|
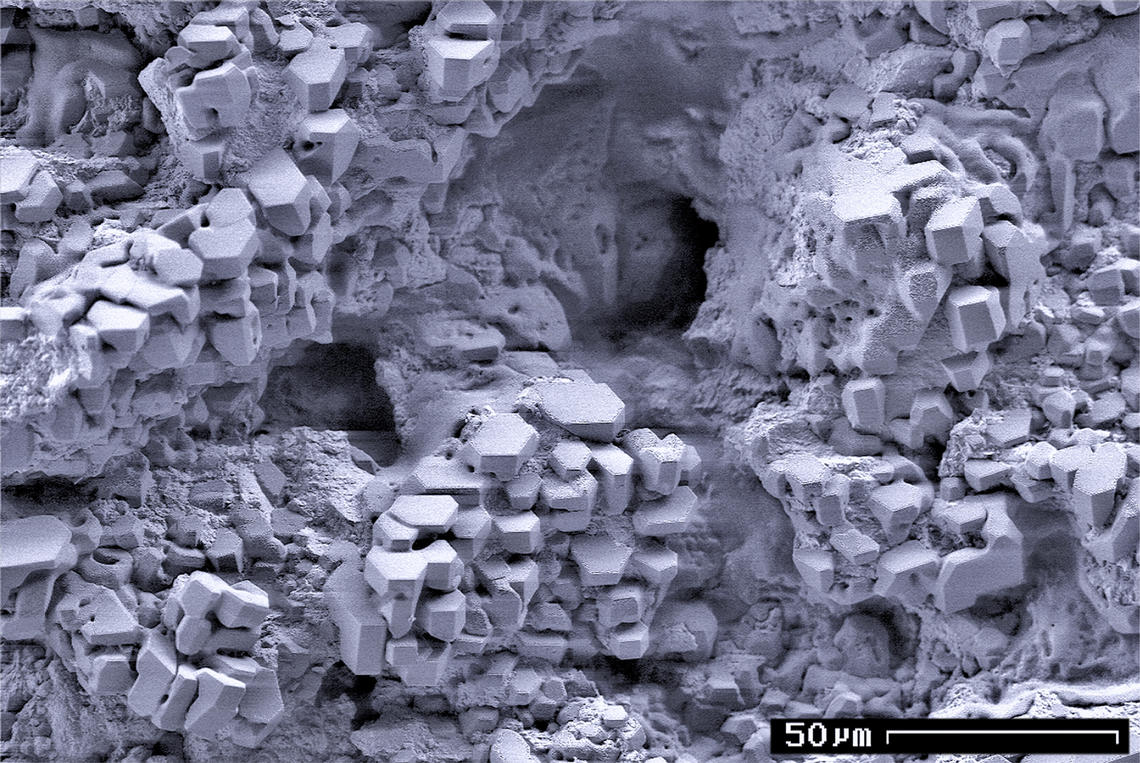
Molecular Sieve
A molecular sieve is a material with pores (very small holes) of uniform size. These pore diameters are similar in size to small molecules, and thus large molecules cannot enter or be adsorbed, while smaller molecules can. As a mixture of molecules migrate through the stationary bed of porous, semi-solid substance referred to as a sieve (or matrix), the components of highest molecular weight (which are unable to pass into the molecular pores) leave the bed first, followed by successively smaller molecules. Some molecular sieves are used in size-exclusion chromatography, a separation technique that sorts molecules based on their size. Other molecular sieves are used as desiccants (some examples include activated charcoal and silica gel). The pore diameter of a molecular sieve is measured in ångströms (Å) or nanometres (nm). According to IUPAC notation, microporous materials have pore diameters of less than 2 nm (20 Å) and macroporous materials have pore diameters of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Sieve5
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not consider ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Minerals
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Minerals'; p. 1. In the series ''Geology: Landforms, Minerals, and Rocks''. Rosen Publishing Group. The geological definition of mineral normally excludes compounds that occur only in living organisms. However, some minerals are often biogenic (such as calcite) or are organic compounds in the sense of chemistry (such as mellite). Moreover, living organisms often synthesize inorganic minerals (such as hydroxylapatite) that also occur in rocks. The concept of mineral is distinct from rock, which is any bulk solid geologic material that is relatively homogeneous at a large enough scale. A rock may consist of one type of mineral, or may be an aggregate of two or more different types of minerals, spacially segregated into distin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents. Nucleophilic alkylating agents Nucleophilic alkylating agents deliver the equivalent of an alkyl anion ( carbanion). The formal "alkyl anion" attacks an electrophile, forming a new cova ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomerisation
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomerization. When the isomerization occurs intramolecularly it may be called a rearrangement reaction. When the activation energy for the isomerization reaction is sufficiently small, both isomers will exist in a temperature-dependent equilibrium with each other. Many values of the standard free energy difference, \Delta G^\circ, have been calculated, with good agreement between observed and calculated data. Examples and applications Alkanes Skeletal isomerization occurs in the cracking process, used in the petrochemical industry. As well as reducing the average chain length, straight-chain hydrocarbons are converted to branched isomers in the process, as illustrated the following reaction of ''n''-butane to ''i''-butane. :\overset -> \o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methane Clathrate
Methane clathrate (CH4·5.75H2O) or (8CH4·46H2O), also called methane hydrate, hydromethane, methane ice, fire ice, natural gas hydrate, or gas hydrate, is a solid clathrate compound (more specifically, a clathrate hydrate) in which a large amount of methane is trapped within a crystal structure of water, forming a solid similar to ice. Originally thought to occur only in the outer regions of the Solar System, where temperatures are low and water ice is common, significant deposits of methane clathrate have been found under sediments on the ocean floors of the Earth. Methane hydrate is formed when hydrogen-bonded water and methane gas come into contact at high pressures and low temperatures in oceans. Methane clathrates are common constituents of the shallow marine geosphere and they occur in deep sedimentary structures and form outcrops on the ocean floor. Methane hydrates are believed to form by the precipitation or crystallisation of methane migrating from deep along geol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parts-per Notation
In science and engineering, the parts-per notation is a set of pseudo-units to describe small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction. Since these fractions are quantity-per-quantity measures, they are pure numbers with no associated units of measurement. Commonly used are parts-per-million (ppm, ), parts-per-billion (ppb, ), parts-per-trillion (ppt, ) and parts-per-quadrillion (ppq, ). This notation is not part of the International System of Units (SI) system and its meaning is ambiguous. Overview Parts-per notation is often used describing dilute solutions in chemistry, for instance, the relative abundance of dissolved minerals or pollutants in water. The quantity "1 ppm" can be used for a mass fraction if a water-borne pollutant is present at one-millionth of a gram per gram of sample solution. When working with aqueous solutions, it is common to assume that the density of water is 1.00 g/mL. Therefore, it is common to e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Petroleum
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crude oil and petroleum products that consist of refined crude oil. A fossil fuel, petroleum is formed when large quantities of dead organisms, mostly zooplankton and algae, are buried underneath sedimentary rock and subjected to both prolonged heat and pressure. Petroleum is primarily recovered by oil drilling. Drilling is carried out after studies of structural geology, sedimentary basin analysis, and reservoir characterisation. Recent developments in technologies have also led to exploitation of other unconventional reserves such as oil sands and oil shale. Once extracted, oil is refined and separated, most easily by distillation, into innumerable products for direct use or use in manufacturing. Products include fuels such as gasolin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mesoporous Silica
Mesoporous silica is a form of silica that is characterised by its mesoporous structure, that is, having pores that range from 2 nm to 50 nm in diameter. According to IUPAC's terminology, mesoporosity sits between microporous (50 nm). Mesoporous silica is a relatively recent development in nanotechnology. The most common types of mesoporous nanoparticles are MCM-41 and SBA-15. Research continues on the particles, which have applications in catalysis, drug delivery and imaging. Mesoporous ordered silica films have been also obtained with different pore topologies. A compound producing mesoporous silica was patented around 1970. It went almost unnoticed and was reproduced in 1997. Mesoporous silica nanoparticles (MSNs) were independently synthesized in 1990 by researchers in Japan. They were later produced also at Mobil Corporation laboratories and named Mobil Composition of Matter (or Mobil Crystalline Materials, MCM). Six years later, silica nanoparticles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as a synthetic product. Notable examples include fused quartz, fumed silica, silica gel, opal and aerogels. It is used in structural materials, microelectronics (as an electrical insulator), and as components in the food and pharmaceutical industries. Structure In the majority of silicates, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a linear molecule. The starkly different structures of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halloysite
Halloysite is an aluminosilicate clay mineral with the empirical formula Al2Si2O5(OH)4. Its main constituents are oxygen (55.78%), silicon (21.76%), aluminium (20.90%), and hydrogen (1.56%). Halloysite typically forms by hydrothermal alteration of alumino-silicate minerals. It can occur intermixed with dickite, kaolinite, montmorillonite and other clay minerals. X-ray diffraction studies are required for positive identification. It was first described in 1826 and named after the Belgian geologist Omalius d'Halloy. Occurrence The formation of halloysite is due to hydrothermal alteration, and it is often found near carbonate rocks. For example, halloysite samples found in Wagon Wheel Gap, Colorado, United States are suspected to be the weathering product of rhyolite by downward moving waters. In general the formation of clay minerals is highly favoured in tropical and sub-tropical climates due to the immense amounts of water flow. Halloysite has also been found overlaying bas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Montmorillonite
Montmorillonite is a very soft phyllosilicate group of minerals that form when they precipitate from water solution as microscopic crystals, known as clay. It is named after Montmorillon in France. Montmorillonite, a member of the smectite group, is a 2:1 clay, meaning that it has two tetrahedral sheets of silica sandwiching a central octahedral sheet of alumina. The particles are plate-shaped with an average diameter around 1 μm and a thickness of 0.96 nm; magnification of about 25,000 times, using an electron microscope, is required to "see" individual clay particles. Members of this group include, amongst others, saponite, nontronite, beidellite, and hectorite. Montmorillonite is a subclass of smectite, a 2:1 phyllosilicate mineral characterized as having greater than 50% octahedral charge; its cation exchange capacity is due to isomorphous substitution of Mg for Al in the central alumina plane. The substitution of lower valence cations in such instances leaves the nea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clay
Clay is a type of fine-grained natural soil material containing clay minerals (hydrous aluminium phyllosilicates, e.g. kaolin, Al2 Si2 O5( OH)4). Clays develop plasticity when wet, due to a molecular film of water surrounding the clay particles, but become hard, brittle and non–plastic upon drying or firing. Most pure clay minerals are white or light-coloured, but natural clays show a variety of colours from impurities, such as a reddish or brownish colour from small amounts of iron oxide. Clay is the oldest known ceramic material. Prehistoric humans discovered the useful properties of clay and used it for making pottery. Some of the earliest pottery shards have been dated to around 14,000 BC, and clay tablets were the first known writing medium. Clay is used in many modern industrial processes, such as paper making, cement production, and chemical filtering. Between one-half and two-thirds of the world's population live or work in buildings made with clay, ofte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)



