Dideuterium on:
[Wikipedia]
[Google]
[Amazon]
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two
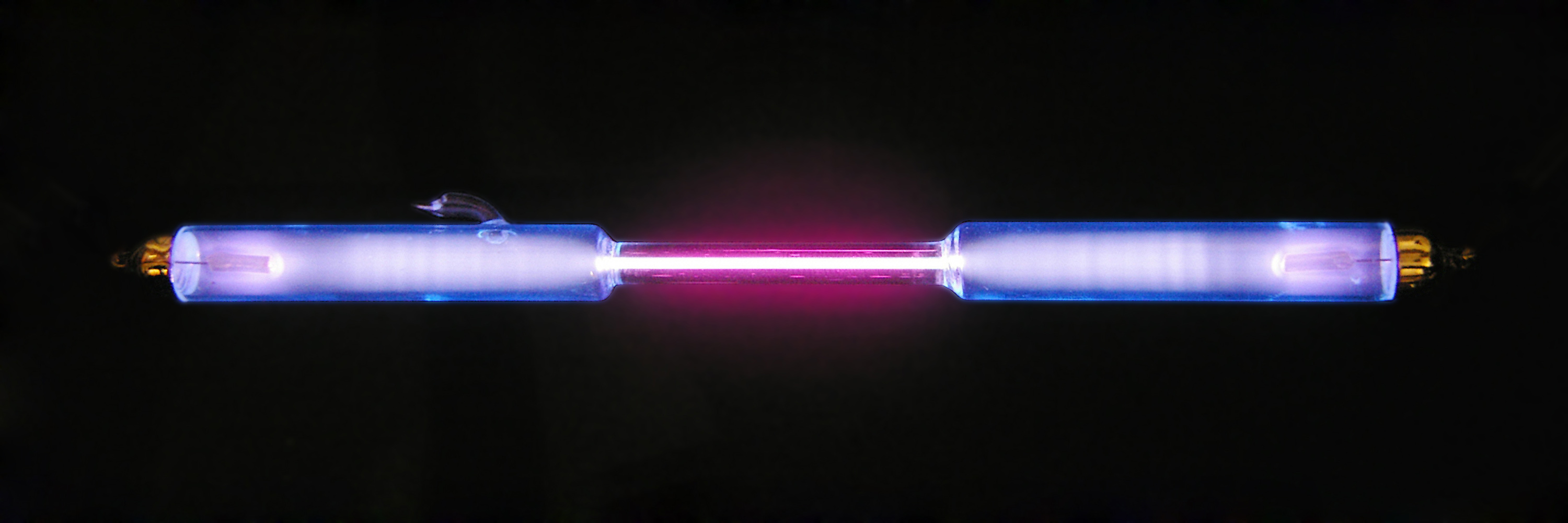 Deuterium is frequently represented by the chemical symbol D. Since it is an isotope of
Deuterium is frequently represented by the chemical symbol D. Since it is an isotope of
stable isotopes
The term stable isotope has a meaning similar to stable nuclide, but is preferably used when speaking of nuclides of a specific element. Hence, the plural form stable isotopes usually refers to isotopes of the same element. The relative abundanc ...
of hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
(the other being protium, or hydrogen-1). The nucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
*Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucle ...
of a deuterium atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
, called a deuteron, contains one proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the protonŌĆōelectron mass ...
and one neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons beh ...
, whereas the far more common protium has no neutrons in the nucleus. Deuterium has a natural abundance
In physics, natural abundance (NA) refers to the abundance of isotopes of a chemical element as naturally found on a planet. The relative atomic mass (a weighted average, weighted by mole-fraction abundance figures) of these isotopes is the atomic ...
in Earth's oceans
The ocean (also the sea or the world ocean) is the body of salt water that covers approximately 70.8% of the surface of Earth and contains 97% of Earth's water. An ocean can also refer to any of the large bodies of water into which the worl ...
of about one atom of deuterium among all atoms of hydrogen (see heavy water). Thus deuterium accounts for approximately 0.0156% by number (0.0312% by mass) of all the naturally occurring hydrogen in the oceans, while protium accounts for more than 99.98%. The abundance of deuterium changes slightly from one kind of natural water to another (see Vienna Standard Mean Ocean Water). ( Tritium is yet another hydrogen isotope, with two neutrons, that is far more rare and is radioactive.)
The name ''deuterium'' is derived from the Greek , meaning "second", to denote the two particles composing the nucleus. Deuterium was discovered and named in 1931 by Harold Urey
Harold Clayton Urey ( ; April 29, 1893 ŌĆō January 5, 1981) was an American physical chemist whose pioneering work on isotopes earned him the Nobel Prize in Chemistry in 1934 for the discovery of deuterium. He played a significant role in the d ...
. When the neutron was discovered in 1932, this made the nuclear structure of deuterium obvious, and Urey won the Nobel Prize
The Nobel Prizes ( ; sv, Nobelpriset ; no, Nobelprisen ) are five separate prizes that, according to Alfred Nobel's will of 1895, are awarded to "those who, during the preceding year, have conferred the greatest benefit to humankind." Alfr ...
in 1934 "for his discovery of heavy hydrogen". Soon after deuterium's discovery, Urey and others produced samples of " heavy water" in which the deuterium content had been highly concentrated.
Deuterium is destroyed in the interiors of stars faster than it is produced. Other natural processes are thought to produce only an insignificant amount of deuterium. Nearly all deuterium found in nature was produced in the Big Bang
The Big Bang event is a physical theory that describes how the universe expanded from an initial state of high density and temperature. Various cosmological models of the Big Bang explain the evolution of the observable universe from the ...
13.8 billion years ago, as the basic or primordial ratio of hydrogen-1 to deuterium (about 26 atoms of deuterium per million hydrogen atoms) has its origin from that time. This is the ratio found in the gas giant planets, such as Jupiter. The analysis of deuteriumŌĆōprotium ratios in comets found results very similar to the mean ratio in Earth's oceans (156 atoms of deuterium per million hydrogen atoms). This reinforces theories that much of Earth's ocean water is of cometary origin. ŌĆö see fig. 7. for a review of D/H ratios in various astronomical objects The deuteriumŌĆōprotium ratio of the comet 67P/ChuryumovŌĆōGerasimenko, as measured by the ''Rosetta
Rosetta or Rashid (; ar, ž▒ž┤┘Ŗž» ' ; french: Rosette ; cop, Ž»Ō▓ŻŌ▓üŽŻŌ▓ōŌ▓¦ ''ti-Rashit'', Ancient Greek: ╬Æ╬┐╬╗╬▓╬╣Žä╬»╬Į╬Ę ''Bolbitin─ō'') is a port city of the Nile Delta, east of Alexandria, in Egypt's Beheira governorate. The Ro ...
'' space probe, is about three times that of Earth water. This figure is the highest yet measured in a comet.
DeuteriumŌĆōprotium ratios thus continue to be an active topic of research in both astronomy and climatology.
Differences from common hydrogen (protium)
Chemical symbol
 Deuterium is frequently represented by the chemical symbol D. Since it is an isotope of
Deuterium is frequently represented by the chemical symbol D. Since it is an isotope of hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
with mass number 2, it is also represented by . IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
allows both D and , although is preferred. A distinct chemical symbol is used for convenience because of the isotope's common use in various scientific processes. Also, its large mass difference with protium (1H) (deuterium has a mass of , compared to the mean
There are several kinds of mean in mathematics, especially in statistics. Each mean serves to summarize a given group of data, often to better understand the overall value (magnitude and sign) of a given data set.
For a data set, the ''arithme ...
hydrogen atomic weight of , and protium's mass of ) confers non-negligible chemical dissimilarities with protium-containing compounds, whereas the isotope weight ratios within other chemical elements are largely insignificant in this regard.
Spectroscopy
Inquantum mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, ...
the energy levels of electrons in atoms depend on the reduced mass of the system of electron and nucleus. For the hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen consti ...
, the role of reduced mass is most simply seen in the Bohr model of the atom, where the reduced mass appears in a simple calculation of the Rydberg constant and Rydberg equation, but the reduced mass also appears in the Schr├Čdinger equation
The Schr├Čdinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of the ...
, and the Dirac equation for calculating atomic energy levels.
The reduced mass of the system in these equations is close to the mass of a single electron, but differs from it by a small amount about equal to the ratio of mass of the electron to the atomic nucleus. For hydrogen, this amount is about , or 1.000545, and for deuterium it is even smaller: , or 1.0002725. The energies of spectroscopic lines for deuterium and light hydrogen ( hydrogen-1) therefore differ by the ratios of these two numbers, which is 1.000272. The wavelengths of all deuterium spectroscopic lines are shorter than the corresponding lines of light hydrogen, by a factor of 1.000272. In astronomical observation, this corresponds to a blue Doppler shift of 0.000272 times the speed of light, or 81.6 km/s.
The differences are much more pronounced in vibrational spectroscopy such as infrared spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or function ...
and Raman spectroscopy
Raman spectroscopy () (named after Indian physicist C. V. Raman) is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman sp ...
, and in rotational spectra such as microwave spectroscopy because the reduced mass of the deuterium is markedly higher than that of protium. In nuclear magnetic resonance spectroscopy, deuterium has a very different NMR frequency (e.g. 61 MHz when protium is at 400 MHz) and is much less sensitive. Deuterated solvents are usually used in protium NMR to prevent the solvent from overlapping with the signal, although deuterium NMR
Deuterium NMR is NMR spectroscopy of deuterium (2H or D), an isotope of hydrogen.
Deuterium is an isotope with spin = 1, unlike hydrogen-1, which has spin = 1/2. The term deuteron NMR, in direct analogy to proton NMR, is also used. Spiess, H. W. ...
on its own right is also possible.
Big Bang nucleosynthesis
Deuterium is thought to have played an important role in setting the number and ratios of the elements that were formed in theBig Bang
The Big Bang event is a physical theory that describes how the universe expanded from an initial state of high density and temperature. Various cosmological models of the Big Bang explain the evolution of the observable universe from the ...
. Combining thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of the ...
and the changes brought about by cosmic expansion, one can calculate the fraction of protons
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the protonŌĆōelectron mass ...
and neutrons
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave ...
based on the temperature at the point that the universe cooled enough to allow formation of nuclei. This calculation indicates seven protons for every neutron at the beginning of nucleogenesis
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons (protons and neutrons) and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in ...
, a ratio that would remain stable even after nucleogenesis was over. This fraction was in favor of protons initially, primarily because the lower mass of the proton favored their production. As the Universe expanded, it cooled. Free neutrons and protons are less stable than helium
Helium (from el, ß╝ź╬╗╬╣╬┐Žé, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
nuclei, and the protons and neutrons had a strong energetic reason to form helium-4
Helium-4 () is a stable isotope of the element helium. It is by far the more abundant of the two naturally occurring isotopes of helium, making up about 99.99986% of the helium on Earth. Its nucleus is identical to an alpha particle, and consis ...
. However, forming helium-4 requires the intermediate step of forming deuterium.
Through much of the few minutes after the Big Bang during which nucleosynthesis could have occurred, the temperature was high enough that the mean energy per particle was greater than the binding energy of weakly bound deuterium; therefore any deuterium that was formed was immediately destroyed. This situation is known as the deuterium bottleneck. The bottleneck delayed formation of any helium-4 until the Universe became cool enough to form deuterium (at about a temperature equivalent to 100 keV). At this point, there was a sudden burst of element formation (first deuterium, which immediately fused to helium). However, very shortly thereafter, at twenty minutes after the Big Bang, the Universe became too cool for any further nuclear fusion and nucleosynthesis to occur. At this point, the elemental abundances were nearly fixed, with the only change as some of the radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
products of Big Bang nucleosynthesis (such as tritium) decay. The deuterium bottleneck in the formation of helium, together with the lack of stable ways for helium to combine with hydrogen or with itself (there are no stable nuclei with mass numbers of five or eight) meant that an insignificant amount of carbon, or any elements heavier than carbon, formed in the Big Bang. These elements thus required formation in stars. At the same time, the failure of much nucleogenesis during the Big Bang ensured that there would be plenty of hydrogen in the later universe available to form long-lived stars, such as our Sun.
Abundance
Deuterium occurs in trace amounts naturally as deuteriumgas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
, written 2 or D2, but most of the naturally occurring atoms in the Universe
The universe is all of space and time and their contents, including planets, stars, galaxies, and all other forms of matter and energy. The Big Bang theory is the prevailing cosmological description of the development of the universe. Acc ...
are bonded with a typical atom, a gas called hydrogen deuteride (HD or ).
The existence of deuterium on Earth, elsewhere in the Solar System
The Solar SystemCapitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar S ...
(as confirmed by planetary probes), and in the spectra of star
A star is an astronomical object comprising a luminous spheroid of plasma (physics), plasma held together by its gravity. The List of nearest stars and brown dwarfs, nearest star to Earth is the Sun. Many other stars are visible to the naked ...
s, is also an important datum in cosmology
Cosmology () is a branch of physics and metaphysics dealing with the nature of the universe. The term ''cosmology'' was first used in English in 1656 in Thomas Blount (lexicographer), Thomas Blount's ''Glossographia'', and in 1731 taken up in ...
. Gamma radiation from ordinary nuclear fusion dissociates deuterium into protons and neutrons, and there are no known natural processes other than the Big Bang nucleosynthesis that might have produced deuterium at anything close to its observed natural abundance. Deuterium is produced by the rare cluster decay
Cluster decay, also named heavy particle radioactivity or heavy ion radioactivity, is a rare type of nuclear decay in which an atomic nucleus emits a small "cluster" of neutrons and protons, more than in an alpha particle, but less than a typic ...
, and occasional absorption of naturally occurring neutrons by light hydrogen, but these are trivial sources. There is thought to be little deuterium in the interior of the Sun and other stars, as at these temperatures the nuclear fusion reaction
Nuclear fusion is a reaction in which two or more atomic nuclei are combined to form one or more different atomic nuclei and subatomic particles (neutrons or protons). The difference in mass between the reactants and products is manifeste ...
s that consume deuterium happen much faster than the proton-proton reaction that creates deuterium. However, deuterium persists in the outer solar atmosphere at roughly the same concentration as in Jupiter, and this has probably been unchanged since the origin of the Solar System. The natural abundance of deuterium seems to be a very similar fraction of hydrogen, wherever hydrogen is found, unless there are obvious processes at work that concentrate it.
The existence of deuterium at a low but constant primordial fraction in all hydrogen is another one of the arguments in favor of the Big Bang
The Big Bang event is a physical theory that describes how the universe expanded from an initial state of high density and temperature. Various cosmological models of the Big Bang explain the evolution of the observable universe from the ...
theory over the Steady State theory of the Universe. The observed ratios of hydrogen to helium to deuterium in the universe are difficult to explain except with a Big Bang model. It is estimated that the abundances of deuterium have not evolved significantly since their production about 13.8 billion years ago. Measurements of Milky Way galactic deuterium from ultraviolet spectral analysis show a ratio of as much as 23 atoms of deuterium per million hydrogen atoms in undisturbed gas clouds, which is only 15% below the WMAP
The Wilkinson Microwave Anisotropy Probe (WMAP), originally known as the Microwave Anisotropy Probe (MAP and Explorer 80), was a NASA spacecraft operating from 2001 to 2010 which measured temperature differences across the sky in the cosmic mic ...
estimated primordial ratio of about 27 atoms per million from the Big Bang. This has been interpreted to mean that less deuterium has been destroyed in star formation in our galaxy than expected, or perhaps deuterium has been replenished by a large in-fall of primordial hydrogen from outside the galaxy. In space a few hundred light years from the Sun, deuterium abundance is only 15 atoms per million, but this value is presumably influenced by differential adsorption of deuterium onto carbon dust grains in interstellar space.
The abundance of deuterium in the atmosphere of Jupiter
Jupiter is the fifth planet from the Sun and the List of Solar System objects by size, largest in the Solar System. It is a gas giant with a mass more than two and a half times that of all the other planets in the Solar System combined, but ...
has been directly measured by the Galileo space probe
''Galileo'' was an American robotic space probe that studied the planet Jupiter and Moons of Jupiter, its moons, as well as the asteroids 951 Gaspra, Gaspra and 243 Ida, Ida. Named after the Italian astronomer Galileo Galilei, it consisted of ...
as 26 atoms per million hydrogen atoms. ISO-SWS observations find 22 atoms per million hydrogen atoms in Jupiter. and this abundance is thought to represent close to the primordial solar system ratio. This is about 17% of the terrestrial deuterium-to-hydrogen ratio of 156 deuterium atoms per million hydrogen atoms.
Cometary bodies such as Comet Hale-Bopp
A comet is an icy, small Solar System body that, when passing close to the Sun, warms and begins to release gases, a process that is called outgassing. This produces a visible atmosphere or coma, and sometimes also a tail. These phenomena are ...
and Halley's Comet have been measured to contain relatively more deuterium (about 200 atoms D per million hydrogens), ratios which are enriched with respect to the presumed protosolar nebula ratio, probably due to heating, and which are similar to the ratios found in Earth seawater. The recent measurement of deuterium amounts of 161 atoms D per million hydrogen in Comet 103P/Hartley
Comet Hartley 2, designated as 103P/Hartley by the Minor Planet Center, is a small periodic comet with an orbital period of 6.46 years. It was discovered by Malcolm Hartley in 1986 at the Schmidt Telescope Unit, Siding Spring Observatory, Au ...
(a former Kuiper belt
The Kuiper belt () is a circumstellar disc in the outer Solar System, extending from the orbit of Neptune at 30 astronomical units (AU) to approximately 50 AU from the Sun. It is similar to the asteroid belt, but is far largerŌĆö20 times ...
object), a ratio almost exactly that in Earth's oceans, emphasizes the theory that Earth's surface water may be largely comet-derived. Most recently the deuteriumŌĆōprotium (DŌĆōH) ratio of 67P/ChuryumovŌĆōGerasimenko as measured by ''Rosetta'' is about three times that of Earth water, a figure that is high. This has caused renewed interest in suggestions that Earth's water may be partly of asteroidal origin.
Deuterium has also been observed to be concentrated over the mean solar abundance in other terrestrial planets, in particular Mars and Venus.
Production
Deuterium is produced for industrial, scientific and military purposes, by starting with ordinary waterŌĆöa small fraction of which is naturally-occurring heavy waterŌĆöand then separating out the heavy water by theGirdler sulfide process
The Girdler sulfide (GS) process, also known as the GeibSpevack (GS) process, is an industrial production method for filtering out of natural water the heavy water (deuterium oxide = D2O) which is used in particle research, in deuterium NMR sp ...
, distillation, or other methods.
In theory, deuterium for heavy water could be created in a nuclear reactor, but separation from ordinary water is the cheapest bulk production process.
The world's leading supplier of deuterium was Atomic Energy of Canada Limited until 1997, when the last heavy water plant was shut down. Canada uses heavy water as a neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, ideally without capturing any, leaving them as thermal neutrons with only minimal (thermal) kinetic energy. These thermal neutrons are immensely mo ...
for the operation of the CANDU reactor design.
Another major producer of heavy water is India. All but one of India's atomic energy plants are pressurised heavy water plants, which use natural (i.e., not enriched) uranium. India has eight heavy water plants, of which seven are in operation. Six plants, of which five are in operation, are based on DŌĆōH exchange in ammonia gas. The other two plants extract deuterium from natural water in a process that uses hydrogen sulphide gas at high pressure.
While India is self-sufficient in heavy water for its own use, India now also exports reactor-grade heavy water.
Properties
Data for molecular deuterium
Formula: D2 or 2 * Density: at STP (, ). * Atomic weight: . * Mean abundance in ocean water (fromVSMOW
Vienna Standard Mean Ocean Water (VSMOW) is an isotopic standard for water. Despite the name, VSMOW is pure water with no salt or other chemicals found in the oceans. The VSMOW standard was promulgated by the International Atomic Energy Agency ...
) 155.76 ┬▒ 0.1 ppm (a ratio of 1 part per approximately 6420 parts), that is, about of the atoms in a sample (by number, not weight)
Data at approximately for D2 (triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at which the subli ...
):
* Density:
** Liquid:
** Gas:
* Viscosity: at (gas phase)
* Specific heat capacity at constant pressure ''cp'':
** Solid:
** Gas:
Physical properties
Compared to hydrogen in its natural composition on Earth, pure deuterium (D2) has a highermelting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends ...
(18.72 K vs. 13.99 K), a higher boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
(23.64 K vs. 20.27 K), a higher critical
Critical or Critically may refer to:
*Critical, or critical but stable, medical states
**Critical, or intensive care medicine
*Critical juncture, a discontinuous change studied in the social sciences.
*Critical Software, a company specializing in ...
temperature (38.3 K vs. 32.94 K) and a higher critical pressure (1.6496 MPa vs. 1.2858 MPa).
The physical properties of deuterium compounds can exhibit significant kinetic isotope effect
In physical organic chemistry, a kinetic isotope effect (KIE) is the change in the reaction rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes. Formally, it is the ratio of rate constants for th ...
s and other physical and chemical property differences from the protium analogs. DO, for example, is more viscous
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inter ...
than . Chemically, there are differences in bond energy and length for compounds of heavy hydrogen isotopes compared to protium, which are larger than the isotopic differences in any other element. Bonds involving deuterium and tritium are somewhat stronger than the corresponding bonds in protium, and these differences are enough to cause significant changes in biological reactions. Pharmaceutical firms are interested in the fact that deuterium is harder to remove from carbon than protium.
Deuterium can replace protium in water molecules to form heavy water (D2O), which is about 10.6% denser than normal water (so that ice made from it sinks in ordinary water). Heavy water is slightly toxic in eukaryotic
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
animals, with 25% substitution of the body water causing cell division problems and sterility, and 50% substitution causing death by cytotoxic syndrome (bone marrow failure and gastrointestinal lining failure). Prokaryotic organisms, however, can survive and grow in pure heavy water, though they develop slowly. Despite this toxicity, consumption of heavy water under normal circumstances does not pose a health threat
A health risk assessment (also referred to as a health risk appraisal and health & well-being assessment) is a questionnaire about a person's medical history, demographic characteristics and lifestyle. It is one of the most widely used screening t ...
to humans. It is estimated that a person might drink of heavy water without serious consequences. Small doses of heavy water (a few grams in humans, containing an amount of deuterium comparable to that normally present in the body) are routinely used as harmless metabolic tracers in humans and animals.
Quantum properties
The deuteron hasspin
Spin or spinning most often refers to:
* Spinning (textiles), the creation of yarn or thread by twisting fibers together, traditionally by hand spinning
* Spin, the rotation of an object around a central axis
* Spin (propaganda), an intentionally b ...
+1 (" triplet state") and is thus a boson. The NMR frequency of deuterium is significantly different from common light hydrogen. Infrared spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or function ...
also easily differentiates many deuterated compounds, due to the large difference in IR absorption frequency seen in the vibration of a chemical bond containing deuterium, versus light hydrogen. The two stable isotopes of hydrogen can also be distinguished by using mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is use ...
.
The triplet deuteron nucleon is barely bound at EB = , and none of the higher energy states are bound. The singlet deuteron is a virtual state, with a negative binding energy of . There is no such stable particle, but this virtual particle transiently exists during neutron-proton inelastic scattering, accounting for the unusually large neutron scattering cross-section of the proton.
Nuclear properties (the deuteron)
Deuteron mass and radius
The nucleus of deuterium is called a deuteron. It has a mass of (just over ). Thecharge radius
The rms charge radius is a measure of the size of an atomic nucleus, particularly the proton distribution. It can be measured by the scattering of electrons by the nucleus. Relative changes in the mean squared nuclear charge distribution can be ...
of the deuteron is .
Like the proton radius
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the protonŌĆōelectron mass ...
, measurements using muon
A muon ( ; from the Greek letter mu (╬╝) used to represent it) is an elementary particle similar to the electron, with an electric charge of −1 '' e'' and a spin of , but with a much greater mass. It is classified as a lepton. As wi ...
ic deuterium produce a smaller result: .
Spin and energy
Deuterium is one of only five stablenuclide
A nuclide (or nucleide, from nucleus, also known as nuclear species) is a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was coined by Truman ...
s with an odd number of protons and an odd number of neutrons. (, , , , ; also, the long-lived radioactive nuclides , , , occur naturally.) Most odd-odd nuclei
In nuclear physics, properties of a nucleus depend on evenness or oddness of its atomic number (proton number) ''Z'', neutron number ''N'' and, consequently, of their sum, the mass number ''A''. Most importantly, oddness of both ''Z'' and ''N'' ...
are unstable with respect to beta decay
In nuclear physics, beta decay (╬▓-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
, because the decay products are even-even, and are therefore more strongly bound, due to nuclear pairing effects. Deuterium, however, benefits from having its proton and neutron coupled to a spin-1 state, which gives a stronger nuclear attraction; the corresponding spin-1 state does not exist in the two-neutron or two-proton system, due to the Pauli exclusion principle
In quantum mechanics, the Pauli exclusion principle states that two or more identical particles with half-integer spins (i.e. fermions) cannot occupy the same quantum state within a quantum system simultaneously. This principle was formulated ...
which would require one or the other identical particle with the same spin to have some other different quantum number, such as orbital angular momentum. But orbital angular momentum of either particle gives a lower binding energy
In physics and chemistry, binding energy is the smallest amount of energy required to remove a particle from a system of particles or to disassemble a system of particles into individual parts. In the former meaning the term is predominantly use ...
for the system, primarily due to increasing distance of the particles in the steep gradient of the nuclear force. In both cases, this causes the diproton
Although there are nine known isotopes of helium (2He) (standard atomic weight: ), only helium-3 () and helium-4 () are stable. All radioisotopes are short-lived, the longest-lived being with a half-life of . The least stable is , with a half-lif ...
and dineutron nucleus to be unstable.
The proton and neutron making up deuterium can be dissociated
Dissociation in chemistry is a general process in which molecules (or ionic compounds such as salts, or complexes) separate or split into other things such as atoms, ions, or radicals, usually in a reversible manner. For instance, when an acid ...
through neutral current
Weak neutral current interactions are one of the ways in which subatomic particles can interact by means of the weak force. These interactions are mediated by the Z boson. The discovery of weak neutral currents was a significant step towar ...
interactions with neutrinos. The cross section
Cross section may refer to:
* Cross section (geometry)
** Cross-sectional views in architecture & engineering 3D
*Cross section (geology)
* Cross section (electronics)
* Radar cross section, measure of detectability
* Cross section (physics)
**Abs ...
for this interaction is comparatively large, and deuterium was successfully used as a neutrino target in the Sudbury Neutrino Observatory experiment.
Diatomic deuterium (D2) has ortho and para nuclear spin isomers like diatomic hydrogen, but with differences in the number and population of spin states and rotational levels, which occur because the deuteron is a boson with nuclear spin equal to one.
Isospin singlet state of the deuteron
Due to the similarity in mass and nuclear properties between the proton and neutron, they are sometimes considered as two symmetric types of the same object, a nucleon. While only the proton has an electric charge, this is often negligible due to the weakness of the electromagnetic interaction relative to the strong nuclear interaction. The symmetry relating the proton and neutron is known as isospin and denoted ''I'' (or sometimes ''T''). Isospin is an SU(2) symmetry, like ordinaryspin
Spin or spinning most often refers to:
* Spinning (textiles), the creation of yarn or thread by twisting fibers together, traditionally by hand spinning
* Spin, the rotation of an object around a central axis
* Spin (propaganda), an intentionally b ...
, so is completely analogous to it. The proton and neutron, each of which have iso spin-, form an isospin doublet (analogous to a spin doublet), with a "down" state (Ōåō) being a neutron and an "up" state (Ōåæ) being a proton. A pair of nucleons can either be in an antisymmetric state of isospin called singlet, or in a symmetric state called triplet
A triplet is a set of three items, which may be in a specific order, or unordered. It may refer to:
Science
* A series of three nucleotide bases forming an element of the Genetic code
* J-coupling as part of Nuclear magnetic resonance spectrosc ...
. In terms of the "down" state and "up" state, the singlet is
:, which can also be written :
This is a nucleus with one proton and one neutron, i.e. a deuterium nucleus. The triplet is
:
and thus consists of three types of nuclei, which are supposed to be symmetric: a deuterium nucleus (actually a highly excited state
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to a ...
of it), a nucleus with two protons, and a nucleus with two neutrons. These states are not stable.
Approximated wavefunction of the deuteron
The deuteron wavefunction must be antisymmetric if the isospin representation is used (since a proton and a neutron are not identical particles, the wavefunction need not be antisymmetric in general). Apart from their isospin, the two nucleons also have spin and spatial distributions of their wavefunction. The latter is symmetric if the deuteron is symmetric underparity
Parity may refer to:
* Parity (computing)
** Parity bit in computing, sets the parity of data for the purpose of error detection
** Parity flag in computing, indicates if the number of set bits is odd or even in the binary representation of the r ...
(i.e. have an "even" or "positive" parity), and antisymmetric if the deuteron is antisymmetric under parity (i.e. have an "odd" or "negative" parity). The parity is fully determined by the total orbital angular momentum of the two nucleons: if it is even then the parity is even (positive), and if it is odd then the parity is odd (negative).
The deuteron, being an isospin singlet, is antisymmetric under nucleons exchange due to isospin, and therefore must be symmetric under the double exchange of their spin and location. Therefore, it can be in either of the following two different states:
*Symmetric spin and symmetric under parity. In this case, the exchange of the two nucleons will multiply the deuterium wavefunction by (ŌłÆ1) from isospin exchange, (+1) from spin exchange and (+1) from parity (location exchange), for a total of (ŌłÆ1) as needed for antisymmetry.
*Antisymmetric spin and antisymmetric under parity. In this case, the exchange of the two nucleons will multiply the deuterium wavefunction by (ŌłÆ1) from isospin exchange, (ŌłÆ1) from spin exchange and (ŌłÆ1) from parity (location exchange), again for a total of (ŌłÆ1) as needed for antisymmetry.
In the first case the deuteron is a spin triplet, so that its total spin ''s'' is 1. It also has an even parity and therefore even orbital angular momentum ''l'' ; The lower its orbital angular momentum, the lower its energy. Therefore, the lowest possible energy state has , .
In the second case the deuteron is a spin singlet, so that its total spin ''s'' is 0. It also has an odd parity and therefore odd orbital angular momentum ''l''. Therefore, the lowest possible energy state has , .
Since gives a stronger nuclear attraction, the deuterium ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. ...
is in the , state.
The same considerations lead to the possible states of an isospin triplet having , or , . Thus the state of lowest energy has , , higher than that of the isospin singlet.
The analysis just given is in fact only approximate, both because isospin is not an exact symmetry, and more importantly because the strong nuclear interaction between the two nucleons is related to angular momentum
In physics, angular momentum (rarely, moment of momentum or rotational momentum) is the rotational analog of linear momentum. It is an important physical quantity because it is a conserved quantityŌĆöthe total angular momentum of a closed syst ...
in spinŌĆōorbit interaction
In quantum physics, the spinŌĆōorbit interaction (also called spinŌĆōorbit effect or spinŌĆōorbit coupling) is a relativistic interaction of a particle's spin with its motion inside a potential. A key example of this phenomenon is the spinŌĆōorbi ...
that mixes different ''s'' and ''l'' states. That is, ''s'' and ''l'' are not constant in time (they do not commute
Commute, commutation or commutative may refer to:
* Commuting, the process of travelling between a place of residence and a place of work
Mathematics
* Commutative property, a property of a mathematical operation whose result is insensitive to th ...
with the Hamiltonian
Hamiltonian may refer to:
* Hamiltonian mechanics, a function that represents the total energy of a system
* Hamiltonian (quantum mechanics), an operator corresponding to the total energy of that system
** Dyall Hamiltonian, a modified Hamiltonian ...
), and over time a state such as , may become a state of , . Parity is still constant in time so these do not mix with odd ''l'' states (such as , ). Therefore, the quantum state
In quantum physics, a quantum state is a mathematical entity that provides a probability distribution for the outcomes of each possible measurement on a system. Knowledge of the quantum state together with the rules for the system's evolution in ...
of the deuterium is a superposition (a linear combination) of the , state and the , state, even though the first component is much bigger. Since the total angular momentum ''j'' is also a good quantum number
In quantum physics and chemistry, quantum numbers describe values of conserved quantities in the dynamics of a quantum system. Quantum numbers correspond to eigenvalues of operators that commute with the HamiltonianŌĆöquantities that can be kno ...
(it is a constant in time), both components must have the same ''j'', and therefore . This is the total spin of the deuterium nucleus.
To summarize, the deuterium nucleus is antisymmetric in terms of isospin, and has spin 1 and even (+1) parity. The relative angular momentum of its nucleons ''l'' is not well defined, and the deuteron is a superposition of mostly with some .
Magnetic and electric multipoles
In order to find theoretically the deuteriummagnetic dipole moment
In electromagnetism, the magnetic moment is the magnetic strength and orientation of a magnet or other object that produces a magnetic field. Examples of objects that have magnetic moments include loops of electric current (such as electromagnets ...
''╬╝'', one uses the formula for a nuclear magnetic moment
:
with
:
''g''(''l'') and ''g''(''s'') are ''g''-factors of the nucleons.
Since the proton and neutron have different values for ''g''(''l'') and ''g''(''s''), one must separate their contributions. Each gets half of the deuterium orbital angular momentum and spin . One arrives at
:
where subscripts p and n stand for the proton and neutron, and .
By using the same identities as here
Here is an adverb that means "in, on, or at this place". It may also refer to:
Software
* Here Technologies, a mapping company
* Here WeGo (formerly Here Maps), a mobile app and map website by Here
Television
* Here TV (formerly "here!"), a TV ...
and using the value , we arrive at the following result, in units of the nuclear magneton ''╬╝''N
: