|
Fractional Distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used. It is used to refine crude oil. Laboratory setup Fractional distillation in a laboratory makes use of common laboratory glassware and apparatuses, typically including a Bunsen burner, a round-bottomed flask and a condenser, as well as the single-purpose fractionating column. As an example, consider the distillation of a mixture of water and ethanol. Ethanol boils at while water boils at . So, by heating the mixture, the most volatile component (ethanol) will concen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Separation Process
A separation process is a method that converts a mixture or a solution of chemical substances into two or more distinct product mixtures, a scientific process of separating two or more substance in order to obtain purity. At least one product mixture from the separation is enriched in one or more of the source mixture's constituents. In some cases, a separation may fully divide the mixture into pure constituents. Separations exploit differences in chemical properties or physical properties (such as size, shape, mass, density, or chemical affinity) between the constituents of a mixture. Processes are often classified according to the particular properties they exploit to achieve separation. If no single difference can be used to accomplish the desired separation, multiple operations can often be combined to achieve the desired end. With a few exceptions, elements or compounds exist in nature in an impure state. Often these raw materials must go through a separation before t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
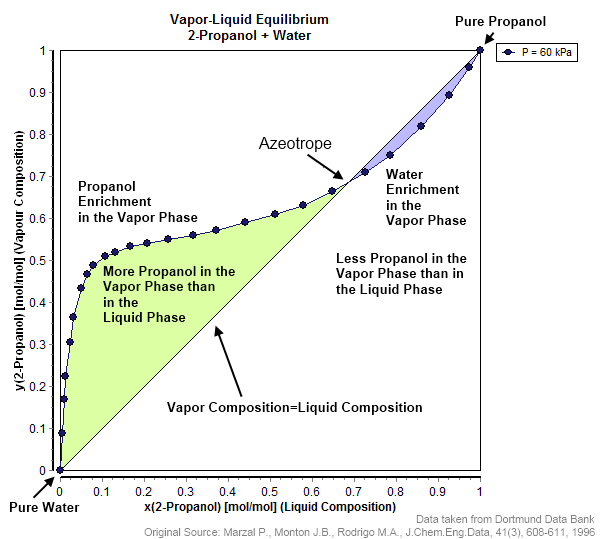
Azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be altered or changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This happens when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Because their composition is unchanged by distillation, azeotropes are also called (especially in older texts) ''constant boiling point mixtures''. Some azeotropic mixtures of pairs of compounds are known, and many azeotropes of three or more compounds are also known. In such a case it is not possible to separate the components by fractional distillation and azeotropic distillation is usually used instead. There are two types of azeotropes: minimum boiling azeotrope and maximum boiling azeotrope. A Solution (chemistry), solution that shows greater positive deviation from Raoult's law forms a minimum boiling azeotrope at a speci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graham Condenser
In chemistry, a condenser is laboratory apparatus used to condense vaporsthat is, turn them into liquidsby cooling them down. Condensers are routinely used in laboratory operations such as distillation, reflux, and extraction. In distillation, a mixture is heated until the more volatile components boil off, the vapors are condensed, and collected in a separate container. In reflux, a reaction involving volatile liquids is carried out at their boiling point, to speed it up; and the vapors that inevitably come off are condensed and returned to the reaction vessel. In Soxhlet extraction, a hot solvent is infused onto some powdered material, such as ground seeds, to leach out some poorly soluble component; the solvent is then automatically distilled out of the resulting solution, condensed, and infused again. Many different types of condensers have been developed for different applications and processing volumes. The simplest and oldest condenser is just a long tube through w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Jacket
A water jacket is a water-filled casing surrounding a device, typically a metal sheath having intake and outlet vents to allow water to be pumped through and circulated. The flow of water to an external heating or cooling device allows precise temperature control of the device. Applications Water jackets are often used in watercooling. They are also used in laboratory glassware: Liebig, Graham, and Allihn condensers. Water jackets were used to cool the barrels of machine guns until several years after the First World War, but modern machine guns are air-cooled to conserve weight and hence increase portability. In a reciprocating piston internal combustion engine the water jacket is a series of holes either cast or bored through the main engine block and connected by inlet and outlet valves to a radiator. Equipment such as tissue culture Tissue culture is the growth of tissues or cells in an artificial medium separate from the parent organism. This technique is also cal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liebig Condenser
The Liebig condenser (, ) or straight condenser is a piece of laboratory equipment, specifically a condenser consisting of a straight glass tube surrounded by a water jacket. In typical laboratory operation, such as distillation, the condenser is clamped to a retort stand in vertical or oblique orientation. The hot vapor of some liquid is introduced at the upper end of the inner tube, and condenses in contact with its colder walls. Water (or some other fluid) is constantly circulated in the jacket to carry away the heat of vaporization released by the condensing vapor, keeping the tube below the liquid's boiling point. The condensed liquid drips out of the lower end of the inner tube. The Liebig condenser can also be used in reflux or Soxhlet extraction operations, although other condenser types are better suited to those tasks. In this usage, the condenser is held vertically above the recipient with the boiling liquid. The vapor is admitted to the inner tube through the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silvering
