|
Ozone Hole Recovery
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lower atmosphere to (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the latter, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation. Ozone's odour is reminiscent of chlorine, and detectable by many people at concentrations of as little as in air. Ozone's O3 structure was determined in 1865. The molecule was later proven to have a bent structure and to be weakly diamagnetic. In standard conditions, ozone is a pale blue gas that condenses at cryogenic temperatures to a dark blue liquid and finally a violet-black solid. Ozo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Tetrachloride
Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVACR) is an organic compound with the chemical formula CCl4. It is a colourless liquid with a "sweet" smell that can be detected at low levels. It is practically incombustible at lower temperatures. It was formerly widely used in fire extinguishers, as a precursor to refrigerants and as a cleaning agent, but has since been phased out because of environmental and safety concerns. Exposure to high concentrations of carbon tetrachloride (including vapor) can affect the central nervous system and degenerate the liver and kidneys. Prolonged exposure can be fatal. Properties In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedron, tetrahedral configuration joined to a central carbon atom by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Earth's Atmosphere
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing for liquid water to exist on the Earth's surface, absorbing ultraviolet solar radiation, warming the surface through heat retention (greenhouse effect), and reducing temperature extremes between day and night (the diurnal temperature variation). By mole fraction (i.e., by number of molecules), dry air contains 78.08% nitrogen, 20.95% oxygen, 0.93% argon, 0.04% carbon dioxide, and small amounts of other gases. Air also contains a variable amount of water vapor, on average around 1% at sea level, and 0.4% over the entire atmosphere. Air composition, temperature, and atmospheric pressure vary with altitude. Within the atmosphere, air suitable for use in photosynthesis by terrestrial plants and breathing of terrestrial animals is found only in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Preferred IUPAC Name
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among the possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choosing between multiple possibilities in situations where it is important to decide on a unique name. It is intended for use in legal and regulatory situations. Preferred IUPAC names are applicable only for organic compounds, to which the IUPAC has the definition as compounds which contain at least a single carbon atom but no alkali, alkaline earth or transition metals and can be named by the nomenclature of organic compounds (see below). Rules for the remaining organic and inorganic compounds are still under development. The concept of PINs is defined in the introductory chapter (freely accessible) and chapter 5 of the ''"Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013"'', which replace two former publicat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trivial Name
In chemistry, a trivial name is a nonsystematic name for a chemical substance. That is, the name is not recognized according to the rules of any formal system of chemical nomenclature such as IUPAC nomenclature of inorganic chemistry, IUPAC inorganic or IUPAC nomenclature of organic chemistry, IUPAC organic nomenclature. A trivial name is not a chemical nomenclature, formal name and is usually a common name. Generally, trivial names are not useful in describing the essential properties of the thing being named. Properties such as the molecular structure of a chemical compound are not indicated. And, in some cases, trivial names can be ambiguous or will carry different meanings in different industries or in different geographic regions (for example, a trivial name such as ''white metal'' can mean various things). Trivial names are simpler. As a result, a limited number of trivial chemical names are retained names, an accepted part of the nomenclature. Trivial names often arise in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ground Level Ozone
Ground-level ozone (O3), also known as surface-level ozone and tropospheric ozone, is a trace gas in the troposphere (the lowest level of the atmosphere of Earth, Earth's atmosphere), with an average concentration of 20–30 parts per billion by volume (ppbv), with close to 100 ppbv in polluted areas. Ozone is also an important constituent of the stratosphere, where the ozone layer (2 to 8 parts per million ozone) exists which is located between 10 and 50 kilometers above the Earth's surface. The troposphere extends from the ground up to a variable height of approximately 14 kilometers above sea level. Ozone is least concentrated in the ground layer (or planetary boundary layer) of the troposphere. Ground-level or tropospheric ozone is created by chemical reactions between NOx gases (oxides of nitrogen produced by combustion) and volatile organic compounds (VOCs). The combination of these chemicals in the presence of sunlight form ozone. Its concentration increases as height above s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidizing Agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ). In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide and the halogens. In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an Redox, oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Chemical & Engineering Data
The Journal of Chemical & Engineering Data is a peer-reviewed scientific journal, published since 1956 by the American Chemical Society. ''JCED'' is currently indexed in: Chemical Abstracts Service (CAS), SCOPUS, EBSCOhost, ProQuest, British Library, PubMed, Ovid Pūblius Ovidius Nāsō (; 20 March 43 BC – 17/18 AD), known in English as Ovid ( ), was a Roman poet who lived during the reign of Augustus. He was a contemporary of the older Virgil and Horace, with whom he is often ranked as one of the th ..., Web of Science, and SwetsWise. The current Editor is J. Ilja Siepmann. References External links * {{DEFAULTSORT:Journal Of Chemical and Engineering Data American Chemical Society academic journals Publications established in 1956 Monthly journals Chemical engineering journals English-language journals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid
Solid is one of the State of matter#Four fundamental states, four fundamental states of matter (the others being liquid, gas, and Plasma (physics), plasma). The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural rigidity and resistance to a force applied to the surface. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire available volume like a gas. The atoms in a solid are bound to each other, either in a regular geometric lattice (crystal, crystalline solids, which include metals and ordinary ice), or irregularly (an amorphous solid such as common window glass). Solids cannot be compressed with little pressure whereas gases can be compressed with little pressure because the molecules in a gas are loosely packed. The branch of physics that deals with solids is called solid-state physics, and is the main branch of condens ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, and plasma), and is the only state with a definite volume but no fixed shape. A liquid is made up of tiny vibrating particles of matter, such as atoms, held together by intermolecular bonds. Like a gas, a liquid is able to flow and take the shape of a container. Most liquids resist compression, although others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly constant density. A distinctive property of the liquid state is surface tension, leading to wetting phenomena. Water is by far the most common liquid on Earth. The density of a liquid is usually close to that of a solid, and much higher than that of a gas. Therefore, liquid and solid are both termed condensed matte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cryogenic Fuel
Cryogenic fuels are fuels that require storage at extremely low temperatures in order to maintain them in a liquid state. These fuels are used in machinery that operates in space (e.g. rockets and satellites) where ordinary fuel cannot be used, due to the very low temperatures often encountered in space, and the absence of an environment that supports combustion (on Earth, oxygen is abundant in the atmosphere, whereas human-explorable space is a vacuum where oxygen is virtually non-existent). Cryogenic fuels most often constitute liquefied gases such as liquid hydrogen. Some rocket engines use regenerative cooling, the practice of circulating their cryogenic fuel around the nozzles before the fuel is pumped into the combustion chamber and ignited. This arrangement was first suggested by Eugen Sänger in the 1940s. All engines in the Saturn V rocket that sent the first manned missions to the Moon used this design element, which is still in use today for liquid fueled engines. Qu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Standard Conditions
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union of Pure and Applied Chemistry (IUPAC) and the National Institute of Standards and Technology (NIST), although these are not universally accepted standards. Other organizations have established a variety of alternative definitions for their standard reference conditions. In chemistry, IUPAC changed its definition of standard temperature and pressure in 1982: * Until 1982, STP was defined as a temperature of 273.15 K (0 °C, 32 °F) and an absolute pressure of exactly 1 atm (101.325 kPa). * Since 1982, STP has been defined as a temperature of 273.15 K (0 °C, 32 °F) and an absolute pressure of exactly 105 Pa (100 kPa, 1 bar). STP should not be confused with the standard state comm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
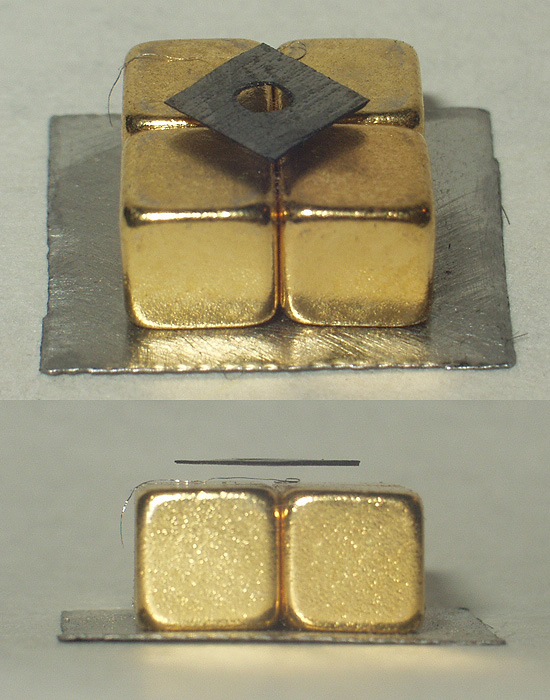
Diamagnetism
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted by a magnetic field. Diamagnetism is a quantum mechanical effect that occurs in all materials; when it is the only contribution to the magnetism, the material is called diamagnetic. In paramagnetic and ferromagnetic substances, the weak diamagnetic force is overcome by the attractive force of magnetic dipoles in the material. The magnetic permeability of diamagnetic materials is less than the permeability of vacuum, ''μ''0. In most materials, diamagnetism is a weak effect which can be detected only by sensitive laboratory instruments, but a superconductor acts as a strong diamagnet because it repels a magnetic field entirely from its interior. Diamagnetism was first discovered when Anton Brugmans observed in 1778 that bismuth was repel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |