Distilling on:
[Wikipedia]
[Google]
[Amazon]
 Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective
 Early evidence of distillation was found on
Early evidence of distillation was found on
''Economic History of Medieval India, 1200–1500''
 ]
]



 As
As
 Heating an ideal mixture of two volatile substances, A and B, with A having the higher volatility, or lower boiling point, in a batch distillation setup (such as in an apparatus depicted in the opening figure) until the mixture is boiling results in a vapor above the liquid that contains a mixture of A and B. The ratio between A and B in the vapor will be different from the ratio in the liquid. The ratio in the liquid will be determined by how the original mixture was prepared, while the ratio in the vapor will be enriched in the more volatile compound, A (due to Raoult's Law, see above). The vapor goes through the condenser and is removed from the system. This, in turn, means that the ratio of compounds in the remaining liquid is now different from the initial ratio (i.e., more enriched in B than in the starting liquid).
The result is that the ratio in the liquid mixture is changing, becoming richer in component B. This causes the boiling point of the mixture to rise, which results in a rise in the temperature in the vapor, which results in a changing ratio of A : B in the gas phase (as distillation continues, there is an increasing proportion of B in the gas phase). This results in a slowly changing ratio of A : B in the distillate.
If the difference in vapour pressure between the two components A and B is large – generally expressed as the difference in boiling points – the mixture in the beginning of the distillation is highly enriched in component A, and when component A has distilled off, the boiling liquid is enriched in component B.
Heating an ideal mixture of two volatile substances, A and B, with A having the higher volatility, or lower boiling point, in a batch distillation setup (such as in an apparatus depicted in the opening figure) until the mixture is boiling results in a vapor above the liquid that contains a mixture of A and B. The ratio between A and B in the vapor will be different from the ratio in the liquid. The ratio in the liquid will be determined by how the original mixture was prepared, while the ratio in the vapor will be enriched in the more volatile compound, A (due to Raoult's Law, see above). The vapor goes through the condenser and is removed from the system. This, in turn, means that the ratio of compounds in the remaining liquid is now different from the initial ratio (i.e., more enriched in B than in the starting liquid).
The result is that the ratio in the liquid mixture is changing, becoming richer in component B. This causes the boiling point of the mixture to rise, which results in a rise in the temperature in the vapor, which results in a changing ratio of A : B in the gas phase (as distillation continues, there is an increasing proportion of B in the gas phase). This results in a slowly changing ratio of A : B in the distillate.
If the difference in vapour pressure between the two components A and B is large – generally expressed as the difference in boiling points – the mixture in the beginning of the distillation is highly enriched in component A, and when component A has distilled off, the boiling liquid is enriched in component B.
 In simple distillation, the vapor is immediately channeled into a condenser. Consequently, the distillate is not pure but rather its composition is identical to the composition of the vapors at the given temperature and pressure. That concentration follows Raoult's law.
As a result, simple distillation is effective only when the liquid boiling points differ greatly (rule of thumb is 25 °C) or when separating liquids from non-volatile solids or oils. For these cases, the vapor pressures of the components are usually different enough that the distillate may be sufficiently pure for its intended purpose.
A cutaway schematic of a simple distillation operation is shown at right. The starting liquid 15 in the boiling flask 2 is heated by a combined hotplate and magnetic stirrer 13 via a
In simple distillation, the vapor is immediately channeled into a condenser. Consequently, the distillate is not pure but rather its composition is identical to the composition of the vapors at the given temperature and pressure. That concentration follows Raoult's law.
As a result, simple distillation is effective only when the liquid boiling points differ greatly (rule of thumb is 25 °C) or when separating liquids from non-volatile solids or oils. For these cases, the vapor pressures of the components are usually different enough that the distillate may be sufficiently pure for its intended purpose.
A cutaway schematic of a simple distillation operation is shown at right. The starting liquid 15 in the boiling flask 2 is heated by a combined hotplate and magnetic stirrer 13 via a


 Short path distillation is a distillation technique that involves the distillate travelling a short distance, often only a few centimeters, and is normally done at reduced pressure. A classic example would be a distillation involving the distillate travelling from one glass bulb to another, without the need for a condenser separating the two chambers. This technique is often used for compounds which are unstable at high temperatures or to purify small amounts of compound. The advantage is that the heating temperature can be considerably lower (at reduced pressure) than the boiling point of the liquid at standard pressure, and the distillate only has to travel a short distance before condensing. A short path ensures that little compound is lost on the sides of the apparatus. The Kugelrohr apparatus is a kind of short path distillation method which often contains multiple chambers to collect distillate fractions.
Short path distillation is a distillation technique that involves the distillate travelling a short distance, often only a few centimeters, and is normally done at reduced pressure. A classic example would be a distillation involving the distillate travelling from one glass bulb to another, without the need for a condenser separating the two chambers. This technique is often used for compounds which are unstable at high temperatures or to purify small amounts of compound. The advantage is that the heating temperature can be considerably lower (at reduced pressure) than the boiling point of the liquid at standard pressure, and the distillate only has to travel a short distance before condensing. A short path ensures that little compound is lost on the sides of the apparatus. The Kugelrohr apparatus is a kind of short path distillation method which often contains multiple chambers to collect distillate fractions.
 Large scale industrial distillation applications include both batch and continuous fractional, vacuum, azeotropic, extractive, and steam distillation. The most widely used industrial applications of continuous, steady-state fractional distillation are in petroleum refineries,
Large scale industrial distillation applications include both batch and continuous fractional, vacuum, azeotropic, extractive, and steam distillation. The most widely used industrial applications of continuous, steady-state fractional distillation are in petroleum refineries,  Industrial towers use reflux to achieve a more complete separation of products. Reflux refers to the portion of the condensed overhead liquid product from a distillation or fractionation tower that is returned to the upper part of the tower as shown in the schematic diagram of a typical, large-scale industrial distillation tower. Inside the tower, the downflowing reflux liquid provides cooling and condensation of the upflowing vapors thereby increasing the efficiency of the distillation tower. The more reflux that is provided for a given number of theoretical plates, the better the tower's separation of lower boiling materials from higher boiling materials. Alternatively, the more reflux that is provided for a given desired separation, the fewer the number of theoretical plates required.
Industrial towers use reflux to achieve a more complete separation of products. Reflux refers to the portion of the condensed overhead liquid product from a distillation or fractionation tower that is returned to the upper part of the tower as shown in the schematic diagram of a typical, large-scale industrial distillation tower. Inside the tower, the downflowing reflux liquid provides cooling and condensation of the upflowing vapors thereby increasing the efficiency of the distillation tower. The more reflux that is provided for a given number of theoretical plates, the better the tower's separation of lower boiling materials from higher boiling materials. Alternatively, the more reflux that is provided for a given desired separation, the fewer the number of theoretical plates required. 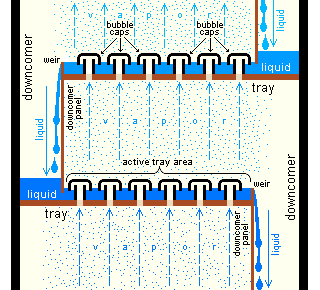 Design and operation of a distillation tower depends on the feed and desired products. Given a simple, binary component feed, analytical methods such as the
Design and operation of a distillation tower depends on the feed and desired products. Given a simple, binary component feed, analytical methods such as the  This packing material can either be random dumped packing ( wide) such as Raschig rings or structured sheet metal. Liquids tend to wet the surface of the packing and the vapors pass across this wetted surface, where
This packing material can either be random dumped packing ( wide) such as Raschig rings or structured sheet metal. Liquids tend to wet the surface of the packing and the vapors pass across this wetted surface, where
''Science and Civilisation in China''
Cambridge University Press. .
* {{Authority control Unit operations Alchemical processes Separation processes Laboratory techniques Phase transitions Gas technologies Ancient inventions
boiling
Boiling is the rapid vaporization of a liquid, which occurs when a liquid is heated to its boiling point, the temperature at which the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere. T ...
and condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
, usually inside an apparatus known as a still
A still is an apparatus used to distill liquid mixtures by heating to selectively boil and then cooling to condense the vapor. A still uses the same concepts as a basic distillation apparatus, but on a much larger scale. Stills have been u ...
. Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids); this may involve chemical changes such as destructive distillation
Destruction may refer to:
Concepts
* Destruktion, a term from the philosophy of Martin Heidegger
* Destructive narcissism, a pathological form of narcissism
* Self-destructive behaviour, a widely used phrase that ''conceptualises'' certain ki ...
or cracking. Distillation may result in essentially complete separation (resulting in nearly pure components), or it may be a partial separation that increases the concentration of selected components; in either case, the process exploits differences in the relative volatility Relative volatility is a measure comparing the vapor pressures of the components in a liquid mixture of chemicals. This quantity is widely used in designing large industrial distillation processes. In effect, it indicates the ease or difficulty of u ...
of the mixture's components. In industrial applications, distillation is a unit operation of practically universal importance, but is a physical separation process, not a chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and break ...
. An installation used for distillation, especially of distilled beverages
Liquor (or a spirit) is an alcoholic drink produced by distillation of grains, fruits, vegetables, or sugar, that have already gone through alcoholic fermentation. Other terms for liquor include: spirit drink, distilled beverage or hard ...
, is a distillery. Distillation includes the following applications:
* The distillation of fermented
Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food p ...
products produces distilled beverages with a high alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
content, or separates other fermentation products of commercial value.
* Distillation is an effective and traditional method of desalination
Desalination is a process that takes away mineral components from saline water. More generally, desalination refers to the removal of salts and minerals from a target substance, as in soil desalination, which is an issue for agriculture. Salt ...
.
* In the petroleum industry
The petroleum industry, also known as the oil industry or the oil patch, includes the global processes of hydrocarbon exploration, exploration, extraction of petroleum, extraction, oil refinery, refining, Petroleum transport, transportation (of ...
, oil stabilization is a form of partial distillation that reduces the vapor pressure of crude oil, thereby making it safe for storage and transport as well as reducing the atmospheric emissions of volatile hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
. In midstream operations at oil refineries
An oil refinery or petroleum refinery is an industrial process plant where petroleum (crude oil) is transformed and refined into useful products such as gasoline (petrol), diesel fuel, asphalt base, fuel oils, heating oil, kerosene, liquef ...
, fractional distillation is a major class of operation
Operation or Operations may refer to:
Arts, entertainment and media
* ''Operation'' (game), a battery-operated board game that challenges dexterity
* Operation (music), a term used in musical set theory
* ''Operations'' (magazine), Multi-Man ...
for transforming crude oil into fuel
A fuel is any material that can be made to react with other substances so that it releases energy as thermal energy or to be used for work. The concept was originally applied solely to those materials capable of releasing chemical energy bu ...
s and chemical feed stocks.
* Cryogenic
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of “cryogenics” and “cr ...
distillation leads to the separation of air into its components – notably oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ...
, nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seve ...
, and argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as a ...
– for industrial use.
* In the chemical industry, large amounts of crude liquid products of chemical synthesis
As a topic of chemistry, chemical synthesis (or combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In mo ...
are distilled to separate them, either from other products, from impurities, or from unreacted starting materials.
History
 Early evidence of distillation was found on
Early evidence of distillation was found on Akkadian Akkadian or Accadian may refer to:
* Akkadians, inhabitants of the Akkadian Empire
* Akkadian language, an extinct Eastern Semitic language
* Akkadian literature, literature in this language
* Akkadian cuneiform, early writing system
* Akkadian myt ...
tablets dated c. 1200 BCE describing perfumery operations. The tablets provided textual evidence that an early primitive form of distillation was known to the Babylonia
Babylonia (; Akkadian: , ''māt Akkadī'') was an ancient Akkadian-speaking state and cultural area based in the city of Babylon in central-southern Mesopotamia (present-day Iraq and parts of Syria). It emerged as an Amorite-ruled state ...
ns of ancient Mesopotamia
Mesopotamia ''Mesopotamíā''; ar, بِلَاد ٱلرَّافِدَيْن or ; syc, ܐܪܡ ܢܗܪ̈ܝܢ, or , ) is a historical region of Western Asia situated within the Tigris–Euphrates river system, in the northern part of the F ...
. Early evidence of distillation was also found related to alchemists working in Alexandria
Alexandria ( or ; ar, ٱلْإِسْكَنْدَرِيَّةُ ; grc-gre, Αλεξάνδρεια, Alexándria) is the second largest city in Egypt, and the largest city on the Mediterranean coast. Founded in by Alexander the Great, Alexandr ...
in Roman Egypt in the 1st century CE.
Distillation was practiced in the ancient Indian subcontinent
The Indian subcontinent is a physiographical region in Southern Asia. It is situated on the Indian Plate, projecting southwards into the Indian Ocean from the Himalayas. Geopolitically, it includes the countries of Bangladesh, Bhutan, India ...
, which is evident from baked clay retort
In a chemistry laboratory, a retort is a device used for distillation or dry distillation of substances. It consists of a spherical vessel with a long downward-pointing neck. The liquid to be distilled is placed in the vessel and heated. Th ...
s and receivers found at Taxila
Taxila or Takshashila (; sa, तक्षशिला; pi, ; , ; , ) is a city in Punjab, Pakistan. Located in the Taxila Tehsil of Rawalpindi District, it lies approximately northwest of the Islamabad–Rawalpindi metropolitan area ...
, Shaikhan Dheri
Pushkalavati ( ps, پشکلاوتي; Urdu: ; Sanskrit: ; Prākrit: ; grc, Πευκελαῶτις ) or Pushkaravati (Sanskrit: ; Pāli: ), and later Shaikhan Dheri ( ps, شېخان ډېرۍ; ur, ), was the capital of the Gandhara kingdom, ...
, Charsadda
Chārsadda ( ps, چارسده; ; ur, ; ) is a town and headquarters of Charsadda District, in the Khyber Pakhtunkhwa province of Pakistan.
in Pakistan
Pakistan ( ur, ), officially the Islamic Republic of Pakistan ( ur, , label=none), is a country in South Asia. It is the world's List of countries and dependencies by population, fifth-most populous country, with a population of almost 24 ...
and Rang Mahal in India
India, officially the Republic of India ( Hindi: ), is a country in South Asia. It is the seventh-largest country by area, the second-most populous country, and the most populous democracy in the world. Bounded by the Indian Ocean on the ...
dating to the early centuries of the Common Era
Common Era (CE) and Before the Common Era (BCE) are year notations for the Gregorian calendar (and its predecessor, the Julian calendar), the world's most widely used calendar era. Common Era and Before the Common Era are alternatives to the ...
. Allchin notes these terracotta distill tubes were "made to imitate bamboo". These "Gandhara
Gandhāra is the name of an ancient region located in the northwestern region of the Indian subcontinent, more precisely in present-day north-west Pakistan and parts of south-east Afghanistan. The region centered around the Peshawar Val ...
stills" were only capable of producing very weak liquor
Liquor (or a spirit) is an alcoholic drink produced by distillation of grains, fruits, vegetables, or sugar, that have already gone through alcoholic fermentation. Other terms for liquor include: spirit drink, distilled beverage or ha ...
, as there was no efficient means of collecting the vapors at low heat. Habib, Irfan (2011)''Economic History of Medieval India, 1200–1500''
Pearson Education
Pearson Education is a British-owned education publishing and assessment service to schools and corporations, as well for students directly. Pearson owns educational media brands including Addison–Wesley, Peachpit, Prentice Hall, eColleg ...
. p. 55. Distilled water
Distilled water is water that has been boiled into vapor and condensed back into liquid in a separate container. Impurities in the original water that do not boil below or near the boiling point of water remain in the original container. Thus, dis ...
has been in use since at least c. 200 CE, when Alexander of Aphrodisias
Alexander of Aphrodisias ( grc-gre, Ἀλέξανδρος ὁ Ἀφροδισιεύς, translit=Alexandros ho Aphrodisieus; AD) was a Peripatetic philosopher and the most celebrated of the Ancient Greek commentators on the writings of Aristotle ...
described the process. Work on distilling other liquids continued in early Byzantine Egypt
, conventional_long_name = Roman Egypt
, common_name = Egypt
, subdivision = Province
, nation = the Roman Empire
, era = Late antiquity
, capital = Alexandria
, title_leader = Praefectus Augustalis
, image_map = Roman E ...
under Zosimus of Panopolis in the 3rd century.
Distillation in China may have begun during the Eastern Han
The Han dynasty (, ; ) was an imperial dynasty of China (202 BC – 9 AD, 25–220 AD), established by Liu Bang (Emperor Gao) and ruled by the House of Liu. The dynasty was preceded by the short-lived Qin dynasty (221–207 BC) and a wa ...
dynasty (1st–2nd centuries CE), but the distillation of beverages began in the Jin (12th–13th centuries) and Southern Song
The Song dynasty (; ; 960–1279) was an imperial dynasty of China that began in 960 and lasted until 1279. The dynasty was founded by Emperor Taizu of Song following his usurpation of the throne of the Later Zhou. The Song conquered the res ...
(10th–13th centuries) dynasties, according to archaeological evidence.
Medieval Muslim chemists such as Jābir ibn Ḥayyān (Latin: Geber, ninth century) and Abū Bakr al-Rāzī
Abū Bakr al-Rāzī (full name: ar, أبو بکر محمد بن زکریاء الرازي, translit=Abū Bakr Muḥammad ibn Zakariyyāʾ al-Rāzī, label=none), () rather than ar, زکریاء, label=none (), as for example in , or in . In m ...
(Latin: Rhazes, ) experimented extensively with the distillation of various substances.
The distillation of wine is attested in Arabic works attributed to al-Kindī (c. 801–873 CE) and to al-Fārābī
Abu Nasr Muhammad Al-Farabi ( fa, ابونصر محمد فارابی), ( ar, أبو نصر محمد الفارابي), known in the West as Alpharabius; (c. 872 – between 14 December, 950 and 12 January, 951)PDF version was a renowned early Isla ...
(c. 872–950), and in the 28th book of al-Zahrāwī's (Latin: Abulcasis, 936–1013) ''Kitāb al-Taṣrīf'' (later translated into Latin as ''Liber servatoris''). In the twelfth century, recipes for the production of ''aqua ardens'' ("burning water", i.e., ethanol) by distilling wine with salt started to appear in a number of Latin works, and by the end of the thirteenth century it had become a widely known substance among Western European chemists. The works of Taddeo Alderotti
Taddeo Alderotti (Latin: Thaddaeus Alderottus, French : Thaddée de Florence), born in Florence between 1206 and 1215, died in 1295, was an Italian doctor and professor of medicine at the University of Bologna, who made important contributions t ...
(1223–1296) describe a method for concentrating alcohol involving repeated distillation through a water-cooled still, by which an alcohol purity of 90% could be obtained.
The fractional distillation of organic substances plays an important role in the works attributed to Jābir ibn Ḥayyān, such as in the ('The Book of Seventy'), translated into Latin by Gerard of Cremona
Gerard of Cremona (Latin: ''Gerardus Cremonensis''; c. 1114 – 1187) was an Italian translator of scientific books from Arabic into Latin. He worked in Toledo, Kingdom of Castile and obtained the Arabic books in the libraries at Toledo. Some of ...
(c. 1114–1187) under the title . The Jabirian experiments with fractional distillation of animal and vegetable substances, and to a lesser degree also of mineral substances, is the main topic of the , an originally Arabic work falsely attributed to Avicenna
Ibn Sina ( fa, ابن سینا; 980 – June 1037 CE), commonly known in the West as Avicenna (), was a Persian polymath who is regarded as one of the most significant physicians, astronomers, philosophers, and writers of the Islam ...
that was translated into Latin and would go on to form the most important alchemical source for Roger Bacon ().
A still was found in an archaeological site in Qinglong, Hebei
Hebei or , (; alternately Hopeh) is a northern province of China. Hebei is China's sixth most populous province, with over 75 million people. Shijiazhuang is the capital city. The province is 96% Han Chinese, 3% Manchu, 0.8% Hui, and ...
province, in China, dating back to the 12th century. Distilled beverages were common during the Yuan dynasty
The Yuan dynasty (), officially the Great Yuan (; xng, , , literally "Great Yuan State"), was a Mongols, Mongol-led Dynasties in Chinese history, imperial dynasty of China and a successor state to the Mongol Empire after Division of the M ...
(13th–14th centuries).
In 1500, German alchemist Hieronymus Braunschweig
Hieronymus Brunschwig or Hieronymus Brunschwygk (c. 1450c. 1512) was a German surgeon ("Wundarzt"), alchemist and botanist. He was notable for his methods of treatment of gunshot wounds and for his early work on distillation techniques. His mos ...
published ''Liber de arte destillandi'' (''The Book of the Art of Distillation''), the first book solely dedicated to the subject of distillation, followed in 1512 by a much expanded version. In 1651, John French published ''The Art of Distillation'', the first major English compendium on the practice, but it has been claimed that much of it derives from Braunschweig's work. This includes diagrams with people in them showing the industrial rather than bench scale of the operation.
 ]
]



 As
As alchemy
Alchemy (from Arabic: ''al-kīmiyā''; from Ancient Greek: χυμεία, ''khumeía'') is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practiced in China, India, the Muslim world ...
evolved into the science of chemistry, vessels called retort
In a chemistry laboratory, a retort is a device used for distillation or dry distillation of substances. It consists of a spherical vessel with a long downward-pointing neck. The liquid to be distilled is placed in the vessel and heated. Th ...
s became used for distillations. Both alembic
An alembic (from ar, الإنبيق, al-inbīq, originating from grc, ἄμβιξ, ambix, 'cup, beaker') is an alchemical still consisting of two vessels connected by a tube, used for distillation of liquids.
Description
The complete di ...
s and retorts are forms of glassware
upTypical drinkware
The list of glassware includes drinking vessels (drinkware) and tableware used to set a table for eating a meal, general glass items such as vases, and glasses used in the catering industry. It does not include laboratory glas ...
with long necks pointing to the side at a downward angle to act as air-cooled condensers to condense the distillate and let it drip downward for collection. Later, copper alembics were invented. Riveted joints were often kept tight by using various mixtures, for instance a dough made of rye flour. These alembics often featured a cooling system around the beak, using cold water, for instance, which made the condensation of alcohol more efficient. These were called pot still
A pot still is a type of distillation apparatus or still used to distill liquors such as whisky or brandy. In modern (post-1850s) practice, they are not used to produce rectified spirit, because they do not separate congeners from ethanol a ...
s. Today, the retorts and pot stills have been largely supplanted by more efficient distillation methods in most industrial processes. However, the pot still is still widely used for the elaboration of some fine alcohols, such as cognac
Cognac ( , also , ) is a variety of brandy named after the commune of Cognac, France. It is produced in the surrounding wine-growing region in the departments of Charente and Charente-Maritime.
Cognac production falls under French appellat ...
, Scotch whisky
Scotch whisky (; sco, Scots whisky/whiskie, whusk(e)y; often simply called whisky or Scotch) is malt whisky or grain whisky (or a blend of the two), made in Scotland.
All Scotch whisky was originally made from malted barley. Commercial dist ...
, Irish whiskey
Irish whiskey ( ga, Fuisce or ''uisce beatha'') is whiskey made on the island of Ireland. The word 'whiskey' (or whisky) comes from the Irish , meaning ''water of life''. Irish whiskey was once the most popular spirit in the world, though a lo ...
, tequila
Tequila (; ) is a distilled beverage made from the blue agave plant, primarily in the area surrounding the city of Tequila northwest of Guadalajara, and in the Jaliscan Highlands (''Los Altos de Jalisco'') of the central western Mexican state ...
, rum, cachaça
''Cachaça'' () is a distilled spirit made from fermented sugarcane juice. Also known as ''pinga'', ''caninha'', and other names, it is the most popular spirit among distilled alcoholic beverages in Brazil.Cavalcante, Messias Soares. Todos os n ...
, and some vodka
Vodka ( pl, wódka , russian: водка , sv, vodka ) is a clear distilled alcoholic beverage. Different varieties originated in Poland, Russia, and Sweden. Vodka is composed mainly of water and ethanol but sometimes with traces of impuriti ...
s. Pot stills made of various materials (wood, clay, stainless steel) are also used by bootleggers in various countries. Small pot stills are also sold for use in the domestic production of flower water or essential oils
An essential oil is a concentrated hydrophobic liquid containing volatile (easily evaporated at normal temperatures) chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the o ...
.
Early forms of distillation involved batch processes using one vaporization and one condensation. Purity was improved by further distillation of the condensate. Greater volumes were processed by simply repeating the distillation. Chemists reportedly carried out as many as 500 to 600 distillations in order to obtain a pure compound.Othmer, D. F. (1982) "Distillation – Some Steps in its Development", in W. F. Furter (ed) ''A Century of Chemical Engineering''.
In the early 19th century, the basics of modern techniques, including pre-heating and reflux, were developed. In 1822, Anthony Perrier developed one of the first continuous stills, and then, in 1826, Robert Stein improved that design to make his patent still. In 1830, Aeneas Coffey got a patent for improving the design even further. Coffey's continuous still may be regarded as the archetype
The concept of an archetype (; ) appears in areas relating to behavior, historical psychology, and literary analysis.
An archetype can be any of the following:
# a statement, pattern of behavior, prototype, "first" form, or a main model that ...
of modern petrochemical units. The French engineer Armand Savalle developed his steam regulator around 1846. In 1877, Ernest Solvay was granted a U.S. Patent for a tray column for ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogeno ...
distillation, and the same and subsequent years saw developments in this theme for oils and spirits.
With the emergence of chemical engineering
Chemical engineering is an engineering field which deals with the study of operation and design of chemical plants as well as methods of improving production. Chemical engineers develop economical commercial processes to convert raw materials in ...
as a discipline at the end of the 19th century, scientific rather than empirical methods could be applied. The developing petroleum
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crude ...
industry in the early 20th century provided the impetus for the development of accurate design methods, such as the McCabe–Thiele method
The McCabe–Thiele method is a chemical engineering technique for the analysis of binary distillation. It uses the fact that the composition at each theoretical tray (or equilibrium stage) is completely determined by the mole fraction of one of ...
by Ernest Thiele and the Fenske equation. The first industrial plant in the United States to use distillation as a means of ocean desalination opened in Freeport, Texas
Freeport is a city in Brazoria County, Texas, United States, located on the Gulf of Mexico. According to the 2020 census, the city population was 10,696, down from 12,049 in 2010.
History
Freeport was founded as a European-American settlement ...
in 1961 with the hope of bringing water security
Water security is the focused goal of water policy and water management. A society with a high level of water security makes the most of water's benefits for humans and ecosystems and limits the risk of destructive impacts associated with water. T ...
to the region.
The availability of powerful computers has allowed direct computer simulation
Computer simulation is the process of mathematical modelling, performed on a computer, which is designed to predict the behaviour of, or the outcome of, a real-world or physical system. The reliability of some mathematical models can be dete ...
s of distillation columns.
Applications
The application of distillation can roughly be divided into four groups: laboratory scale, industrial distillation, distillation of herbs for perfumery and medicinals ( herbal distillate), andfood processing
Food processing is the transformation of agricultural products into food, or of one form of food into other forms. Food processing includes many forms of processing foods, from grinding grain to make raw flour to home cooking to complex industr ...
. The latter two are distinctively different from the former two in that distillation is not used as a true purification method but more to transfer all volatiles
Volatiles are the group of chemical elements and chemical compounds that can be readily vaporized. In contrast with volatiles, elements and compounds that are not readily vaporized are known as refractory substances.
On planet Earth, the term ...
from the source materials to the distillate in the processing of beverages and herbs.
The main difference between laboratory scale distillation and industrial distillation are that laboratory scale distillation is often performed on a batch basis, whereas industrial distillation often occurs continuously. In batch distillation, the composition of the source material, the vapors of the distilling compounds, and the distillate change during the distillation. In batch distillation, a still is charged (supplied) with a batch of feed mixture, which is then separated into its component fractions, which are collected sequentially from most volatile to less volatile, with the bottoms – remaining least or non-volatile fraction – removed at the end. The still can then be recharged and the process repeated.
In continuous distillation
Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously (without interruption) fed into the process and separated fractions are removed continuously as output streams. Distillation is the sepa ...
, the source materials, vapors, and distillate are kept at a constant composition by carefully replenishing the source material and removing fractions from both vapor and liquid in the system. This results in a more detailed control of the separation process.
Idealized model
The boiling point of a liquid is the temperature at which thevapor pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phase ...
of the liquid equals the pressure around the liquid, enabling bubbles to form without being crushed. A special case is the normal boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
, where the vapor pressure of the liquid equals the ambient atmospheric pressure
Atmospheric pressure, also known as barometric pressure (after the barometer), is the pressure within the atmosphere of Earth. The standard atmosphere (symbol: atm) is a unit of pressure defined as , which is equivalent to 1013.25 millibar ...
.
It is a misconception that in a liquid mixture at a given pressure, each component boils at the boiling point corresponding to the given pressure, allowing the vapors of each component to collect separately and purely. However, this does not occur, even in an idealized system. Idealized models of distillation are essentially governed by Raoult's law and Dalton's law and assume that vapor–liquid equilibria are attained.
Raoult's law states that the vapor pressure of a solution is dependent on 1) the vapor pressure of each chemical component in the solution and 2) the fraction of solution each component makes up, a.k.a. the mole fraction
In chemistry, the mole fraction or molar fraction (''xi'' or ) is defined as unit of the amount of a constituent (expressed in moles), ''ni'', divided by the total amount of all constituents in a mixture (also expressed in moles), ''n''tot. This ...
. This law applies to ideal solution
In chemistry, an ideal solution or ideal mixture is a solution that exhibits thermodynamic properties analogous to those of a mixture of ideal gases. The enthalpy of mixing is zero as is the volume change on mixing by definition; the closer to zero ...
s, or solutions that have different components but whose molecular interactions are the same as or very similar to pure solutions.
Dalton's law states that the total pressure is the sum of the partial pressures of each individual component in the mixture. When a multi-component liquid is heated, the vapor pressure of each component will rise, thus causing the total vapor pressure to rise. When the total vapor pressure reaches the pressure surrounding the liquid, boiling
Boiling is the rapid vaporization of a liquid, which occurs when a liquid is heated to its boiling point, the temperature at which the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere. T ...
occurs and liquid turns to gas throughout the bulk of the liquid. A mixture with a given composition has one boiling point at a given pressure when the components are mutually soluble. A mixture of constant composition does not have multiple boiling points.
An implication of one boiling point is that lighter components never cleanly "boil first". At boiling point, all volatile components boil, but for a component, its percentage in the vapor is the same as its percentage of the total vapor pressure. Lighter components have a higher partial pressure and, thus, are concentrated in the vapor, but heavier volatile components also have a (smaller) partial pressure and necessarily vaporize also, albeit at a lower concentration in the vapor. Indeed, batch distillation and fractionation succeed by varying the composition of the mixture. In batch distillation, the batch vaporizes, which changes its composition; in fractionation, liquid higher in the fractionation column contains more lights and boils at lower temperatures. Therefore, starting from a given mixture, it appears to have a boiling range instead of a boiling point, although this is because its composition changes: each intermediate mixture has its own, singular boiling point.
The idealized model is accurate in the case of chemically similar liquids, such as benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen ato ...
and toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) ...
. In other cases, severe deviations from Raoult's law and Dalton's law are observed, most famously in the mixture of ethanol and water. These compounds, when heated together, form an azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be altered or changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This ...
, which is when the vapor phase and liquid phase contain the same composition. Although there are computational methods that can be used to estimate the behavior of a mixture of arbitrary components, the only way to obtain accurate vapor–liquid equilibrium
In thermodynamics and chemical engineering, the vapor–liquid equilibrium (VLE) describes the distribution of a chemical species between the vapor phase and a liquid phase.
The concentration of a vapor in contact with its liquid, especially a ...
data is by measurement.
It is not possible to completely purify a mixture of components by distillation, as this would require each component in the mixture to have a zero partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal g ...
. If ultra-pure products are the goal, then further chemical separation
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., wit ...
must be applied. When a binary mixture is vaporized and the other component, e.g., a salt, has zero partial pressure for practical purposes, the process is simpler.
Batch or differential distillation
Continuous distillation
Continuous distillation is an ongoing distillation in which a liquid mixture is continuously (without interruption) fed into the process and separated fractions are removed continuously as output streams occur over time during the operation. Continuous distillation produces a minimum of two output fractions, including at least one volatile distillate fraction, which has boiled and been separately captured as a vapor and then condensed to a liquid. There is always a bottoms (or residue) fraction, which is the least volatile residue that has not been separately captured as a condensed vapor. Continuous distillation differs from batch distillation in the respect that concentrations should not change over time. Continuous distillation can be run at asteady state
In systems theory, a system or a process is in a steady state if the variables (called state variables) which define the behavior of the system or the process are unchanging in time. In continuous time, this means that for those properties ' ...
for an arbitrary amount of time. For any source material of specific composition, the main variables that affect the purity of products in continuous distillation are the reflux ratio and the number of theoretical equilibrium stages, in practice determined by the number of trays or the height of packing. Reflux is a flow from the condenser back to the column, which generates a recycle that allows a better separation with a given number of trays. Equilibrium stages are ideal steps where compositions achieve vapor–liquid equilibrium, repeating the separation process and allowing better separation given a reflux ratio. A column with a high reflux ratio may have fewer stages, but it refluxes a large amount of liquid, giving a wide column with a large holdup. Conversely, a column with a low reflux ratio must have a large number of stages, thus requiring a taller column.
General improvements
Both batch and continuous distillations can be improved by making use of afractionating column
A fractionating column or fractional column is an essential item used in the distillation of liquid mixtures to separate the mixture into its component parts, or fractions, based on the differences in volatilities. Fractionating columns are used in ...
on top of the distillation flask. The column improves separation by providing a larger surface area for the vapor and condensate to come into contact. This helps it remain at equilibrium for as long as possible. The column can even consist of small subsystems ('trays' or 'dishes') which all contain an enriched, boiling liquid mixture, all with their own vapor–liquid equilibrium.
There are differences between laboratory-scale and industrial-scale fractionating columns, but the principles are the same. Examples of laboratory-scale fractionating columns (in increasing efficiency) include
* Air condenser
* Vigreux column (usually laboratory scale only)
* Packed column
In chemical processing, a packed bed is a hollow tube, pipe, or other vessel that is filled with a packing material. The packing can be randomly filled with small objects like Raschig rings or else it can be a specifically designed structured ...
(packed with glass beads, metal pieces, or other chemically inert material)
* Spinning band distillation
Spinning band distillation is a technique used to separate liquid mixtures which are similar in boiling points. When liquids with similar boiling points are distilled, the vapors are mixtures, and not pure compounds. Fractionating columns help sepa ...
system.
Laboratory procedures
Laboratory scale distillations are almost exclusively run as batch distillations. The device used in distillation, sometimes referred to as a ''still
A still is an apparatus used to distill liquid mixtures by heating to selectively boil and then cooling to condense the vapor. A still uses the same concepts as a basic distillation apparatus, but on a much larger scale. Stills have been u ...
'', consists at a minimum of a reboiler or ''pot'' in which the source material is heated, a condenser in which the heated vapor
In physics, a vapor (American English) or vapour (British English and Canadian English; see spelling differences) is a substance in the gas phase at a temperature lower than its critical temperature,R. H. Petrucci, W. S. Harwood, and F. G. H ...
is cooled back to the liquid state
State may refer to:
Arts, entertainment, and media Literature
* ''State Magazine'', a monthly magazine published by the U.S. Department of State
* ''The State'' (newspaper), a daily newspaper in Columbia, South Carolina, United States
* '' Our ...
, and a receiver in which the concentrated or purified liquid, called the distillate, is collected. Several laboratory scale techniques for distillation exist (see also distillation types).
A completely sealed distillation apparatus could experience extreme and rapidly varying internal pressure, which could cause it to burst open at the joints. Therefore, some path is usually left open (for instance, at the receiving flask) to allow the internal pressure to equalize with atmospheric pressure. Alternatively, a vacuum pump
A vacuum pump is a device that draws gas molecules from a sealed volume in order to leave behind a partial vacuum. The job of a vacuum pump is to generate a relative vacuum within a capacity. The first vacuum pump was invented in 1650 by Otto ...
may be used to keep the apparatus at a lower than atmospheric pressure. If the substances involved are air- or moisture-sensitive, the connection to the atmosphere can be made through one or more drying tubes packed with materials that scavenge the undesired air components, or through bubbler
A drinking fountain, also called a water fountain or water bubbler, is a fountain designed to provide drinking water. It consists of a basin with either continuously running water or a tap. The drinker bends down to the stream of water and ...
s that provide a movable liquid barrier. Finally, the entry of undesired air components can be prevented by pumping a low but steady flow of suitable inert gas, like nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seve ...
, into the apparatus.
Simple distillation
silicone oil
A silicone oil is any liquid polymerized siloxane with organic side chains. The most important member is polydimethylsiloxane. These polymers are of commercial interest because of their relatively high thermal stability, lubricating, and Liquid di ...
bath (orange, 14). The vapor flows through a short Vigreux column 3, then through a Liebig condenser 5, is cooled by water (blue) that circulates through ports 6 and 7. The condensed liquid drips into the receiving flask 8, sitting in a cooling bath (blue, 16). The adapter 10 has a connection 9 that may be fitted to a vacuum pump. The components are connected by ground glass joints.
Fractional distillation
For many cases, the boiling points of the components in the mixture will be sufficiently close that Raoult's law must be taken into consideration. Therefore, fractional distillation must be used in order to separate the components by repeated vaporization-condensation cycles within a packed fractionating column. This separation, by successive distillations, is also referred to as rectification. As the solution to be purified is heated, its vapors rise to thefractionating column
A fractionating column or fractional column is an essential item used in the distillation of liquid mixtures to separate the mixture into its component parts, or fractions, based on the differences in volatilities. Fractionating columns are used in ...
. As it rises, it cools, condensing on the condenser walls and the surfaces of the packing material. Here, the condensate continues to be heated by the rising hot vapors; it vaporizes once more. However, the composition of the fresh vapors are determined once again by Raoult's law. Each vaporization-condensation cycle (called a '' theoretical plate'') will yield a purer solution of the more volatile component. In reality, each cycle at a given temperature does not occur at exactly the same position in the fractionating column; ''theoretical plate'' is thus a concept rather than an accurate description.
More theoretical plates lead to better separations. A spinning band distillation
Spinning band distillation is a technique used to separate liquid mixtures which are similar in boiling points. When liquids with similar boiling points are distilled, the vapors are mixtures, and not pure compounds. Fractionating columns help sepa ...
system uses a spinning band of Teflon
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene that has numerous applications. It is one of the best-known and widely applied PFAS. The commonly known brand name of PTFE-based composition is Teflon by Chem ...
or metal to force the rising vapors into close contact with the descending condensate, increasing the number of theoretical plates.
Steam distillation
Likevacuum distillation
Vacuum distillation is distillation performed under reduced pressure, which allows the purification of compounds not readily distilled at ambient pressures or simply to save time or energy. This technique separates compounds based on differences i ...
, steam distillation is a method for distilling compounds which are heat-sensitive. The temperature of the steam is easier to control than the surface of a heating element
A heating element converts electrical energy into heat through the process of Joule heating. Electric current through the element encounters resistance, resulting in heating of the element. Unlike the Peltier effect, this process is independe ...
, and allows a high rate of heat transfer without heating at a very high temperature. This process involves bubbling steam through a heated mixture of the raw material. By Raoult's law, some of the target compound will vaporize (in accordance with its partial pressure). The vapor mixture is cooled and condensed, usually yielding a layer of oil and a layer of water.
Steam distillation of various aromatic herbs and flowers can result in two products; an essential oil as well as a watery herbal distillate. The essential oils
An essential oil is a concentrated hydrophobic liquid containing volatile (easily evaporated at normal temperatures) chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the o ...
are often used in perfumery and aromatherapy
Aromatherapy is based on the usage of aromatic materials including essential oils and other aroma compounds, with claims for improving psychological and physical well-being. It is offered as a complementary therapy or as a form of alternative ...
while the watery distillates have many applications in aromatherapy
Aromatherapy is based on the usage of aromatic materials including essential oils and other aroma compounds, with claims for improving psychological and physical well-being. It is offered as a complementary therapy or as a form of alternative ...
, food processing
Food processing is the transformation of agricultural products into food, or of one form of food into other forms. Food processing includes many forms of processing foods, from grinding grain to make raw flour to home cooking to complex industr ...
and skin care
Skin care is a range of practices that support skin integrity, enhance its appearance, and relieve skin conditions. They can include nutrition, avoidance of excessive sun exposure, and appropriate use of emollients. Practices that enhance appea ...
.

Vacuum distillation
Some compounds have very high boiling points. To boil such compounds, it is often better to lower the pressure at which such compounds are boiled instead of increasing the temperature. Once the pressure is lowered to the vapor pressure of the compound (at the given temperature), boiling and the rest of the distillation process can commence. This technique is referred to as vacuum distillation and it is commonly found in the laboratory in the form of the rotary evaporator. This technique is also very useful for compounds which boil beyond their decomposition temperature at atmospheric pressure and which would therefore be decomposed by any attempt to boil them under atmospheric pressure.Short path and molecular distillation
Molecular distillation Molecular distillation is a type of short-path vacuum distillation, characterized by an extremely low vacuum pressure, 0.01 torr or below, which is performed using a molecular still. It is a process of separation, purification and concentration o ...
is vacuum distillation below the pressure of 0.01 torr
The torr (symbol: Torr) is a unit of pressure based on an absolute scale, defined as exactly of a standard atmosphere (). Thus one torr is exactly (≈ ).
Historically, one torr was intended to be the same as one "millimeter of mercur ...
. 0.01 torr is one order of magnitude above high vacuum
''High Vacuum'' is a science fiction novel by Charles Eric Maine. It was first published in 1957 by Hodder & Stoughton.
Synopsis
The first crewed Moon ship, ''Alpha'', runs out of fuel just before landing in the Mare Imbrium and crashes, killin ...
, where fluids are in the free molecular flow regime, i.e. the mean free path
In physics, mean free path is the average distance over which a moving particle (such as an atom, a molecule, or a photon) travels before substantially changing its direction or energy (or, in a specific context, other properties), typically as a ...
of molecules is comparable to the size of the equipment. The gaseous phase no longer exerts significant pressure on the substance to be evaporated, and consequently, rate of evaporation no longer depends on pressure. That is, because the continuum assumptions of fluid dynamics no longer apply, mass transport is governed by molecular dynamics rather than fluid dynamics. Thus, a short path between the hot surface and the cold surface is necessary, typically by suspending a hot plate covered with a film of feed next to a cold plate with a line of sight in between. Molecular distillation is used industrially for purification of oils.
Air-sensitive vacuum distillation
Some compounds have high boiling points as well as being air sensitive. A simple vacuum distillation system as exemplified above can be used, whereby the vacuum is replaced with an inert gas after the distillation is complete. However, this is a less satisfactory system if one desires to collect fractions under a reduced pressure. To do this a "cow" or "pig" adaptor can be added to the end of the condenser, or for better results or for very air sensitive compounds a Perkin triangle apparatus can be used. The Perkin triangle, has means via a series of glass orTeflon
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene that has numerous applications. It is one of the best-known and widely applied PFAS. The commonly known brand name of PTFE-based composition is Teflon by Chem ...
taps to allows fractions to be isolated from the rest of the still
A still is an apparatus used to distill liquid mixtures by heating to selectively boil and then cooling to condense the vapor. A still uses the same concepts as a basic distillation apparatus, but on a much larger scale. Stills have been u ...
, without the main body of the distillation being removed from either the vacuum or heat source, and thus can remain in a state of reflux. To do this, the sample is first isolated from the vacuum by means of the taps, the vacuum over the sample is then replaced with an inert gas (such as nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seve ...
or argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as a ...
) and can then be stoppered and removed. A fresh collection vessel can then be added to the system, evacuated and linked back into the distillation system via the taps to collect a second fraction, and so on, until all fractions have been collected.
Zone distillation
Zone distillation is a distillation process in a long container with partial melting of refined matter in moving liquid zone and condensation of vapor in the solid phase at condensate pulling in cold area. The process is worked in theory. When zone heater is moving from the top to the bottom of the container then solid condensate with irregular impurity distribution is forming. Then most pure part of the condensate may be extracted as product. The process may be iterated many times by moving (without turnover) the received condensate to the bottom part of the container on the place of refined matter. The irregular impurity distribution in the condensate (that is efficiency of purification) increases with the number of iterations. Zone distillation is the distillation analog of zone recrystallization. Impurity distribution in the condensate is described by known equations of zone recrystallization – with the replacement of the distribution co-efficient k of crystallization - for the separation factor α of distillation.Closed-system vacuum distillation (cryovap)
Non-condensable gas can be expelled from the apparatus by the vapor of relatively volatile co-solvent, which spontaneously evaporates during initial pumping, and this can be achieved with regular oil or diaphragm pump.Other types
* The process of reactive distillation involves using the reaction vessel as the still. In this process, the product is usually significantly lower-boiling than its reactants. As the product is formed from the reactants, it is vaporized and removed from the reaction mixture. This technique is an example of a continuous vs. a batch process; advantages include less downtime to charge the reaction vessel with starting material, and less workup. Distillation "over a reactant" could be classified as a reactive distillation. It is typically used to remove volatile impurity from the distallation feed. For example, a little lime may be added to remove carbon dioxide from water followed by a second distillation with a little sulfuric acid added to remove traces of ammonia. * Catalytic distillation is the process by which the reactants are catalyzed while being distilled to continuously separate the products from the reactants. This method is used to assist equilibrium reactions in reaching completion. * Pervaporation is a method for the separation of mixtures of liquids by partial vaporization through a non-porousmembrane
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. ...
.
* Extractive distillation is defined as distillation in the presence of a miscible, high boiling, relatively non-volatile component, the solvent, that forms no azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be altered or changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This ...
with the other components in the mixture.
* Flash evaporation (or partial evaporation) is the partial vaporization
Vaporization (or vaporisation) of an element or compound is a phase transition from the liquid phase to vapor. There are two types of vaporization: evaporation and boiling. Evaporation is a surface phenomenon, whereas boiling is a bulk phenomenon. ...
that occurs when a saturated liquid stream undergoes a reduction in pressure by passing through a throttling valve
A valve is a device or natural object that regulates, directs or controls the flow of a fluid (gases, liquids, fluidized solids, or slurries) by opening, closing, or partially obstructing various passageways. Valves are technically fitting ...
or other throttling device. This process is one of the simplest unit operations, being equivalent to a distillation with only one equilibrium stage.
* Codistillation is distillation which is performed on mixtures in which the two compounds are not miscible. In the laboratory, the Dean-Stark apparatus is used for this purpose to remove water from synthesis products. The Bleidner apparatus is another example with two refluxing solvents.
* Membrane distillation is a type of distillation in which vapors of a mixture to be separated are passed through a membrane, which selectively permeates one component of mixture. Vapor pressure difference is the driving force. It has potential applications in seawater desalination and in removal of organic and inorganic components.
The unit process of evaporation may also be called "distillation":
* In rotary evaporation a vacuum distillation apparatus is used to remove bulk solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
s from a sample. Typically the vacuum is generated by a water aspirator or a membrane pump.
* In a Kugelrohr apparatus a short path distillation apparatus is typically used (generally in combination with a (high) vacuum) to distill high boiling (> 300 °C) compounds. The apparatus consists of an oven in which the compound to be distilled is placed, a receiving portion which is outside of the oven, and a means of rotating the sample. The vacuum is normally generated by using a high vacuum pump.
Other uses:
* Dry distillation or destructive distillation
Destruction may refer to:
Concepts
* Destruktion, a term from the philosophy of Martin Heidegger
* Destructive narcissism, a pathological form of narcissism
* Self-destructive behaviour, a widely used phrase that ''conceptualises'' certain ki ...
, despite the name, is not truly distillation, but rather a chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and break ...
known as pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''p ...
in which solid substances are heated in an inert or reducing atmosphere and any volatile fractions, containing high-boiling liquids and products of pyrolysis, are collected. The destructive distillation of wood
Wood is a porous and fibrous structural tissue found in the stems and roots of trees and other woody plants. It is an organic materiala natural composite of cellulose fibers that are strong in tension and embedded in a matrix of ligni ...
to give methanol is the root of its common name – ''wood alcohol''.
* Freeze distillation is an analogous method of purification using freezing
Freezing is a phase transition where a liquid turns into a solid when its temperature is lowered below its freezing point. In accordance with the internationally established definition, freezing means the solidification phase change of a liquid o ...
instead of evaporation. It is not truly distillation, but a recrystallization where the product is the mother liquor, and does not produce products equivalent to distillation. This process is used in the production of ice beer
Ice beer is a beer that has undergone some degree of freezing during production. These beers generally have a higher alcohol content, and lower price relative to it.
The process of "icing" beer involves lowering the temperature until ice cryst ...
and ice wine to increase ethanol and sugar content, respectively. It is also used to produce applejack. Unlike distillation, freeze distillation concentrates poisonous congeners rather than removing them; As a result, many countries prohibit such applejack as a health measure. Also, distillation by evaporation can separate these since they have different boiling points.
*Distillation by filtration: In early alchemy and chemistry, otherwise known as natural philosophy, a form of "distillation" by capillary filtration was known as a form of distillation at the time. In this, a series of cups or bowls were set upon a stepped support with a "wick" of cotton or felt-like material, which had been wetted with water or a clear liquid with each step dripping down through the wetted cloth through capillary action in succeeding steps, creating a "purification" of the liquid, leaving solid materials behind in the upper bowls and purifying the succeeding product through capillary action through the moistened cloth. This was called "distillatio" by filtration by those using the method.
Azeotropic process
Interactions between the components of the solution create properties unique to the solution, as most processes entail non-ideal mixtures, where Raoult's law does not hold. Such interactions can result in a constant-boilingazeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be altered or changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This ...
which behaves as if it were a pure compound (i.e., boils at a single temperature instead of a range). At an azeotrope, the solution contains the given component in the same proportion as the vapor, so that evaporation does not change the purity, and distillation does not effect separation. For example, ethyl alcohol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a h ...
and water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
form an azeotrope of 95.6% at 78.1 °C.
If the azeotrope is not considered sufficiently pure for use, there exist some techniques to break the azeotrope to give a pure distillate. This set of techniques are known as azeotropic distillation. Some techniques achieve this by "jumping" over the azeotropic composition (by adding another component to create a new azeotrope, or by varying the pressure). Others work by chemically or physically removing or sequestering the impurity. For example, to purify ethanol beyond 95%, a drying agent (or desiccant
A desiccant is a hygroscopic substance that is used to induce or sustain a state of dryness (desiccation) in its vicinity; it is the opposite of a humectant. Commonly encountered pre-packaged desiccants are solids that absorb water. Desiccants ...
, such as potassium carbonate
Potassium carbonate is the inorganic compound with the formula K2 CO3. It is a white salt, which is soluble in water. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used in the production of soap and gl ...
) can be added to convert the soluble water into insoluble water of crystallization
In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is ...
. Molecular sieve
A molecular sieve is a material with pores (very small holes) of uniform size. These pore diameters are similar in size to small molecules, and thus large molecules cannot enter or be adsorbed, while smaller molecules can. As a mixture of molec ...
s are often used for this purpose as well.
Immiscible liquids, such as water and toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) ...
, easily form azeotropes. Commonly, these azeotropes are referred to as a low boiling azeotrope because the boiling point of the azeotrope is lower than the boiling point of either pure component. The temperature and composition of the azeotrope is easily predicted from the vapor pressure of the pure components, without use of Raoult's law. The azeotrope is easily broken in a distillation set-up by using a liquid–liquid separator (a decanter) to separate the two liquid layers that are condensed overhead. Only one of the two liquid layers is refluxed to the distillation set-up.
High boiling azeotropes, such as a 20 percent by weight mixture of hydrochloric acid in water, also exist. As implied by the name, the boiling point of the azeotrope is greater than the boiling point of either pure component.
To break azeotropic distillations and cross distillation boundaries, such as in the DeRosier Problem, it is necessary to increase the composition of the light key in the distillate.
Breaking an azeotrope with unidirectional pressure manipulation
The boiling points of components in an azeotrope overlap to form a band. By exposing an azeotrope to a vacuum or positive pressure, it's possible to bias the boiling point of one component away from the other by exploiting the differing vapor pressure curves of each; the curves may overlap at the azeotropic point, but are unlikely to remain identical further along the pressure axis to either side of the azeotropic point. When the bias is great enough, the two boiling points no longer overlap and so the azeotropic band disappears. This method can remove the need to add other chemicals to a distillation, but it has two potential drawbacks. Under negative pressure, power for a vacuum source is needed and the reduced boiling points of the distillates requires that the condenser be run cooler to prevent distillate vapors being lost to the vacuum source. Increased cooling demands will often require additional energy and possibly new equipment or a change of coolant. Alternatively, if positive pressures are required, standard glassware can not be used, energy must be used for pressurization and there is a higher chance of side reactions occurring in the distillation, such as decomposition, due to the higher temperatures required to effect boiling. A unidirectional distillation will rely on a pressure change in one direction, either positive or negative.Pressure-swing distillation
Pressure-swing distillation is essentially the same as the unidirectional distillation used to break azeotropic mixtures, but here both positive and negative pressures may be employed. This improves the selectivity of the distillation and allows a chemist to optimize distillation by avoiding extremes of pressure and temperature that waste energy. This is particularly important in commercial applications. One example of the application of pressure-swing distillation is during the industrial purification ofethyl acetate
Ethyl acetate ( systematically ethyl ethanoate, commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues ...
after its catalytic synthesis from ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a h ...
.
Industrial process
 Large scale industrial distillation applications include both batch and continuous fractional, vacuum, azeotropic, extractive, and steam distillation. The most widely used industrial applications of continuous, steady-state fractional distillation are in petroleum refineries,
Large scale industrial distillation applications include both batch and continuous fractional, vacuum, azeotropic, extractive, and steam distillation. The most widely used industrial applications of continuous, steady-state fractional distillation are in petroleum refineries, petrochemical
Petrochemicals (sometimes abbreviated as petchems) are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable ...
and chemical plant
A chemical plant is an industrial process plant that manufactures (or otherwise processes) chemicals, usually on a large scale. The general objective of a chemical plant is to create new material wealth via the chemical or biological transfor ...
s and natural gas processing
Natural-gas processing is a range of industrial processes designed to purify raw natural gas by removing impurities, contaminants and higher molecular mass hydrocarbons to produce what is known as ''pipeline quality'' dry natural gas. Natural gas ...
plants.
To control and optimize such industrial distillation, a standardized laboratory method, ASTM D86, is established. This test method extends to the atmospheric distillation of petroleum products using a laboratory batch distillation unit to quantitatively determine the boiling range characteristics of petroleum products.
Industrial distillation is typically performed in large, vertical cylindrical columns known as distillation towers or distillation columns with diameters ranging from about and heights ranging from about or more. When the process feed has a diverse composition, as in distilling crude oil, liquid outlets at intervals up the column allow for the withdrawal of different ''fractions'' or products having different boiling points or boiling ranges. The "lightest" products (those with the lowest boiling point) exit from the top of the columns and the "heaviest" products (those with the highest boiling point) exit from the bottom of the column and are often called the bottoms.
Chemical engineer
In the field of engineering, a chemical engineer is a professional, equipped with the knowledge of chemical engineering, who works principally in the chemical industry to convert basic raw materials into a variety of products and deals with the ...
s must choose what combination of reflux rate and number of plates is both economically and physically feasible for the products purified in the distillation column.
Such industrial fractionating towers are also used in cryogenic
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of “cryogenics” and “cr ...
air separation
An air separation plant separates atmospheric air into its primary components, typically nitrogen and oxygen, and sometimes also argon and other rare inert gases.
The most common method for air separation is fractional distillation. Cryogenic ai ...
, producing liquid oxygen
Liquid oxygen—abbreviated LOx, LOX or Lox in the aerospace, submarine and gas industries—is the liquid form of molecular oxygen. It was used as the oxidizer in the first liquid-fueled rocket invented in 1926 by Robert H. Goddard, an a ...
, liquid nitrogen
Liquid nitrogen—LN2—is nitrogen in a liquid state at low temperature. Liquid nitrogen has a boiling point of about . It is produced industrially by fractional distillation of liquid air. It is a colorless, low viscosity liquid that is wi ...
, and high purity argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as a ...
. Distillation of chlorosilanes also enables the production of high-purity silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ...
for use as a semiconductor
A semiconductor is a material which has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity falls as its temperature rises; metals behave in the opposite way. ...
.
McCabe–Thiele method
The McCabe–Thiele method is a chemical engineering technique for the analysis of binary distillation. It uses the fact that the composition at each theoretical tray (or equilibrium stage) is completely determined by the mole fraction of one of ...
or the Fenske equation can be used. For a multi-component feed, simulation
A simulation is the imitation of the operation of a real-world process or system over time. Simulations require the use of models; the model represents the key characteristics or behaviors of the selected system or process, whereas the ...
models are used both for design and operation. Moreover, the efficiencies of the vapor–liquid contact devices (referred to as "plates" or "trays") used in distillation towers are typically lower than that of a theoretical 100% efficient equilibrium stage. Hence, a distillation tower needs more trays than the number of theoretical vapor–liquid equilibrium stages. A variety of models have been postulated to estimate tray efficiencies.
In modern industrial uses, a packing material is used in the column instead of trays when low pressure drops across the column are required. Other factors that favor packing are: vacuum systems, smaller diameter columns, corrosive systems, systems prone to foaming, systems requiring low liquid holdup, and batch distillation. Conversely, factors that favor plate columns are: presence of solids in feed, high liquid rates, large column diameters, complex columns, columns with wide feed composition variation, columns with a chemical reaction, absorption columns, columns limited by foundation weight tolerance, low liquid rate, large turn-down ratio and those processes subject to process surges.
 This packing material can either be random dumped packing ( wide) such as Raschig rings or structured sheet metal. Liquids tend to wet the surface of the packing and the vapors pass across this wetted surface, where
This packing material can either be random dumped packing ( wide) such as Raschig rings or structured sheet metal. Liquids tend to wet the surface of the packing and the vapors pass across this wetted surface, where mass transfer
Mass transfer is the net movement of mass from one location (usually meaning stream, phase, fraction or component) to another. Mass transfer occurs in many processes, such as absorption, evaporation, drying, precipitation, membrane filtra ...
takes place. Unlike conventional tray distillation in which every tray represents a separate point of vapor–liquid equilibrium, the vapor–liquid equilibrium curve in a packed column is continuous. However, when modeling packed columns, it is useful to compute a number of "theoretical stages" to denote the separation efficiency of the packed column with respect to more traditional trays. Differently shaped packings have different surface areas and void space between packings. Both of these factors affect packing performance.
Another factor in addition to the packing shape and surface area that affects the performance of random or structured packing is the liquid and vapor distribution entering the packed bed. The number of theoretical stages required to make a given separation is calculated using a specific vapor to liquid ratio. If the liquid and vapor are not evenly distributed across the superficial tower area as it enters the packed bed, the liquid to vapor ratio will not be correct in the packed bed and the required separation will not be achieved. The packing will appear to not be working properly. The height equivalent to a theoretical plate (HETP) will be greater than expected. The problem is not the packing itself but the mal-distribution of the fluids entering the packed bed. Liquid mal-distribution is more frequently the problem than vapor. The design of the liquid distributors used to introduce the feed and reflux to a packed bed is critical to making the packing perform to it maximum efficiency. Methods of evaluating the effectiveness of a liquid distributor to evenly distribute the liquid entering a packed bed can be found in references.Moore, F., Rukovena, F. (August 1987) ''Random Packing, Vapor and Liquid Distribution: Liquid and gas distribution in commercial packed towers'', Chemical Plants & Processing, Edition Europe, pp. 11–15 Considerable work has been done on this topic by Fractionation Research, Inc. (commonly known as FRI).
Multi-effect distillation
The goal of multi-effect distillation is to increase the energy efficiency of the process, for use in desalination, or in some cases one stage in the production of ultrapure water. The number of effects is inversely proportional to the kW·h/m3 of water recovered figure, and refers to the volume of water recovered per unit of energy compared with single-effect distillation. One effect is roughly 636 kW·h/m3. * Multi-stage flash distillation can achieve more than 20 effects with thermal energy input, as mentioned in the article. * Vapor compression evaporation – Commercial large-scale units can achieve around 72 effects with electrical energy input, according to manufacturers. There are many other types of multi-effect distillation processes, including one referred to as simply multi-effect distillation (MED), in which multiple chambers, with intervening heat exchangers, are employed.In food processing
Beverages
Carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or ...
-containing plant materials are allowed to ferment, producing a dilute solution of ethanol in the process. Spirits such as whiskey
Whisky or whiskey is a type of distilled alcoholic beverage made from fermented grain mash. Various grains (which may be malted) are used for different varieties, including barley, corn, rye, and wheat. Whisky is typically aged in wooden c ...
and rum are prepared by distilling these dilute solutions of ethanol. Components other than ethanol, including water, esters, and other alcohols, are collected in the condensate, which account for the flavor of the beverage. Some of these beverages are then stored in barrels or other containers to acquire more flavor compounds and characteristic flavors.
Gallery
See also
* Atmospheric distillation of crude oil * Clyssus * Fragrance extraction *Low-temperature distillation
The low-temperature distillation (LTD) technology is the first implementation of the direct spray distillation (DSD) process. The first large-scale units are now in operation for desalination. The process was first developed by scientists at the ...
* Microdistillery
* Sublimation
Sublimation or sublimate may refer to:
* ''Sublimation'' (album), by Canvas Solaris, 2004
* Sublimation (phase transition), directly from the solid to the gas phase
* Sublimation (psychology), a mature type of defense mechanism
* Sublimate of mer ...
* Dixon rings
* Random column packing
References
Further reading
* * * Needham, Joseph (1980)''Science and Civilisation in China''
Cambridge University Press. .
External links
* {{Authority control Unit operations Alchemical processes Separation processes Laboratory techniques Phase transitions Gas technologies Ancient inventions