Silvering is the chemical process of coating a non-conductive substrate such as glass with a reflective substance, to produce a mirror. While the metal is often silver, the term is used for the application of any reflective metal. Process Most common household mirrors are "back-silvered" or "second-surface", meaning that the light reaches the reflective layer after passing through the glass. A protective layer of paint is usually applied to protect the back side of the reflective surface . This arrangement protects the fragile reflective layer from corrosion, scratches, and other damage. However, the glass layer may absorb some of the light and cause distortions and optical aberrations due to refraction at the front surface, and multiple additional reflections on it, giving rise to "ghost images" (although some optical mirrors such as Mangins, take advantage of it). Therefore, precision optical mirrors normally are "front-silvered" or " first-surface", meaning that the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermometer
A thermometer is a device that measures temperature or a temperature gradient (the degree of hotness or coldness of an object). A thermometer has two important elements: (1) a temperature sensor (e.g. the bulb of a mercury-in-glass thermometer or the pyrometric sensor in an infrared thermometer) in which some change occurs with a change in temperature; and (2) some means of converting this change into a numerical value (e.g. the visible scale that is marked on a mercury-in-glass thermometer or the digital readout on an infrared model). Thermometers are widely used in technology and industry to monitor processes, in meteorology, in medicine, and in scientific research. History While an individual thermometer is able to measure degrees of hotness, the readings on two thermometers cannot be compared unless they conform to an agreed scale. Today there is an absolute thermodynamic temperature scale. Internationally agreed temperature scales are designed to approximate this clo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapor–liquid Equilibrium
In thermodynamics and chemical engineering, the vapor–liquid equilibrium (VLE) describes the distribution of a chemical species between the vapor phase and a liquid phase. The concentration of a vapor in contact with its liquid, especially at equilibrium, is often expressed in terms of vapor pressure, which will be a partial pressure (a part of the total gas pressure) if any other gas(es) are present with the vapor. The equilibrium vapor pressure of a liquid is in general strongly dependent on temperature. At vapor–liquid equilibrium, a liquid with individual components in certain concentrations will have an equilibrium vapor in which the concentrations or partial pressures of the vapor components have certain values depending on all of the liquid component concentrations and the temperature. The converse is also true: if a vapor with components at certain concentrations or partial pressures is in vapor–liquid equilibrium with its liquid, then the component concentrat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to reactions over a long period of time. Reflux in industrial distillation The term ''reflux'' is very widely used in industries that utilize large-scale distillation columns and fractionators such as petroleum refineries, petrochemical and chemical plants, and natural gas processing plants. In that context, reflux refers to the portion of the overhead liquid product from a distillation column or fractionator that is returned to the upper part of the column as shown in the schematic diagram of a typical industrial distillation column. Inside the column, the downflowing reflux liquid provides cooling and condensation of the upflowing vapors thereby increasing the efficiency of the distillation column. The more reflux provided for a given num ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theoretical Tray
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage, or a theoretical tray. The performance of many separation processes depends on having series of equilibrium stages and is enhanced by providing more such stages. In other words, having more theoretical plates increases the efficiency of the separation process be it either a distillation, absorption, chromatographic, adsorption or similar process. Applications The concept of theoretical plates and trays or equilibrium stages is used in the design of many different types of separation. Distillation columns The concept of theoretical plates in designing distillation processes has been discussed in many reference texts. Any physical device that provides good contact between the vapor and liquid phases pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor to liquid water when in contact with a liquid or solid surface or cloud condensation nuclei within the atmosphere. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition. Initiation Condensation is initiated by the formation of atomic/molecular clusters of that species within its gaseous volume—like rain drop or snow flake formation within clouds—or at the contact between such gaseous phase and a liquid or solid surface. In clouds, this can be catalyzed by water-nucleating proteins, produced by atmospheric microbes, which are capable of binding gaseous or liquid water molecules. Reversibility scenarios A few distinct reversibility scenarios emerge here with respect to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature Gradient
A temperature gradient is a physical quantity that describes in which direction and at what rate the temperature changes the most rapidly around a particular location. The temperature gradient is a dimensional quantity expressed in units of degrees (on a particular temperature scale) per unit length. The SI unit is kelvin per meter (K/m). Temperature gradients in the atmosphere are important in the atmospheric sciences (meteorology, climatology and related fields). Mathematical description Assuming that the temperature ''T'' is an intensive quantity, i.e., a single-valued, continuous and differentiable function of three-dimensional space (often called a scalar field), i.e., that :T=T(x,y,z) where ''x'', ''y'' and ''z'' are the coordinates of the location of interest, then the temperature gradient is the vector quantity defined as :\nabla T = \begin , , \end Physical processes Climatology On a global and annual basis, the dynamics of the atmosphere (and the ocea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |