. NIOSH is part of the C ...
has established an Immediately Dangerous to Life and Health Limit (IDLH) of 5 ╬╝mol/mol. Work environments where ozone is used or where it is likely to be produced should have adequate ventilation and it is prudent to have a monitor for ozone that will alarm if the concentration exceeds the OSHA PEL. Continuous monitors for ozone are available from several suppliers.
Elevated ozone exposure can occur on
passenger aircraft
An airliner is a type of aircraft for transporting passengers and air cargo. Such aircraft are most often operated by airlines. Although the definition of an airliner can vary from country to country, an airliner is typically defined as an ai ...
, with levels depending on altitude and atmospheric turbulence.
[Lai, Jennifer (2008-05-08).]
Airplane Air Heavy On The Ozone ŌĆō Daily Brief
. Portfolio.com. Retrieved on 2012-02-01. United States
Federal Aviation Administration
The Federal Aviation Administration (FAA) is the largest transportation agency of the U.S. government and regulates all aspects of civil aviation in the country as well as over surrounding international waters. Its powers include air traffic m ...
regulations set a limit of 250 nmol/mol with a maximum four-hour average of 100 nmol/mol. Some planes are equipped with ozone converters in the ventilation system to reduce passenger exposure.
Production
Ozone generators, or ozonators, are used to produce ozone for cleaning air or removing smoke odours in unoccupied rooms. These ozone generators can produce over 3 g of ozone per hour. Ozone often forms in nature under conditions where O
2 will not react.
[ Ozone used in industry is measured in ╬╝mol/mol (ppm, parts per million), nmol/mol (ppb, parts per billion), ╬╝g/m3, mg/h (milligrams per hour) or weight percent. The regime of applied concentrations ranges from 1% to 5% (in air) and from 6% to 14% (in oxygen) for older generation methods. New electrolytic methods can achieve up 20% to 30% dissolved ozone concentrations in output water.
Temperature and humidity play a large role in how much ozone is being produced using traditional generation methods (such as corona discharge and ultraviolet light). Old generation methods will produce less than 50% of nominal capacity if operated with humid ambient air, as opposed to very dry air. New generators, using electrolytic methods, can achieve higher purity and dissolution through using water molecules as the source of ozone production.
]
Coronal discharge method
 This is the most common type of ozone generator for most industrial and personal uses. While variations of the "hot spark" coronal discharge method of ozone production exist, including medical grade and industrial grade ozone generators, these units usually work by means of a corona discharge tube or ozone plate. They are typically cost-effective and do not require an oxygen source other than the ambient air to produce ozone concentrations of 3ŌĆō6%. Fluctuations in ambient air, due to weather or other environmental conditions, cause variability in ozone production. However, they also produce
This is the most common type of ozone generator for most industrial and personal uses. While variations of the "hot spark" coronal discharge method of ozone production exist, including medical grade and industrial grade ozone generators, these units usually work by means of a corona discharge tube or ozone plate. They are typically cost-effective and do not require an oxygen source other than the ambient air to produce ozone concentrations of 3ŌĆō6%. Fluctuations in ambient air, due to weather or other environmental conditions, cause variability in ozone production. However, they also produce nitrogen oxide Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:
Charge-neutral
*Nitric oxide (NO), nitrogen(II) oxide, or nitrogen monoxide
*Nitrogen dioxide (), nitrogen(IV) oxide
* Nitrogen trioxide (), or n ...
s as a by-product. Use of an air dryer
Compressed air dryers are special types of filter systems that are specifically designed to remove the water that is inherent in compressed air. The process of compressing air raises its temperature and concentrates atmospheric contaminants, primar ...
can reduce or eliminate nitric acid formation by removing water vapor and increase ozone production. At room temperature, nitric acid will form into a vapour that is hazardous if inhaled. Symptoms can include chest pain, shortness of breath, headaches and a dry nose and throat causing a burning sensation. Use of an oxygen concentrator
An oxygen concentrator is a device that concentrates the oxygen from a gas supply (typically ambient air) by selectively removing nitrogen to supply an oxygen-enriched product gas stream. They are used industrially and as medical devices for oxy ...
can further increase the ozone production and further reduce the risk of nitric acid formation by removing not only the water vapor, but also the bulk of the nitrogen.
Ultraviolet light
UV ozone generators, or vacuum-ultraviolet (VUV) ozone generators, employ a light source that generates a narrow-band ultraviolet light, a subset of that produced by the Sun. The Sun's UV sustains the ozone layer in the stratosphere of Earth.
UV ozone generators use ambient air for ozone production, no air prep systems are used (air dryer or oxygen concentrator), therefore these generators tend to be less expensive. However, UV ozone generators usually produce ozone with a concentration of about 0.5% or lower which limits the potential ozone production rate. Another disadvantage of this method is that it requires the ambient air (oxygen) to be exposed to the UV source for a longer amount of time, and any gas that is not exposed to the UV source will not be treated. This makes UV generators impractical for use in situations that deal with rapidly moving air or water streams (in-duct air sterilization, for example). Production of ozone is one of the potential dangers of ultraviolet germicidal irradiation
Ultraviolet germicidal irradiation (UVGI) is a disinfection method that uses short-wavelength ultraviolet (ultraviolet C or UV-C) light to kill or inactivate microorganisms by destroying nucleic acids and disrupting their DNA, leaving them unabl ...
. VUV ozone generators are used in swimming pools and spa
A spa is a location where mineral-rich spring water (and sometimes seawater) is used to give medicinal baths. Spa towns or spa resorts (including hot springs resorts) typically offer various health treatments, which are also known as balneoth ...
applications ranging to millions of gallons of water. VUV ozone generators, unlike corona discharge generators, do not produce harmful nitrogen by-products and also unlike corona discharge systems, VUV ozone generators work extremely well in humid air environments. There is also not normally a need for expensive off-gas mechanisms, and no need for air driers or oxygen concentrators which require extra costs and maintenance.
Cold plasma
In the cold plasma method, pure oxygen gas is exposed to a plasma
Plasma or plasm may refer to:
Science
* Plasma (physics), one of the four fundamental states of matter
* Plasma (mineral), a green translucent silica mineral
* QuarkŌĆōgluon plasma, a state of matter in quantum chromodynamics
Biology
* Blood pla ...
created by DBD. The diatomic oxygen is split into single atoms, which then recombine in triplets to form ozone.
It is common in the industry to mislabel some DBD ozone generators as CD Corona Discharge generators. Typically all solid flat metal electrode ozone generators produce ozone using the dielectric barrier discharge method.
Cold plasma machines use pure oxygen as the input source and produce a maximum concentration of about 24% ozone. They produce far greater quantities of ozone in a given time compared to ultraviolet production that has about 2% efficiency.
The discharges manifest as filamentary transfer of electrons (micro discharges) in a gap between two electrodes. In order to evenly distribute the micro discharges, a dielectric insulator must be used to separate the metallic electrodes and to prevent arcing.
Electrolytic
Electrolytic ozone generation (EOG) splits water molecules into H2, O2, and O3.
In most EOG methods, the hydrogen gas will be removed to leave oxygen and ozone as the only reaction products. Therefore, EOG can achieve higher dissolution
Dissolution may refer to:
Arts and entertainment Books
* ''Dissolution'' (''Forgotten Realms'' novel), a 2002 fantasy novel by Richard Lee Byers
* ''Dissolution'' (Sansom novel), a 2003 historical novel by C. J. Sansom Music
* Dissolution, in mu ...
in water without other competing gases found in corona discharge method, such as nitrogen gases present in ambient air. This method of generation can achieve concentrations of 20ŌĆō30% and is independent of air quality because water is used as the source material. Production of ozone electrolytically is typically unfavorable because of the high overpotential
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly relat ...
required to produce ozone as compared to oxygen. This is why ozone is not produced during typical water electrolysis. However, it is possible to increase the overpotential of oxygen by careful catalyst selection such that ozone is preferentially produced under electrolysis. Catalysts typically chosen for this approach are lead dioxide
Lead(IV) oxide is the inorganic compound with the formula PbO2. It is an oxide where lead is in an oxidation state of +4. It is a dark-brown solid which is insoluble in water. It exists in two crystalline forms. It has several important applicatio ...
or boron-doped diamond.
The ozone to oxygen ratio is improved by increasing current density at the anode, cooling the electrolyte around the anode close to 0 ┬░C, using an acidic electrolyte (such as dilute sulfuric acid) instead of a basic solution, and by applying pulsed current instead of DC.
Special considerations
Ozone cannot be stored and transported like other industrial gases (because it quickly decays into diatomic oxygen) and must therefore be produced on site. Available ozone generators vary in the arrangement and design of the high-voltage electrodes. At production capacities higher than 20 kg per hour, a gas/water tube heat-exchanger may be utilized as ground electrode and assembled with tubular high-voltage electrodes on the gas-side. The regime of typical gas pressures is around absolute in oxygen and absolute in air. Several megawatts of electrical power
Electric power is the rate at which electrical energy is transferred by an electric circuit. The SI unit of power is the watt, one joule per second. Standard prefixes apply to watts as with other SI units: thousands, millions and billions of ...
may be installed in large facilities, applied as single phase AC current
Currents, Current or The Current may refer to:
Science and technology
* Current (fluid), the flow of a liquid or a gas
** Air current, a flow of air
** Ocean current, a current in the ocean
*** Rip current, a kind of water current
** Current (stre ...
at 50 to 8000 Hz and peak voltage
Voltage, also known as electric pressure, electric tension, or (electric) potential difference, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge to m ...
s between 3,000 and 20,000 volts. Applied voltage is usually inversely related to the applied frequency.
The dominating parameter influencing ozone generation efficiency is the gas temperature, which is controlled by cooling water temperature and/or gas velocity. The cooler the water, the better the ozone synthesis. The lower the gas velocity, the higher the concentration (but the lower the net ozone produced). At typical industrial conditions, almost 90% of the effective power is dissipated as heat and needs to be removed by a sufficient cooling water flow.
Because of the high reactivity of ozone, only a few materials may be used like stainless steel
Stainless steel is an alloy of iron that is resistant to rusting and corrosion. It contains at least 11% chromium and may contain elements such as carbon, other nonmetals and metals to obtain other desired properties. Stainless steel's corros ...
(quality 316L), titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in ...
, aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
(as long as no moisture is present), glass
Glass is a non-crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenching) of ...
, polytetrafluorethylene
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene that has numerous applications. It is one of the best-known and widely applied PFAS. The commonly known brand name of PTFE-based composition is Teflon by Chemour ...
, or polyvinylidene fluoride
Polyvinylidene fluoride or polyvinylidene difluoride (PVDF) is a highly non-reactive thermoplastic fluoropolymer produced by the polymerization of vinylidene difluoride.
PVDF is a specialty plastic used in applications requiring the highest pur ...
. Viton may be used with the restriction of constant mechanical forces and absence of humidity (humidity limitations apply depending on the formulation). Hypalon
Hypalon is a chlorosulfonated polyethylene (CSPE) synthetic rubber (CSM) noted for its resistance to chemicals, temperature extremes, and ultraviolet light. It was a product of DuPont Performance Elastomers, a subsidiary of DuPont. Hypalon as it i ...
may be used with the restriction that no water comes in contact with it, except for normal atmospheric levels. Embrittlement
Embrittlement is a significant decrease of ductility of a material, which makes the material Brittleness, brittle. Embrittlement is used to describe any phenomena where the environment compromises a stressed material's mechanical performance, such ...
or shrinkage is the common mode of failure of elastomers with exposure to ozone. Ozone cracking is the common mode of failure of elastomer seals like O-rings
An O-ring, also known as a packing or a toric joint, is a mechanical gasket in the shape of a torus; it is a loop of elastomer with a round cross-section, designed to be seated in a groove and compressed during assembly between two or more par ...
.
Silicone rubber Silicone rubber is an elastomer (rubber-like material) composed of silicone—itself a polymer—containing silicon together with carbon, hydrogen, and oxygen. Silicone rubbers are widely used in industry, and there are multiple formulations ...
s are usually adequate for use as gaskets
image:gaskets.jpg, Some seals and gaskets
A gasket is a Seal (mechanical), mechanical seal which fills the space between two or more mating surfaces, generally to prevent leakage from or into the joined objects while under compression (physical) ...
in ozone concentrations below 1 wt%, such as in equipment for accelerated aging of rubber samples.
Incidental production
Ozone may be formed from by electrical discharges and by action of high energy electromagnetic radiation
In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic field, electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy. It includes radio waves, microwaves, inf ...
. Unsuppressed arcing in electrical contacts, motor brushes, or mechanical switches breaks down the chemical bonds of the atmospheric oxygen surrounding the contacts ŌåÆ 2O Free radicals of oxygen in and around the arc recombine to create ozone []. Certain electrical equipment generate significant levels of ozone. This is especially true of devices using high voltage
High voltage electricity refers to electrical potential large enough to cause injury or damage. In certain industries, ''high voltage'' refers to voltage above a certain threshold. Equipment and conductors that carry high voltage warrant spec ...
s, such as Air ioniser, ionic air purifiers, laser printers, [ hotocopiers, tasers
A taser is an electroshock weapon used to incapacitate people, allowing them to be approached and handled in an unresisting and thus safe manner. It is sold by Axon, formerly TASER International. It fires two small barbed darts intended to ...
and arc welders. Electric motor
An electric motor is an Electric machine, electrical machine that converts electrical energy into mechanical energy. Most electric motors operate through the interaction between the motor's magnetic field and electric current in a Electromagneti ...
s using brush
A brush is a common tool with bristles, wire or other filaments. It generally consists of a handle or block to which filaments are affixed in either a parallel or perpendicular orientation, depending on the way the brush is to be gripped durin ...
es can generate ozone from repeated spark
Spark commonly refers to:
* Spark (fire), a small glowing particle or ember
* Electric spark, a form of electrical discharge
Spark may also refer to:
Places
* Spark Point, a rocky point in the South Shetland Islands
People
* Spark (surname)
* ...
ing inside the unit. Large motors that use brushes, such as those used by elevators or hydraulic pumps, will generate more ozone than smaller motors.
Ozone is similarly formed in the Catatumbo lightning storms phenomenon on the Catatumbo River
The Catatumbo River ( es, R├Ło Catatumbo) is a river rising in northern Colombia, flowing into Lake Maracaibo in Venezuela. The Catatumbo River is approximately long. It forms a part of the international boundary between the two countries. The ...
in Venezuela
Venezuela (; ), officially the Bolivarian Republic of Venezuela ( es, link=no, Rep├║blica Bolivariana de Venezuela), is a country on the northern coast of South America, consisting of a continental landmass and many islands and islets in th ...
, though ozone's instability makes it dubious that it has any effect on the ozonosphere.[┬┐Rel├Īmpagos del Catatumbo regeneran la capa de ozono?](_blank)
. Agencia de noticias de la Universidad del Zulia
The University of Zulia ( es, La Universidad del Zulia, also known as LUZ literally meaning "light" in Spanish), is a public university whose main campus is located in the city of Maracaibo, Venezuela. LUZ is one of the largest and most important ...
.
It is the world's largest single natural generator of ozone, lending calls for it to be designated a UNESCO World Heritage Site
A World Heritage Site is a landmark or area with legal protection by an international convention administered by the United Nations Educational, Scientific and Cultural Organization (UNESCO). World Heritage Sites are designated by UNESCO for h ...
.
Laboratory production
 In the laboratory, ozone can be produced by
In the laboratory, ozone can be produced by electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
using a 9 volt battery
9 (nine) is the natural number following and preceding .
Evolution of the Arabic digit
In the beginning, various Indians wrote a digit 9 similar in shape to the modern closing question mark without the bottom dot. The Kshatrapa, Andhra and ...
, a pencil graphite rod cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in whi ...
, a platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
wire anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic is ...
and a 3 molar sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dis ...
. The half cell
In electrochemistry, a half-cell is a structure that contains a conductive electrode and a surrounding conductive electrolyte separated by a naturally occurring Helmholtz double layer. Chemical reactions within this layer momentarily pump electri ...
reactions taking place are:
: 3 H2O ŌåÆ O3 + 6 H+ + 6 eŌłÆ ( ╬ö''E''┬░ = ŌłÆ1.53 V)
: 6 H+ + 6 eŌłÆ ŌåÆ 3 H2 (╬ö''E''┬░ = 0 V)
: 2 H2O ŌåÆ O2 + 4 H+ + 4 eŌłÆ (╬ö''E''┬░ = 1.23 V)
In the net reaction, three equivalents of water are converted into one equivalent of ozone and three equivalents of hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
. Oxygen formation is a competing reaction.
It can also be generated by a high voltage
High voltage electricity refers to electrical potential large enough to cause injury or damage. In certain industries, ''high voltage'' refers to voltage above a certain threshold. Equipment and conductors that carry high voltage warrant spec ...
arc. In its simplest form, high voltage AC, such as the output of a neon-sign transformer
A neon-sign transformer (NST) is a transformer made for the purpose of powering a neon sign. They convert line voltage from the 120-347 V up to high voltages, in the range of 2 to 15 kV. These transformers supply between 18-30 mA; 60 mA on ...
is connected to two metal rods with the ends placed sufficiently close to each other to allow an arc. The resulting arc will convert atmospheric oxygen to ozone.
It is often desirable to contain the ozone. This can be done with an apparatus consisting of two concentric glass tubes sealed together at the top with gas ports at the top and bottom of the outer tube. The inner core should have a length of metal foil inserted into it connected to one side of the power source. The other side of the power source should be connected to another piece of foil wrapped around the outer tube. A source of dry is applied to the bottom port. When high voltage is applied to the foil leads, electricity
Electricity is the set of physical phenomena associated with the presence and motion of matter that has a property of electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described ...
will discharge between the dry dioxygen in the middle and form and which will flow out the top port. This is called a Siemen's ozoniser. The reaction can be summarized as follows:
Applications
Industry
The largest use of ozone is in the preparation of pharmaceuticals
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and rel ...
, synthetic lubricants
Synthetic oil is a lubricant consisting of chemical compounds that are artificially modified or synthesised. Synthetic lubricants can be manufactured using chemically modified petroleum components rather than whole crude oil, but can also be syn ...
, and many other commercially useful organic compounds
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The s ...
, where it is used to sever carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
-carbon bonds.bleach
Bleach is the generic name for any chemical product that is used industrially or domestically to remove color (whitening) from a fabric or fiber or to clean or to remove stains in a process called bleaching. It often refers specifically, to ...
ing substances and for killing microorganisms in air and water sources. Many municipal drinking water systems kill bacteria with ozone instead of the more common chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
. Ozone has a very high oxidation potential
Redox potential (also known as oxidation / reduction potential, ''ORP'', ''pe'', ''E_'', or E_) is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respe ...
. Ozone does not form organochlorine
An organochloride, organochlorine compound, chlorocarbon, or chlorinated hydrocarbon is an organic compound containing at least one covalent bond, covalently bonded atom of chlorine. The chloroalkane class (alkanes with one or more hydrogens subst ...
compounds, nor does it remain in the water after treatment. Ozone can form the suspected carcinogen bromate
The bromate anion, BrO, is a bromine-based oxoanion. A bromate is a chemical compound that contains this ion. Examples of bromates include sodium bromate, (), and potassium bromate, ().
Bromates are formed many different ways in municipal drinki ...
in source water with high bromide
A bromide ion is the negatively charged form (BrŌłÆ) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retardant ...
concentrations. The U.S. Safe Drinking Water Act
The Safe Drinking Water Act (SDWA) is the principal federal law in the United States intended to ensure safe drinking water for the public. Pursuant to the act, the Environmental Protection Agency (EPA) is required to set standards for drinking w ...
mandates that these systems introduce an amount of chlorine to maintain a minimum of 0.2 ╬╝mol/mol residual free chlorine
Sodium hypochlorite (commonly known in a dilute solution as bleach) is an Inorganic chemistry, inorganic chemical compound with the chemical formula, formula NaOCl (or NaClO), comprising a sodium cation () and a hypochlorite anion (or ). It may ...
in the pipes, based on results of regular testing. Where electrical power
Electric power is the rate at which electrical energy is transferred by an electric circuit. The SI unit of power is the watt, one joule per second. Standard prefixes apply to watts as with other SI units: thousands, millions and billions of ...
is abundant, ozone is a cost-effective method of treating water, since it is produced on demand and does not require transportation and storage of hazardous chemicals. Once it has decayed, it leaves no taste or odour in drinking water.
Although low levels of ozone have been advertised to be of some disinfectant use in residential homes, the concentration of ozone in dry air required to have a rapid, substantial effect on airborne pathogens exceeds safe levels recommended by the U.S. Occupational Safety and Health Administration
The Occupational Safety and Health Administration'' (OSHA ) is a large regulatory agency of the United States Department of Labor that originally had federal visitorial powers to inspect and examine workplaces. Congress established the agenc ...
and Environmental Protection Agency
A biophysical environment is a biotic and abiotic surrounding of an organism or population, and consequently includes the factors that have an influence in their survival, development, and evolution. A biophysical environment can vary in scale f ...
. Humidity control can vastly improve both the killing power of the ozone and the rate at which it decays back to oxygen (more humidity allows more effectiveness). Spore
In biology, a spore is a unit of sexual or asexual reproduction that may be adapted for dispersal and for survival, often for extended periods of time, in unfavourable conditions. Spores form part of the life cycles of many plants, algae, f ...
forms of most pathogens are very tolerant of atmospheric ozone in concentrations at which asthma patients start to have issues.
In 1908 artificial ozonisation the Central Line of the London Underground
The London Underground (also known simply as the Underground or by its nickname the Tube) is a rapid transit system serving Greater London and some parts of the adjacent ceremonial counties of England, counties of Buckinghamshire, Essex and He ...
was introduced as an aerial disinfectant. The process was found to be worthwhile, but was phased out by 1956. However the beneficial effect was maintained by the ozone created incidentally from the electrical discharges of the train motors (see above: Incidental production).
Ozone generators were made available to schools and universities in Wales for the Autumn term 2021, to disinfect classrooms after COVID-19
Coronavirus disease 2019 (COVID-19) is a contagious disease caused by a virus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The first known case was COVID-19 pandemic in Hubei, identified in Wuhan, China, in December ...
outbreaks.
Industrially, ozone is used to:
* Disinfect laundry in hospitals, food factories, care homes etc.;
* Disinfect water in place of chlorineDeodorize
Air fresheners are consumer products that typically emit fragrance and are used in homes or commercial interiors such as restrooms, foyers, hallways, vestibules and other smaller indoor areas, as well as larger areas such as hotel lobbies, auto ...
air and objects, such as after a fire. This process is extensively used in fabric restoration
* Kill bacteria on food or on contact surfaces;dairy
A dairy is a business enterprise established for the harvesting or processing (or both) of animal milk ŌĆō mostly from cows or buffaloes, but also from goats, sheep, horses, or camels ŌĆō for human consumption. A dairy is typically located on ...
plants can make effective use of dissolved ozone as a replacement to chemical sanitizers such as peracetic acid
Peracetic acid (also known as peroxyacetic acid, or PAA) is an organic compound with the formula CH3CO3H. This peroxy acid is a colorless liquid with a characteristic acrid odor reminiscent of acetic acid. It can be highly corrosive.
Peracetic a ...
, hypochlorite
In chemistry, hypochlorite is an anion with the chemical formula ClOŌłÆ. It combines with a number of cations to form hypochlorite salts. Common examples include sodium hypochlorite (household bleach) and calcium hypochlorite (a component of ble ...
or heat.
* Disinfect cooling tower
A cooling tower is a device that rejects waste heat to the atmosphere through the cooling of a coolant stream, usually a water stream to a lower temperature. Cooling towers may either use the evaporation of water to remove process heat and ...
s and control Legionella
''Legionella'' is a genus of pathogenic gram-negative bacteria that includes the species '' L. pneumophila'', causing legionellosis (all illnesses caused by ''Legionella'') including a pneumonia-type illness called Legionnaires' disease and a mi ...
with reduced chemical consumption, water bleed-off and increased performance.
* Sanitize swimming pools and spas
* Kill insects in stored grain
* Scrub yeast and mold spores from the air in food processing plants;
* Wash fresh fruits and vegetables to kill yeast, mold and bacteria;iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
, arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but ...
, hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
, nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
s, and complex organics lumped together as "colour");
* Provide an aid to flocculation
Flocculation, in the field of chemistry, is a process by which colloidal particles come out of suspension to sediment under the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The action differs from pr ...
(agglomeration of molecules, which aids in filtration, where the iron and arsenic are removed);
* Manufacture chemical compounds via chemical synthesis
* Clean and bleach fabrics (the former use is utilized in fabric restoration; the latter use is patented);
* Act as an antichlor in chlorine-based bleaching;
* Assist in processing plastics to allow adhesion of inks;
* Age rubber samples to determine the useful life of a batch of rubber;
* Eradicate water-borne parasites such as ''Giardia lamblia
''Giardia duodenalis'', also known as ''Giardia intestinalis'' and ''Giardia lamblia'', is a flagellated parasitic microorganism of the genus '' Giardia'' that colonizes the small intestine, causing a diarrheal condition known as giardiasis. ...
'' and ''Cryptosporidium
''Cryptosporidium'', sometimes informally called crypto, is a genus of apicomplexan parasitic alveolates that can cause a respiratory and gastrointestinal illness (cryptosporidiosis) that primarily involves watery diarrhea (intestinal cryptosp ...
'' in surface water treatment plants.
Ozone is a reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
in many organic reactions
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical r ...
in the laboratory and in industry. Ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds of alkenes (), alkynes (), or azo compounds () are cleaved with ozone (). Alkenes and alkynes form organic compounds in which the multiple carbonŌĆōcarbon b ...
is the cleavage of an alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbonŌĆōcarbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
to carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
compounds.
Many hospitals around the world use large ozone generators to decontaminate operating rooms between surgeries. The rooms are cleaned and then sealed airtight before being filled with ozone which effectively kills or neutralizes all remaining bacteria.
Ozone is used as an alternative to chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
or chlorine dioxide
Chlorine dioxide is a chemical compound with the formula ClO2 that exists as yellowish-green gas above 11 ┬░C, a reddish-brown liquid between 11 ┬░C and ŌłÆ59 ┬░C, and as bright orange crystals below ŌłÆ59 ┬░C. It is usually ...
in the bleaching of wood pulp
Bleaching of wood pulp is the chemical processing of wood pulp to lighten its color and whiten the pulp. The primary product of wood pulp is paper, for which whiteness (similar to, but distinct from brightness) is an important characteristic. These ...
. It is often used in conjunction with oxygen and hydrogen peroxide to eliminate the need for chlorine-containing compounds in the manufacture of high-quality, white paper
Paper is a thin sheet material produced by mechanically or chemically processing cellulose fibres derived from wood, rags, grasses or other vegetable sources in water, draining the water through fine mesh leaving the fibre evenly distributed ...
.
Ozone can be used to detoxify cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of ...
wastes (for example from gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile met ...
and silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/hŌééerŪĄ-, ''hŌééerŪĄ'': "shiny" or "white") and atomic number 47. A soft, whi ...
mining
Mining is the extraction of valuable minerals or other geological materials from the Earth, usually from an ore body, lode, vein, seam, reef, or placer deposit. The exploitation of these deposits for raw material is based on the economic via ...
) by oxidizing cyanide to cyanate
Cyanate is an anion with the structural formula , usually written . It also refers to any salt containing it, such as ammonium cyanate.
It is an isomer of the much less stable fulminate anion .William R. Martin and David W. Ball (2019): "Small ...
and eventually to carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
.
Water disinfection
Since the invention of Dielectric Barrier Discharge (DBD) plasma reactors, it has been employed for water treatment with ozone. However, with cheaper alternative disinfectants like chlorine, such applications of DBD ozone water decontamination have been limited by high power consumption and bulky equipment.
Consumers
Devices generating high levels of ozone, some of which use ionization, are used to sanitize and deodorize uninhabited buildings, rooms, ductwork, woodsheds, boats and other vehicles.
Ozonated water is used to launder clothes and to sanitize food, drinking water, and surfaces in the home. According to the U.S. Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food ...
(FDA), it is "amending the food additive
Food additives are substances added to food to preserve flavor or enhance taste, appearance, or other sensory qualities. Some additives have been used for centuries as part of an effort to preserve food, for example vinegar (pickling), salt (salt ...
regulations to provide for the safe use of ozone in gaseous and aqueous phases as an antimicrobial agent
An antimicrobial is an agent that kills microorganisms or stops their growth. Antimicrobial medicines can be grouped according to the microorganisms they act primarily against. For example, antibiotics are used against bacteria, and antifungals ar ...
on food, including meat and poultry." Studies at California Polytechnic University demonstrated that 0.3 ╬╝mol/mol levels of ozone dissolved in filtered tapwater can produce a reduction of more than 99.99% in such food-borne microorganisms as salmonella, ''E. coli'' 0157:H7 and ''Campylobacter''. This quantity is 20,000 times the WHO
Who or WHO may refer to:
* Who (pronoun), an interrogative or relative pronoun
* Who?, one of the Five Ws in journalism
* World Health Organization
Arts and entertainment Fictional characters
* Who, a creature in the Dr. Seuss book '' Horton He ...
-recommended limits stated above.pesticide
Pesticides are substances that are meant to control pests. This includes herbicide, insecticide, nematicide, molluscicide, piscicide, avicide, rodenticide, bactericide, insect repellent, animal repellent, microbicide, fungicide, and lampri ...
residues from fruit
In botany, a fruit is the seed-bearing structure in flowering plants that is formed from the ovary after flowering.
Fruits are the means by which flowering plants (also known as angiosperms) disseminate their seeds. Edible fruits in particu ...
s and vegetable
Vegetables are parts of plants that are consumed by humans or other animals as food. The original meaning is still commonly used and is applied to plants collectively to refer to all edible plant matter, including the flowers, fruits, stems, ...
s.halogens
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
. Gaseous ozone created by ultraviolet light or by corona discharge is injected into the water.
Ozone is also widely used in the treatment of water in aquariums and fishponds. Its use can minimize bacterial growth, control parasites, eliminate transmission of some diseases, and reduce or eliminate "yellowing" of the water. Ozone must not come in contact with fishes' gill structures. Natural saltwater (with life forms) provides enough "instantaneous demand" that controlled amounts of ozone activate bromide ions to hypobromous acid
Hypobromous acid is a weak, unstable acid with chemical formula of HOBr. It is mainly produced and handled in an aqueous solution. It is generated both biologically and commercially as a disinfectant. Salts of hypobromite are rarely isolated as ...
, and the ozone entirely decays in a few seconds to minutes. If oxygen-fed ozone is used, the water will be higher in dissolved oxygen and fishes' gill structures will atrophy, making them dependent on oxygen-enriched water.
Aquaculture
Ozonation ŌĆō a process of infusing water with ozone ŌĆō can be used in aquaculture to facilitate organic breakdown. Ozone is also added to recirculating systems to reduce nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
levelsnitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
. If nitrite levels in the water are high, nitrites will also accumulate in the blood and tissues of fish, where it interferes with oxygen transport (it causes oxidation of the heme-group of haemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word ╬▒ß╝Ę╬╝╬▒, ''ha├«ma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocyte ...
from ferrous () to ferric (), making haemoglobin unable to bind ).haddock
The haddock (''Melanogrammus aeglefinus'') is a saltwater ray-finned fish from the family Gadidae, the true cods. It is the only species in the monotypic genus ''Melanogrammus''. It is found in the North Atlantic Ocean and associated seas where ...
and Atlantic halibut eggs against nodavirus. Nodavirus is a lethal and vertically transmitted virus which causes severe mortality in fish. Haddock eggs should not be treated with high ozone level as eggs so treated did not hatch and died after 3ŌĆō4 days.
Agriculture
Ozone application on freshly cut pineapple and banana shows increase in flavonoids and total phenol contents when exposure is up to 20 minutes. Decrease in ascorbic acid
Vitamin C (also known as ascorbic acid and ascorbate) is a water-soluble vitamin found in citrus and other fruits and vegetables, also sold as a dietary supplement and as a topical 'serum' ingredient to treat melasma (dark pigment spots) an ...
(one form of vitamin C
Vitamin C (also known as ascorbic acid and ascorbate) is a water-soluble vitamin found in citrus and other fruits and vegetables, also sold as a dietary supplement and as a topical 'serum' ingredient to treat melasma (dark pigment spots) an ...
) content is observed but the positive effect on total phenol content and flavonoids can overcome the negative effect. Tomatoes upon treatment with ozone shows an increase in ╬▓-carotene, lutein and lycopene. However, ozone application on strawberries in pre-harvest period shows decrease in ascorbic acid content.
Ozone facilitates the extraction of some heavy metals from soil using EDTA
Ethylenediaminetetraacetic acid (EDTA) is an aminopolycarboxylic acid with the formula H2N(CH2CO2H)2sub>2. This white, water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-soluble complexes eve ...
. EDTA forms strong, water-soluble coordination compounds with some heavy metals ( Pb, Zn) thereby making it possible to dissolve them out from contaminated soil. If contaminated soil is pre-treated with ozone, the extraction efficacy of Pb, Am and Pu increases by 11.0ŌĆō28.9%, 43.5%
Unintentional Environmental Effect on Pollinators
Crop pollination is an essential part of an ecosystem. Ozone can have detrimental effects on plant-pollinator interactions.
Alternative medicine
The use of ozone for the treatment of medical conditions is not supported by high quality evidence, and is generally considered alternative medicine
Alternative medicine is any practice that aims to achieve the healing effects of medicine despite lacking biological plausibility, testability, repeatability, or evidence from clinical trials. Complementary medicine (CM), complementary and alt ...
.
See also
* Cyclic ozone
Cyclic ozone is a theoretically predicted form of ozone. Like ordinary ozone (O3), it would have three oxygen atoms. It would differ from ordinary ozone in how those three oxygen atoms are arranged. In ordinary ozone, the atoms are arranged in ...
* Global Ozone Monitoring by Occultation of Stars Global Ozone Monitoring by Occultation of Stars (GOMOS), is an instrument on board the European satellite Envisat launched 1 March 2002. It is the first space instrument dedicated to the study of the atmosphere of the Earth by the technique of s ...
(GOMOS)
* Global warming
In common usage, climate change describes global warmingŌĆöthe ongoing increase in global average temperatureŌĆöand its effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes to E ...
* Greenhouse gas
A greenhouse gas (GHG or GhG) is a gas that Absorption (electromagnetic radiation), absorbs and Emission (electromagnetic radiation), emits radiant energy within the thermal infrared range, causing the greenhouse effect. The primary greenhouse ...
* Chappuis absorption
Chappuis absorption () refers to the absorption (electromagnetic radiation), absorption of electromagnetic radiation by ozone, which is especially noticeable in the ozone layer, which absorbs a small part of sunlight in the visible spectrum, visib ...
* International Day for the Preservation of the Ozone Layer
International Day for the Preservation of the Ozone Layer (informally and simply called Ozone Day) is celebrated on September 16 designed by the United Nations General Assembly. This designation had been made on December 19, 2000, in commemorat ...
(September 16)
* Nitrogen oxides Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:
Charge-neutral
*Nitric oxide (NO), nitrogen(II) oxide, or nitrogen monoxide
*Nitrogen dioxide (), nitrogen(IV) oxide
*Nitrogen trioxide (), or ni ...
* Ozone depletion
Ozone depletion consists of two related events observed since the late 1970s: a steady lowering of about four percent in the total amount of ozone in Earth's atmosphere, and a much larger springtime decrease in stratospheric ozone (the ozone l ...
, including the phenomenon known as the ozone hole
Ozone depletion consists of two related events observed since the late 1970s: a steady lowering of about four percent in the total amount of ozone in Earth's atmosphere, and a much larger springtime decrease in stratospheric ozone (the ozone la ...
.
* Ozone therapy
Ozone therapy is an alternative medical treatment that introduces ozone or ozonides to the body. The United States Food and Drug Administration (FDA) prohibits all medical uses of ozone, "In any medical condition for which there is no proof of s ...
* Ozoneweb
* Ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds of alkenes (), alkynes (), or azo compounds () are cleaved with ozone (). Alkenes and alkynes form organic compounds in which the multiple carbonŌĆōcarbon b ...
* Polymer degradation
Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycle ...
* Sterilization (microbiology)
Sterilization refers to any process that removes, kills, or deactivates all forms of life (particularly microorganisms such as fungi, bacteria, spores, and unicellular eukaryotic organisms) and other biological agents such as prions present in or ...
Provide possible
References
Further reading
*
* Becker, K. H., U. Kogelschatz, K. H. Schoenbach, R. J. Barker (ed.). ''Non-Equilibrium Air Plasmas at Atmospheric Pressure''. Series in Plasma Physics. Bristol and Philadelphia: Institute of Physics Publishing Ltd; ; 2005
* United States Environmental Protection Agency. Risk and Benefits Group. (August 2014).
Health Risk and Exposure Assessment for Ozone: Final Report
'.
External links
International Ozone Association
European Environment Agency's near real-time ozone map (ozoneweb)
Paul Crutzen Interview
ĆöVideo of Nobel Laureate Paul Crutzen talking to Nobel Laureate Harry Kroto by the Vega Science Trust
National Institute of Environmental Health Sciences, Ozone Information
NASA Study Links "Smog" to Arctic Warming
ĆöNASA Goddard
The Goddard Space Flight Center (GSFC) is a major NASA space research laboratory located approximately northeast of Washington, D.C. in Greenbelt, Maryland, United States. Established on May 1, 1959 as NASA's first space flight center, GSFC empl ...
Institute for Space Studies (GISS) study shows the warming effect of ozone in the Arctic during winter and spring.
US EPA report questioning effectiveness or safety of ozone generators sold as air cleaners
{{Authority control
Allotropes of oxygen
Disinfectants
Environmental chemistry
Gases
Greenhouse gases
Industrial gases
Oxidizing agents
Homonuclear triatomic molecules
Gases with color
Pollution
Air pollution

 In 1785, Dutch chemist
In 1785, Dutch chemist 
 Usually ozonolysis is carried out in a solution of
Usually ozonolysis is carried out in a solution of 
 The standard way to express total ozone levels (the amount of ozone in a given vertical column) in the atmosphere is by using
The standard way to express total ozone levels (the amount of ozone in a given vertical column) in the atmosphere is by using  Ozone gas attacks any
Ozone gas attacks any 
 Ozone generators, or ozonators, are used to produce ozone for cleaning air or removing smoke odours in unoccupied rooms. These ozone generators can produce over 3 g of ozone per hour. Ozone often forms in nature under conditions where O2 will not react. Ozone used in industry is measured in ╬╝mol/mol (ppm, parts per million), nmol/mol (ppb, parts per billion), ╬╝g/m3, mg/h (milligrams per hour) or weight percent. The regime of applied concentrations ranges from 1% to 5% (in air) and from 6% to 14% (in oxygen) for older generation methods. New electrolytic methods can achieve up 20% to 30% dissolved ozone concentrations in output water.
Temperature and humidity play a large role in how much ozone is being produced using traditional generation methods (such as corona discharge and ultraviolet light). Old generation methods will produce less than 50% of nominal capacity if operated with humid ambient air, as opposed to very dry air. New generators, using electrolytic methods, can achieve higher purity and dissolution through using water molecules as the source of ozone production.
Ozone generators, or ozonators, are used to produce ozone for cleaning air or removing smoke odours in unoccupied rooms. These ozone generators can produce over 3 g of ozone per hour. Ozone often forms in nature under conditions where O2 will not react. Ozone used in industry is measured in ╬╝mol/mol (ppm, parts per million), nmol/mol (ppb, parts per billion), ╬╝g/m3, mg/h (milligrams per hour) or weight percent. The regime of applied concentrations ranges from 1% to 5% (in air) and from 6% to 14% (in oxygen) for older generation methods. New electrolytic methods can achieve up 20% to 30% dissolved ozone concentrations in output water.
Temperature and humidity play a large role in how much ozone is being produced using traditional generation methods (such as corona discharge and ultraviolet light). Old generation methods will produce less than 50% of nominal capacity if operated with humid ambient air, as opposed to very dry air. New generators, using electrolytic methods, can achieve higher purity and dissolution through using water molecules as the source of ozone production.
 This is the most common type of ozone generator for most industrial and personal uses. While variations of the "hot spark" coronal discharge method of ozone production exist, including medical grade and industrial grade ozone generators, these units usually work by means of a corona discharge tube or ozone plate. They are typically cost-effective and do not require an oxygen source other than the ambient air to produce ozone concentrations of 3ŌĆō6%. Fluctuations in ambient air, due to weather or other environmental conditions, cause variability in ozone production. However, they also produce
This is the most common type of ozone generator for most industrial and personal uses. While variations of the "hot spark" coronal discharge method of ozone production exist, including medical grade and industrial grade ozone generators, these units usually work by means of a corona discharge tube or ozone plate. They are typically cost-effective and do not require an oxygen source other than the ambient air to produce ozone concentrations of 3ŌĆō6%. Fluctuations in ambient air, due to weather or other environmental conditions, cause variability in ozone production. However, they also produce  In the laboratory, ozone can be produced by
In the laboratory, ozone can be produced by