alkali–silica reaction on:
[Wikipedia]
[Google]
[Amazon]
 The alkali–silica reaction (ASR), more commonly known as concrete cancer, is a deleterious swelling reaction that occurs over time in
The alkali–silica reaction (ASR), more commonly known as concrete cancer, is a deleterious swelling reaction that occurs over time in
 To attempt to simplify and to stylize a very complex set of various reactions, the whole ASR reaction, after its complete evolution (ageing process) in the presence of sufficient Ca2+ cations available in solution, could be compared to the
To attempt to simplify and to stylize a very complex set of various reactions, the whole ASR reaction, after its complete evolution (ageing process) in the presence of sufficient Ca2+ cations available in solution, could be compared to the
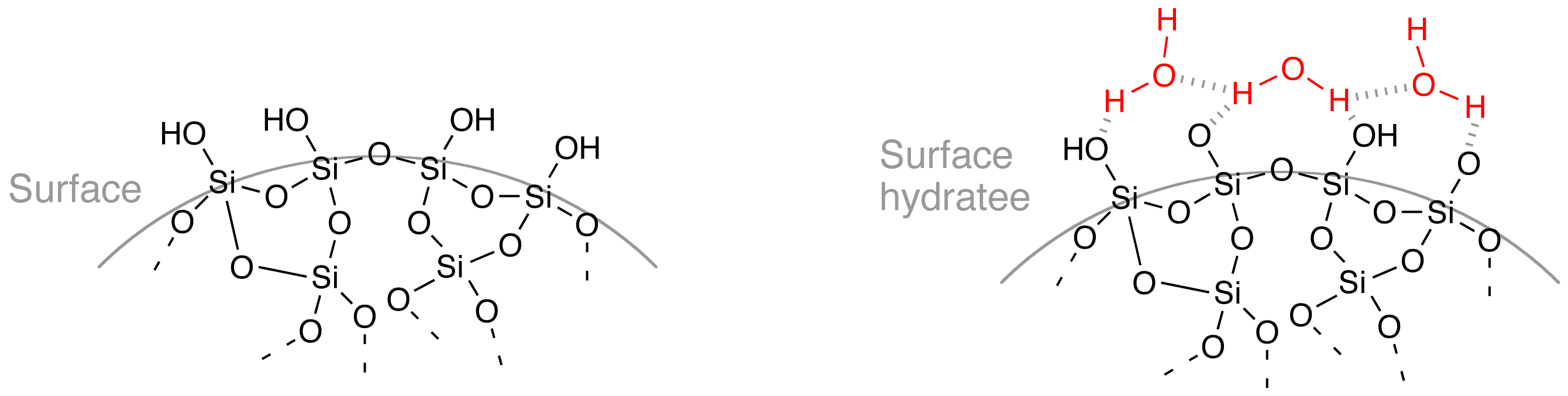 The surface of solid silica in contact with water is covered by
The surface of solid silica in contact with water is covered by  : ≡Si–O–Si≡ + ↔ ≡Si–OH + HO–Si≡
: =Si=O + ↔ =
: ≡Si–O–Si≡ + ↔ ≡Si–OH + HO–Si≡
: =Si=O + ↔ =
 On the other hand,
On the other hand,
The effect of secondary ettringite formation on the durability of concrete: A literature analysis (No. RD108T).
See mainly Chapter 7: Rapid test method for secondary ettringite formation. pp. 81-95 of the PDF file (pp. 69-83 of the hard copy)
Available in open access on the site of Cement.org
/ref> * The concrete microbar test was proposed by Grattan-Bellew ''et al.'' (2003) as a universal accelerated test for alkali-aggregate reaction. * CSA A23.1-14A and CSA A23.2-14A: Canadian CSA standard concrete prism tests for potential expansivity of cement/aggregate combinations.A23.1-14/A23.2-14 Concrete materials and methods of concrete construction / Test methods and standard practices for concrete. Published by CSA Group in 2014, 690 pages.
/ref> CSA A23.2-14A is a long-term test in which concrete prisms are stored under saturated moist conditions at a temperature of 38 °C, for a minimum of 365 days. It is the Canadian standard equivalent to ASTM C1293. * LCPC/IFSTTAR (1997) LPC-44. Alkali reaction in concrete. Residual expansion tests on hardened concrete.LCPC/IFSTTAR (1997) Alcali-réaction du béton. Essai d'expansion résiduelle sur béton durci. Projet de méthode d'essai LCP 44. Février 1997. 15 pp. MethodeDEssai-LCPC-ME44.pdf. https://www.ifsttar.fr/fileadmin/user_upload/editions/lcpc/MethodeDEssai/MethodeDEssai-LCPC-ME44.pdf * RILEM AAR-3 concrete prism method (storage at 38 °C). * RILEM AAR-4 concrete prism method (storage at 60 °C). * RILEM AAR-4 alternative method (storage at 60 °C). * German concrete test method (storage at 40 °C). * Norwegian concrete prism method (storage at 38 °C).

 The alkali–silica reaction (ASR), more commonly known as concrete cancer, is a deleterious swelling reaction that occurs over time in
The alkali–silica reaction (ASR), more commonly known as concrete cancer, is a deleterious swelling reaction that occurs over time in concrete
Concrete is a composite material composed of fine and coarse aggregate bonded together with a fluid cement (cement paste) that hardens (cures) over time. Concrete is the second-most-used substance in the world after water, and is the most ...
between the highly alkaline cement
A cement is a binder, a chemical substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel (aggregate) together. Cement m ...
paste and the reactive amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal.
Etymology
The term comes from the Greek language ...
(''i.e.'', non-crystalline) silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is o ...
found in many common aggregates, given sufficient moisture.
This deleterious chemical reaction causes the expansion of the altered aggregate by the formation of a soluble and viscous gel of sodium silicate
Sodium silicate is a generic name for chemical compounds with the formula or ·, such as sodium metasilicate , sodium orthosilicate , and sodium pyrosilicate . The anions are often polymeric. These compounds are generally colorless transpare ...
(Na2SiO3, also noted Na2H2SiO4, or N-S-H (sodium silicate hydrate), depending on the adopted convention). This hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance ...
gel swells and increases in volume when absorbing water: it exerts an expansive pressure inside the siliceous
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is ...
aggregate, causing spalling
Spall are fragments of a material that are broken off a larger solid body. It can be produced by a variety of mechanisms, including as a result of projectile impact, corrosion, weathering, cavitation, or excessive rolling pressure (as in a ball ...
and loss of strength of the concrete, finally leading to its failure.
ASR can lead to serious cracking in concrete, resulting in critical structural problems that can even force the demolition
Demolition (also known as razing, cartage, and wrecking) is the science and engineering in safely and efficiently tearing down of buildings and other artificial structures. Demolition contrasts with deconstruction, which involves taking a ...
of a particular structure. The expansion of concrete through reaction between cement and aggregates was first studied by Thomas E. Stanton in California during the 1930s with his founding publication in 1940.
Chemistry
 To attempt to simplify and to stylize a very complex set of various reactions, the whole ASR reaction, after its complete evolution (ageing process) in the presence of sufficient Ca2+ cations available in solution, could be compared to the
To attempt to simplify and to stylize a very complex set of various reactions, the whole ASR reaction, after its complete evolution (ageing process) in the presence of sufficient Ca2+ cations available in solution, could be compared to the pozzolanic reaction
The pozzolanic activity is a measure for the degree of reaction over time or the reaction rate between a pozzolan and Ca2+ or calcium hydroxide (Ca(OH)2) in the presence of water. The rate of the pozzolanic reaction is dependent on the intrinsic c ...
which would be catalysed by the undesirable presence of excessive concentrations of alkali hydroxides (NaOH and KOH) in the concrete. It is a mineral acid-base reaction between NaOH
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
or KOH, calcium hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime ( calcium oxide) is mixed or slaked with water. It has m ...
, also known as portlandite
Portlandite is a hydroxide-bearing mineral typically included in the oxide mineral class. It is the naturally occurring form of calcium hydroxide (Ca(OH)2) and the calcium analogue of brucite (Mg(OH)2).
Occurrence
Portlandite occurs in a variety ...
, or (Ca(OH)2), and silicic acid
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one ...
(H4SiO4, or Si(OH)4). For simplifying, after a complete exchange of the alkali cations with the calcium ions released by portlandite, the alkali-silica reaction in its ultimate stage leading to calcium silicate hydrate
Calcium silicate hydrate (or C-S-H) is the main product of the hydration of Portland cement and is primarily responsible for the strength in cement based materials (e.g. concrete).
Preparation
When water is added to cement, each of the compounds u ...
(C-S-H) could be schematically represented as following:
:Ca(OH)2 + H4SiO4 → Ca2+ + H2SiO42− + 2 H2O → CaH2SiO4
Here, the silicic acid H4SiO4, or Si(OH)4, which is equivalent to SiO2 · 2 H2O represents hydrous or amorphous silica for the sake of simplicity in aqueous chemistry.
Indeed, the term ''silicic acid'' has traditionally been used as a synonym
A synonym is a word, morpheme, or phrase that means exactly or nearly the same as another word, morpheme, or phrase in a given language. For example, in the English language, the words ''begin'', ''start'', ''commence'', and ''initiate'' are al ...
for silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is o ...
, SiO2. Strictly speaking, silica is the anhydride
An organic acid anhydride is an acid anhydride that is an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the pa ...
of orthosilicic acid, Si(OH)4.
:SiO2↓ + 2 H2O Si(OH)4
An ancient industrial notation referring to , metasilicic acid, is also often used to depict the alkali-silica reaction. However, the metasilicic acid, , or , is a hypothetic molecule which has never been observed, even in extreme diluted solutions because is unstable and continue to hydrate.
Indeed, contrary to the hydration of CO2 which consumes only one water molecule and stops at H2CO3, the hydration of SiO2 consumes two water molecules and continues one step further to form H4SiO4. The difference in hydration behaviour between SiO2 and CO2 is explained by thermodynamic
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of t ...
reasons (Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work (physics), work that may be performed by a closed system, thermodynamically closed system a ...
) and by bond energy
In chemistry, bond energy (''BE''), also called the mean bond enthalpy or average bond enthalpy is the measure of bond strength in a chemical bond. IUPAC defines bond energy as the average value of the gas-phase bond-dissociation energy (usually a ...
or steric hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
around the central atom of the molecule.
That is why the more correct geochemical notation referring to the orthosilicic acid
Orthosilicic acid () is an inorganic compound with the formula . Although rarely observed, it is the key compound of silica and silicates and the precursor to other silicic acids . Silicic acids play important roles in biomineralization and te ...
really existing in dilute solution is preferred here. However, the main advantage of the now deprecated, but still often used, industrial notation referring to the metasilicate anion (), which also does not exist in aqueous solution, is its greater simplicity and its direct similitude in notation with the carbonate () system.
One will also note that the NaOH and KOH species (alkali hydroxide
The alkali hydroxides are a class of chemical compounds which are composed of an alkali metal cation and the hydroxide anion (OH−). The alkali hydroxides are:
* Lithium hydroxide (LiOH)
* Sodium hydroxide (NaOH)
* Potassium hydroxide (KOH)
* Ru ...
s, also often simply called alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of ...
to refer to their strongly basic character) which catalyze
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
and accelerate the silica dissolution in the alkali-silica reaction do not explicitly appear in this simplified representation of the ultimate reaction with portlandite, because they are continuously regenerated from the cation exchange reaction with portlandite. As a consequence, they disappear from the global mass balance equation of the catalyzed reaction.
Silica dissolution mechanism
 The surface of solid silica in contact with water is covered by
The surface of solid silica in contact with water is covered by siloxane
A siloxane is a functional group in organosilicon chemistry with the Si−O−Si linkage. The parent siloxanes include the oligomeric and polymeric hydrides with the formulae H(OSiH2)''n''OH and (OSiH2)n. Siloxanes also include branched compound ...
bonds (≡Si–O–Si≡) and silanol
A silanol is a functional group in silicon chemistry with the connectivity Si–O–H. It is related to the hydroxy functional group (C–O–H) found in all alcohols. Silanols are often invoked as intermediates in organosilicon ...
groups (≡Si–OH) sensitive to an alkaline attack by ions.
The presence of these oxygen-bearing groups very prone to form hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing ...
s with water molecules explains the affinity of silica for water and makes colloidal silica very hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are ...
.
Siloxane bonds may undergo hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
and condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
reactions as schematically represented hereafter:
 : ≡Si–O–Si≡ + ↔ ≡Si–OH + HO–Si≡
: =Si=O + ↔ =
: ≡Si–O–Si≡ + ↔ ≡Si–OH + HO–Si≡
: =Si=O + ↔ =
 On the other hand,
On the other hand, silanol
A silanol is a functional group in silicon chemistry with the connectivity Si–O–H. It is related to the hydroxy functional group (C–O–H) found in all alcohols. Silanols are often invoked as intermediates in organosilicon ...
groups can also undergo protonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid ...
/deprotonation:
: ≡Si–OH ↔ ≡Si– + .
These equilibria can be shifted towards the right side of the reaction leading to silica dissolution by increasing the concentration of hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water ...
anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
(OH–), i.e., by increasing the pH of the solution.
Alkaline hydrolysis of siloxane bonds occurs by nucleophilic substitution
In chemistry, a nucleophilic substitution is a class of chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass c ...
of OH– onto a silicon atom, while another –O–Si group is leaving to preserve the tetravalent character of Si atom:
: ≡Si–O–Si≡ + → ≡Si–OH + –O–Si≡
: =Si=O + → =
Deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju. ...
of silanol
A silanol is a functional group in silicon chemistry with the connectivity Si–O–H. It is related to the hydroxy functional group (C–O–H) found in all alcohols. Silanols are often invoked as intermediates in organosilicon ...
groups:
: ≡Si–OH + → ≡Si– + .
In the pH range 0 – 7, the solubility of silica is constant, but above pH 8, the hydrolysis of siloxane bonds and deprotonation of silanol groups exponentially increase with pH. This is why glass easily dissolves at high pH and does not withstand extremely basic NaOH/KOH solutions. Therefore, NaOH/KOH released during cement hydration attacks and dissolves the tridimensional network of silica present in the aggregates. Amorphous or poorly crystallized silica, as cryptocrystalline
Cryptocrystalline is a rock texture made up of such minute crystals that its crystalline nature is only vaguely revealed even microscopically in thin section by transmitted polarized light. Among the sedimentary rocks, chert and flint are crypto ...
chalcedony
Chalcedony ( , or ) is a cryptocrystalline form of silica, composed of very fine intergrowths of quartz and moganite. These are both silica minerals, but they differ in that quartz has a trigonal crystal structure, while moganite is monocl ...
or chert
Chert () is a hard, fine-grained sedimentary rock composed of microcrystalline or cryptocrystalline quartz, the mineral form of silicon dioxide (SiO2). Chert is characteristically of biological origin, but may also occur inorganically as a ...
present in flint
Flint, occasionally flintstone, is a sedimentary cryptocrystalline form of the mineral quartz, categorized as the variety of chert that occurs in chalk or marly limestone. Flint was widely used historically to make stone tools and sta ...
s (in chalk
Chalk is a soft, white, porous, sedimentary carbonate rock. It is a form of limestone composed of the mineral calcite and originally formed deep under the sea by the compression of microscopic plankton that had settled to the sea floor. Cha ...
) or rolled river gravel
Gravel is a loose aggregation of rock fragments. Gravel occurs naturally throughout the world as a result of sedimentary and erosive geologic processes; it is also produced in large quantities commercially as crushed stone.
Gravel is classif ...
s, is much more soluble and sensitive to alkaline attack by OH– anions than well crystallized silica such as quartz
Quartz is a hard, crystalline mineral composed of silica ( silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical ...
. Strained (deformed) quartz or chert exposed to freeze-thaw cycles in Canada and Nordic countries are also more sensitive to alkaline (high pH) solutions.
The species responsible for silica dissolution is the hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water ...
anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
(OH–). The high pH conditions are said to be alkaline
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
and one also speaks of the alkalinity
Alkalinity (from ar, القلوي, al-qaly, lit=ashes of the saltwort) is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale.
Alkalinity is the strengt ...
of the basic solutions. For the sake of electroneutrality, (OH–) anions need to be accompanied by positively charged cations, Na+ or K+ in NaOH
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
or KOH solutions respectively. Na and K both belong to the alkali metals column in the Periodic Table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ...
. When speaking of alkali's, one systematically refers to NaOH and KOH basic hydroxides, or their corresponding oxides Na2O and K2O in cement. Therefore, it is the hydroxide, or the oxide, component of the salt which is the only relevant chemical species for silica dissolution, not the alkali metal in itself. However, to determine the alkali equivalent content (Na2Oeq) in cement, because the need to maintain electroneutrality in solids or in solution, one directly measures the contents of cement in Na and K elements and one conservatively considers that their counter ions are the hydroxide ions. As Na+ and K+ cations are hydrated species, they also contribute to retain water in alkali-silica reaction products.
Osmotic processes (Chatterji ''et al.'', 1986, 1987, 1989) and the electrical double layer (EDL) play also a fundamental role in the transport of water towards the concentrated liquid alkali gel explaining their swelling behavior and the deleterious expansion of aggregates responsible of ASR damages in concrete.
Catalysis of ASR by dissolved NaOH or KOH
The ASR reaction significantly differs from the pozzolanic reaction by the fact that it is catalysed by solublealkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of ...
hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water ...
s (NaOH
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
/ KOH) at very high pH. It can be represented as follows using the classical geochemical notation for representing silica by the fully hydrated dissolved silica (Si(OH)4 or silicic acid
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one ...
: H4SiO4), but an older industrial notation also exists (H2SiO3, hemihydrated silica (does not exist), by analogy with carbonic acid):
:2 Na(OH) + H4SiO4 → Na2H2SiO4
:the so-produced soluble alkali silicagel can then react with calcium hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime ( calcium oxide) is mixed or slaked with water. It has m ...
(portlandite
Portlandite is a hydroxide-bearing mineral typically included in the oxide mineral class. It is the naturally occurring form of calcium hydroxide (Ca(OH)2) and the calcium analogue of brucite (Mg(OH)2).
Occurrence
Portlandite occurs in a variety ...
) to precipitate insoluble calcium silicate hydrate
Calcium silicate hydrate (or C-S-H) is the main product of the hydration of Portland cement and is primarily responsible for the strength in cement based materials (e.g. concrete).
Preparation
When water is added to cement, each of the compounds u ...
s (C-S-H phases) and regenerate NaOH for continuing the initial silica dissolution reaction:
:Na2H2SiO4 + Ca(OH)2 → CaH2SiO4 + 2 NaOH
The sum, or the combination, of the two above mentioned reactions gives a general reaction resembling the pozzolanic reaction, but it is important to keep in mind that this reaction is catalysed by the undesirable presence in cement, or other concrete components, of soluble alkaline hydroxydes (NaOH / KOH) responsible for the dissolution of the silica (silicic acid) at high pH:
:Ca(OH)2 + H4SiO4 → CaH2SiO4
Without the presence of dissolved NaOH or KOH, responsible for the high pH (~13.5) of the concrete pore water, the amorphous silica of the reactive aggregates would not be dissolved and the reaction would not evolve. Moreover, the soluble sodium or potassium silicate is very hygroscopic and swells when it absorbs water. When the sodium silicate gel forms and swells inside a porous siliceous aggregate, it first expands and occupies the free porosity. When this latter is completely filled, if the soluble but very viscous gel cannot be easily expelled from the silica network, the hydraulic pressure rises inside the attacked aggregate and leads to its fracture. It is the hydro-mechanical expansion of the damaged siliceous aggregate surrounded by calcium-rich hardened cement paste which is responsible for the development of a network of cracks in concrete. When the sodium silicate expelled from the aggregate encounters grains of portlandite present in the hardened cement paste, an exchange between sodium and calcium cations occurs and hydrated calcium silicate (C-S-H) precipitates with a concomitant release of NaOH. In its turn, the regenerated NaOH can react with the amorphous silica aggregate leading to an increased production of soluble sodium silicate. When a continuous rim of C-S-H completely envelops the external surface of the attacked siliceous aggregate, it behaves as a semi-permeable barrier and hinders the expulsion of the viscous sodium silicate while allowing the NaOH / KOH to diffuse from the hardened cement paste inside the aggregate. This selective barrier of C-S-H contributes to increase the hydraulic pressure inside the aggregate and aggravates the cracking process. It is the expansion of the aggregates which damages concrete in the alkali-silica reaction.
Portlandite (Ca(OH)2) represents the main reserve of OH– anions in the solid phase as suggested by Davies and Oberholster (1988) and emphasized by Wang and Gillott (1991). As long as portlandite, or the siliceous aggregates, has not become completely exhausted, the ASR reaction will continue. The alkali hydroxides are continuously regenerated by the reaction of the sodium silicate with portlandite and thus represent the transmission belt of the ASR reaction driving it to completeness. It is thus impossible to interrupt the ASR reaction. The only way to avoid ASR in the presence of siliceous aggregates and water is to maintain the concentration of soluble alkali (NaOH and KOH) at the lowest possible level in concrete, so that the catalysis mechanism becomes negligible.
Analogy with the soda lime and concrete carbonatation
The alkali-silica reaction mechanism catalysed by a solublestrong base
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. ...
as NaOH or KOH in the presence of Ca(OH)2 (alkalinity buffer present in the solid phase) can be compared with the carbonatation
Carbonatation is a chemical reaction in which calcium hydroxide reacts with carbon dioxide and forms insoluble calcium carbonate:
:Ca(OH)2CO2->CaCO3H_2O
The process of forming a carbonate is sometimes referred to as "carbonation", although th ...
process of soda lime
Soda lime is a mixture of NaOH and CaO chemicals, used in granular form in closed breathing environments, such as general anaesthesia, submarines, rebreathers and recompression chambers, to remove carbon dioxide from breathing gases to preven ...
. The silicic acid
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one ...
( H2SiO3 or SiO2) is simply replaced in the reaction by the carbonic acid ( H2CO3 or CO2).
:
In the presence of water or simply ambient moisture, the strong bases, NaOH or KOH, readily dissolve in their hydration water (hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance ...
substances, deliquescence
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance ...
phenomenon) and this greatly facilitates the catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycl ...
process because the reaction in aqueous solution occurs much faster than in the dry solid phase. The moist NaOH impregnates the surface and the porosity
Porosity or void fraction is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0% and 100%. Strictly speaking, some tests measure ...
of calcium hydroxide grains with a high specific surface area. Soda lime is commonly used in closed-circuit diving rebreathers and in anesthesia
Anesthesia is a state of controlled, temporary loss of sensation or awareness that is induced for medical or veterinary purposes. It may include some or all of analgesia (relief from or prevention of pain), paralysis (muscle relaxation), a ...
systems.
The same catalytic effect by the alkali hydroxide
The alkali hydroxides are a class of chemical compounds which are composed of an alkali metal cation and the hydroxide anion (OH−). The alkali hydroxides are:
* Lithium hydroxide (LiOH)
* Sodium hydroxide (NaOH)
* Potassium hydroxide (KOH)
* Ru ...
s (function of the Na2Oeq content of cement
A cement is a binder, a chemical substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel (aggregate) together. Cement m ...
) also contributes to the carbonatation of portlandite
Portlandite is a hydroxide-bearing mineral typically included in the oxide mineral class. It is the naturally occurring form of calcium hydroxide (Ca(OH)2) and the calcium analogue of brucite (Mg(OH)2).
Occurrence
Portlandite occurs in a variety ...
by atmospheric CO2 in concrete
Concrete is a composite material composed of fine and coarse aggregate bonded together with a fluid cement (cement paste) that hardens (cures) over time. Concrete is the second-most-used substance in the world after water, and is the most ...
although the rate of propagation of the reaction front is there essentially limited by the CO2 diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
within the concrete matrix less porous
Porosity or void fraction is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0% and 100%. Strictly speaking, some tests measure ...
.
The soda lime carbonatation reaction can be directly translated into the ancient industrial notation of silicate (referring to the never observed metasilicic acid) simply by substituting a C atom by a Si atom in the mass balance equations (''i.e.'', by replacing a carbonate by a metasilicate anion). This gives the following set of reactions also commonly encountered in the literature to schematically depict the continuous regeneration of NaOH in ASR:
:
If NaOH is clearly deficient in the system under consideration (soda lime or alkali-silica reaction), it is formally possible to write the same reactions sets by simply replacing the CO32- anions by HCO3− and the SiO32- anions by HSiO3−, the principle of catalysis remaining the same, even if the number of intermediate species differs.
Main sources of in hardened cement paste
One can distinguish several sources of hydroxide anions () in hardened cement paste (HCP) from the family ofPortland cement
Portland cement is the most common type of cement in general use around the world as a basic ingredient of concrete, mortar, stucco, and non-specialty grout. It was developed from other types of hydraulic lime in England in the early 19th cen ...
(pure OPC, with BFS, or with cementitious additions, FA or SF).
Direct sources
anions can be directly present in the HCP pore water or be slowly released from the solid phase (main buffer, or solid stock) by the dissolution of (portlandite) when its solubility increases when high pH value starts to drop. Beside these two main sources, ions exchange reactions and precipitation of poorly soluble calcium salts can also contribute to release into solution. Alkali hydroxides, NaOH and KOH, arise from the direct dissolution of and oxides produced by the pyrolysis of the raw materials at high temperature (1450 °C) in thecement kiln
Cement kilns are used for the pyroprocessing stage of manufacture of portland and other types of hydraulic cement, in which calcium carbonate reacts with silica-bearing minerals to form a mixture of calcium silicates. Over a billion tonnes of ...
. The presence of minerals with high Na and K contents in the raw materials can thus be problematic. The ancient wet manufacturing process of cement, consuming more energy (water evaporation) that the modern dry process, had the advantage to eliminate much of the soluble Na and K salts present in the raw material.
As previously described in the two sections dealing respectively with ASR catalysis by alkali hydroxides and soda lime carbonatation, soluble NaOH and KOH are continuously regenerated and released into solution when the soluble alkali silicate reacts with to precipitate insoluble calcium silicate. As suggested by Davies and Oberholster (1988), the alkali-silica reaction is self-perpetuating as the alkali hydroxides are continuously regenerated in the system. Therefore, portlandite is the main buffer of in the solid phase. As long as the stock of hydroxides in the solid phase is not exhausted, the alkali-silica reaction can continue to proceed until complete disparition of one of the reagents ( or ) involved in the pozzolanic reaction
The pozzolanic activity is a measure for the degree of reaction over time or the reaction rate between a pozzolan and Ca2+ or calcium hydroxide (Ca(OH)2) in the presence of water. The rate of the pozzolanic reaction is dependent on the intrinsic c ...
.
Indirect sources
There exist also other indirect sources of , all related to the presence of soluble Na and K salts in the pore water of hardened cement paste (HCP). The first category contains soluble Na and K salts whose corresponding anions can precipitate an insoluble calcium salts, e.g., , , , , , ... . Hereafter, an example forcalcium sulfate
Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris, ...
(gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard or sidewalk chalk, and dr ...
, anhydrite
Anhydrite, or anhydrous calcium sulfate, is a mineral with the chemical formula CaSO4. It is in the orthorhombic crystal system, with three directions of perfect cleavage parallel to the three planes of symmetry. It is not isomorphous with t ...
) precipitation releasing sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkal ...
:
: + → + 2 NaOH
or, the reaction of sodium carbonate with portlandite, also important for the catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycl ...
of the alkali–carbonate reaction as emphasized by Fournier and Bérubé (2000) and Bérubé ''et al.'' (2005):Fournier, B., & Bérubé, M. A. (2000). Alkali-aggregate reaction in concrete: a review of basic concepts and engineering implications. Canadian Journal of Civil Engineering, 27(2), 167-191. See the chemical equations on p. 168.Bérubé, M. A., Smaoui, N., Bissonnette, B., & Fournier, B. (2005). Outil d'évaluation et de gestion des ouvrages d'art affectés de réactions alcalis-silice (RAS). Études et Recherches en Transport, Ministère des Transports du Québec. See the chemical equations on pp. 3-4.
: + → + 2 NaOH
However, not all Na or K soluble salts can precipitate insoluble calcium salts, such as, ''e.g.'', NaCl-based deicing salts:
: 2 + ← + 2 NaOH
As calcium chloride is a soluble salt, the reaction cannot occur and the chemical equilibrium regresses to the left side of the reaction.
So, a question arises: can NaCl or KCl from deicing salts still possibly play a role in the alkali-silica reaction? and cations in themselves cannot attack silica (the culprit is their counter ion ) and soluble alkali chlorides cannot produce soluble alkali hydroxide by interacting with calcium hydroxide. So, does it exist another route to still produce hydroxide anions in the hardened cement paste (HCP)?
Beside portlandite, other hydrated solid phases are present in HCP. The main phases are the calcium silicate hydrate
Calcium silicate hydrate (or C-S-H) is the main product of the hydration of Portland cement and is primarily responsible for the strength in cement based materials (e.g. concrete).
Preparation
When water is added to cement, each of the compounds u ...
s (C-S-H) (the "''glue''" in cement paste), calcium sulfo-aluminate phases ( AFm and AFt, ettringite
Ettringite is a hydrous calcium aluminium sulfate mineral with formula: . It is a colorless to yellow mineral crystallizing in the trigonal system. The prismatic crystals are typically colorless, turning white on partial dehydration. It is part o ...
) and hydrogarnet. C-S-H phases are less soluble (~ 10−5 M) than portlandite (CH) (~ 2.2 10−2 M at 25 °C) and therefore are expected to play a negligible role for the calcium ions release.
An anion-exchange reaction between chloride ions and the hydroxide anions contained in the lattice of some calcium aluminate hydrates (C-A-H), or related phases (C-A-S-H, AFm, AFt), is suspected to also contribute to the release of hydroxide anions into solution. The principle mechanism is schematically illustrated hereafter for C-A-H phases:
: + (C-A-H)–OH → (C-A-H)–Cl +
As a simple, but robust, conclusion, the presence of soluble Na and K salts can also cause, by precipitation of poorly soluble calcium salt (with portlandite, CH) or anion exchange reactions (with phases related to C-A-H), the release of anions into solution. Therefore, the presence of any salts of Na and K in cement pore water is undesirable and the measurements of Na and K elements is a good proxy (indicator
Indicator may refer to:
Biology
* Environmental indicator of environmental health (pressures, conditions and responses)
* Ecological indicator of ecosystem health (ecological processes)
* Health indicator, which is used to describe the health o ...
) for the maximal concentration of in pore solution. This is why the total alkali equivalent content () of cement can simply rely on the measurements of Na and K (''e.g.'', by ICP-AES, AAS, XRF measurements techniques).
Alkali gel evolution and ageing
The maturation process of the fluid alkali silicagel found in exudations into less soluble solid products found in gel pastes or in efflorescences is described hereafter. Four distinct steps are considered in this progressive transformation. 1. dissolution and formation (here, explicitly written in the ancient industrial metasilicate notation (based on the non-existing metasilicic acid, ) to also illustrate the frequent use of this later in the literature): :2 NaOH + → · (young N-S-H gel) :this reaction is accompanied by hydration and swelling of the alkali gel leading to the expansion of the affected aggregates. The pH of the fresh alkali gel is very high and it has often a characteristic amber color. The high pH of young alkali gel exudations often precludes the growth of mosses at the surface of concrete crack infilling. 2. Maturation of the alkali gel: polymerisation and gelation by thesol–gel process
In materials science, the sol–gel process is a method for producing solid materials from small molecules. The method is used for the fabrication of metal oxides, especially the oxides of silicon (Si) and titanium (Ti). The process involves conv ...
. Condensation of silicate monomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
s or oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relat ...
s dispersed in a colloidal solution (sol) into a biphasic aqueous polymeric network of silicagel. divalent cations released by calcium hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime ( calcium oxide) is mixed or slaked with water. It has m ...
(portlandite
Portlandite is a hydroxide-bearing mineral typically included in the oxide mineral class. It is the naturally occurring form of calcium hydroxide (Ca(OH)2) and the calcium analogue of brucite (Mg(OH)2).
Occurrence
Portlandite occurs in a variety ...
) when the pH starts to slightly drop may influence the gelation process.
3. Cation exchange with calcium hydroxide (portlandite) and precipitation of amorphous calcium silicate hydrate
Calcium silicate hydrate (or C-S-H) is the main product of the hydration of Portland cement and is primarily responsible for the strength in cement based materials (e.g. concrete).
Preparation
When water is added to cement, each of the compounds u ...
s (C-S-H) accompanied by NaOH regeneration:
: + → + 2 NaOH
:Amorphous non-stoechiometric calcium silicate hydrates (C-S-H, the non-stoechiometry being denoted here by the use of dashes) can recrystallize into rosettes similar to these of gyrolite
Gyrolite, NaCa16(Si23Al)O60(OH)8·14H2O, is a rare silicate mineral (basic sodium calcium silicate hydrate: N-C-S-H, in cement chemist notation) belonging to the class of phyllosilicates. Gyrolite is also often associated with zeolites. It is mos ...
. The C-S-H formed at this stage can be considered an evolved calcium silicate hydrate.
4. Carbonation of the C-S-H leading to precipitation of calcium carbonate and amorphous SiO2 stylized as follows:
: + → +
As long as the alkali gel () has not yet reacted with ions released from portlandite dissolution, it remains fluid and can easily exude from broken aggregates or through open cracks in the damage concrete structure. This can lead to visible yellow viscous liquid exudations (amber liquid droplets) at the surface of affected concrete.
When pH slowly drops due to the progress of the silica dissolution reaction, solubility of calcium hydroxide increases and the alkali gel reacts with ions. Its viscosity increases due to gelation process and its mobility (fluidity) strongly decreases when C-S-H phases start to precipitate after reaction with calcium hydroxide (portlandite). At this moment, the calcified gel becomes hard, hindering therefore the alkali gel transport in concrete.
When the C-S-H gel is exposed to atmospheric carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
, it undergoes a rapid carbonation and white/yellow efflorescence
In chemistry, efflorescence (which means "to flower out" in French) is the migration of a salt to the surface of a porous material, where it forms a coating. The essential process involves the dissolving of an internally held salt in water, or ...
s appear at the surface of concrete. When the relatively fluid alkali gel continue to exude below the hardened superficial gel layer, it pushes the efflorescences out of the crack surface making them to appear in relief. Because the gel drying and carbonation reactions rates are faster than the gel exudation velocity (liquid gel expulsion rate through open cracks), in most of the cases, fresh liquid alkali exudates are not frequently encountered at the surface of civil engineering concrete structures. Decompressed concrete cores can sometimes let observe fresh yellow liquid alkali exudations (viscous amber droplets) just after their drilling.
Mechanism of concrete deterioration
The mechanism of ASR causing the deterioration of concrete can thus be described in four steps as follows: # The very basic solution (NaOH / KOH) attacks the siliceous aggregates (silicic acid dissolution at high pH), converting the poorly crystallised or amorphous silica to a soluble but very viscous alkali silicate gel (N-S-H, K-S-H). # The consumption of NaOH / KOH by the dissolution reaction of amorphous silica decreases the pH of the pore water of the hardened cement paste. This allows the dissolution of Ca(OH)2 (portlandite
Portlandite is a hydroxide-bearing mineral typically included in the oxide mineral class. It is the naturally occurring form of calcium hydroxide (Ca(OH)2) and the calcium analogue of brucite (Mg(OH)2).
Occurrence
Portlandite occurs in a variety ...
) and increases the concentration of Ca2+ ions into the cement pore water. Calcium ions then react with the soluble sodium silicate gel to convert it into solid calcium silicate hydrates (C-S-H). The C-S-H forms a continuous poorly permeable coating at the external surface of the aggregate.
# The penetrated alkaline solution (NaOH / KOH) converts the remaining siliceous minerals into bulky soluble alkali silicate gel. The resulting expansive pressure increases in the core of the aggregate.
# The accumulated pressure cracks the aggregate and the surrounding cement paste when the pressure exceeds the tolerance of the aggregate.
Structural effects of ASR
The cracking caused by ASR can have several negative impacts on concrete, including: # Expansion: The swelling nature of ASR gel increases the chance of expansion in concrete elements. #Compressive strength
In mechanics, compressive strength or compression strength is the capacity of a material or structure to withstand loads tending to reduce size (as opposed to tensile strength which withstands loads tending to elongate). In other words, compre ...
: The effect of ASR on compressive strength can be minor for low expansion levels, to relatively higher degrees at larger expansion. (Swamy R.N. 1986) points out that the compressive strength is not very accurate parameter to study the severity of ASR; however, the test is done because of its simplicity.
# Tensile strength
Ultimate tensile strength (UTS), often shortened to tensile strength (TS), ultimate strength, or F_\text within equations, is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials ...
/ Flexural capacity: Researches show that ASR cracking can significantly reduce the tensile strength of concrete; therefore reducing the flexural capacity of beams. Some research on bridge structures indicate about 85% loss of capacity as a result of ASR.
# Modulus of elasticity
An elastic modulus (also known as modulus of elasticity) is the unit of measurement of an object's or substance's resistance to being deformed elastically (i.e., non-permanently) when a stress is applied to it. The elastic modulus of an object is ...
/UPV: The effect of ASR on elastic properties of concrete and ultrasound pulse velocity (UPV) is very similar to tensile capacity. The modulus of elasticity is shown to be more sensitive to ASR than pulse velocity.
# Fatigue
Fatigue describes a state of tiredness that does not resolve with rest or sleep. In general usage, fatigue is synonymous with extreme tiredness or exhaustion that normally follows prolonged physical or mental activity. When it does not resolve ...
: ASR reduces the load bearing capacity and the fatigue life of concrete (Ahmed T. 2000).
# Shear strength
In engineering, shear strength is the strength of a material or component against the type of yield or structural failure when the material or component fails in shear. A shear load is a force that tends to produce a sliding failure on a mater ...
: ASR enhances the shear capacity of reinforced concrete with and without shear reinforcement (Ahmed T. 2000).
Mitigation
ASR can be mitigated in new concrete by several approaches: # ''Limit the alkali metal content of the cement''. Many standards impose limits on the "Equivalent Na2O" content of cement. # ''Limit the reactive silica content of the aggregate''. Certain volcanic rocks are particularly susceptible to ASR because they contain volcanic glass ( obsidian) and should not be used as aggregate. The use of calcium carbonate aggregates can avoid this. In principle,limestone
Limestone ( calcium carbonate ) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of . Limestone forms wh ...
(CaCO3) the level of silica depends on its purity. Some siliceous limestones (a.o., '' Kieselkalk'' found in Switzerland) may be cemented by amorphous or poorly crystalline silica and can be very sensitive to the ASR reaction, as also observed with some Tournaisian siliceous limestones exploited in quarries in the area of Tournai
Tournai or Tournay ( ; ; nl, Doornik ; pcd, Tornai; wa, Tornè ; la, Tornacum) is a city and municipality of Wallonia located in the province of Hainaut, Belgium. It lies southwest of Brussels on the river Scheldt. Tournai is part of Eu ...
in Belgium
Belgium, ; french: Belgique ; german: Belgien officially the Kingdom of Belgium, is a country in Northwestern Europe. The country is bordered by the Netherlands to the north, Germany to the east, Luxembourg to the southeast, France to ...
. The use of limestone as aggregate is not a guarantee against ASR in itself. In Canada, the Spratt siliceous limestone is also particularly well known in studies dealing with ASR and is commonly used as the Canadian ASR reference aggregate.
# Add very fine siliceous materials to neutralize the excessive alkalinity
Alkalinity (from ar, القلوي, al-qaly, lit=ashes of the saltwort) is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale.
Alkalinity is the strengt ...
of cement with silicic acid
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one ...
by a controlled pozzolanic reaction
The pozzolanic activity is a measure for the degree of reaction over time or the reaction rate between a pozzolan and Ca2+ or calcium hydroxide (Ca(OH)2) in the presence of water. The rate of the pozzolanic reaction is dependent on the intrinsic c ...
at the early stage of the cement setting. Pozzolanic materials to add to the mix may be, ''e.g.'', pozzolan
Pozzolans are a broad class of siliceous and aluminous materials which, in themselves, possess little or no cementitious value but which will, in finely divided form and in the presence of water, react chemically with calcium hydroxide (Ca(OH)2) ...
, silica fume
Silica fume, also known as microsilica, (CAS number 69012-64-2, EINECS number 273-761-1) is an amorphous (non-crystalline) polymorph of silicon dioxide, silica. It is an ultrafine powder collected as a by-product of the silicon and ferrosilicon a ...
, fly ash
Fly ash, flue ash, coal ash, or pulverised fuel ash (in the UK) plurale tantum: coal combustion residuals (CCRs)is a coal combustion product that is composed of the particulates (fine particles of burned fuel) that are driven out of coal-fired ...
, or metakaolin
Metakaolin is the anhydrous calcined form of the clay mineral kaolinite. Minerals that are rich in kaolinite are known as china clay or kaolin, traditionally used in the manufacture of porcelain. The particle size of metakaolin is smaller than c ...
. These react preferentially with the cement alkalis without formation of an expansive pressure, because siliceous minerals in fine particles convert to alkali silicate and then to calcium silicate without formation of semipermeable reaction rims.
#Limit the external alkalis that come in contact with the system.
A prompt reaction initiated at the early stage of concrete hardening on very fine silica particles will help to suppress a slow and delayed reaction with larger siliceous aggregates on the long term. Following the same principle, the fabrication of low-pH cement also implies the addition of finely divided pozzolanic materials rich in silicic acid to the concrete mix to decrease its alkalinity. Beside initially lowering the pH value of the concrete pore water, the main working mechanism of silica fume addition is to consume portlandite (the reservoir of hydroxyde (OH–) in the solid phase) and to decrease the porosity of the hardened cement paste by the formation of calcium silicate hydrates (C-S-H). However, silica fume has to be very finely dispersed in the concrete mix because agglomerated flakes of compacted silica fume can themselves also induce ASR if the dispersion process is insufficient. This can be the case in laboratory studies made on cement pastes alone in the absence of aggregates. Silica fume is sufficiently dispersed during mixing operations of large batches of fresh concrete by the presence of coarse and fine aggregates.
As part of a study conducted by the Federal Highway Administration
The Federal Highway Administration (FHWA) is a division of the United States Department of Transportation that specializes in highway transportation. The agency's major activities are grouped into two programs, the Federal-aid Highway Program ...
, a variety of methods have been applied to field structures suffering from ASR-affected expansion and cracking. Some methods, such as the application of silane
Silane is an inorganic compound with chemical formula, . It is a colourless, pyrophoric, toxic gas with a sharp, repulsive smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Sila ...
s, have shown significant promise, especially when applied to elements such as small columns and highway barriers. The topical application of lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid ...
compounds, have shown little or no promise in reducing ASR-induced expansion and cracking.
Curative treatment
There are no curative treatments in general for ASR affected structures. Repair in damaged sections is possible, but the reaction will continue. In some cases, when a sufficient drying of thin components (walls, slabs) of a structure is possible, and is followed by the installation of a watertightmembrane
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. ...
, the evolution of the reaction can be slowed down, and sometimes stopped, due to the lack of water needed to continue fueling the reaction. Indeed, water plays a triple role in the alkali-silica reaction: solvent for the reaction taking place, transport medium for the dissolved species reacting, and finally also reagent consumed by the reaction itself.
However, concrete at the center of thick concrete components or structures can never dry because water transport in saturated or unsaturated conditions is always limited by diffusion in the concrete pores (water present under the liquid form, or under the vapor state). The water diffusion time is thus proportional to the square of its transport distance. As a consequence, the water saturation degree inside thick concrete structures often remains higher than 80%, a level sufficient to provide enough water to the system and to maintain the alkali-silica reaction on going.
Massive structures such as dams pose particular problems: they cannot be easily replaced, and the swelling can block spillway
A spillway is a structure used to provide the controlled release of water downstream from a dam or levee, typically into the riverbed of the dammed river itself. In the United Kingdom, they may be known as overflow channels. Spillways ensure t ...
gates or turbine
A turbine ( or ) (from the Greek , ''tyrbē'', or Latin ''turbo'', meaning vortex) is a rotary mechanical device that extracts energy from a fluid flow and converts it into useful work. The work produced by a turbine can be used for generating ...
operations. Cutting slots across the structure can relieve some pressure, and help restore geometry and function.
Heavy aggregates for nuclear shielding concrete
Two types of heavy aggregates are commonly used for nuclear shielding concrete in order to efficiently absorbgamma-ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically sh ...
s: baryte
Baryte, barite or barytes ( or ) is a mineral consisting of barium sulfate ( Ba S O4). Baryte is generally white or colorless, and is the main source of the element barium. The ''baryte group'' consists of baryte, celestine (strontium sulfate), ...
(, density = 4.3 – 4.5) and various types of iron oxide
Iron oxides are chemical compounds composed of iron and oxygen. Several iron oxides are recognized. All are black magnetic solids. Often they are non-stoichiometric. Oxyhydroxides are a related class of compounds, perhaps the best known of w ...
s, mainly magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With ...
(, density = 5.2) and hematite
Hematite (), also spelled as haematite, is a common iron oxide compound with the formula, Fe2O3 and is widely found in rocks and soils. Hematite crystals belong to the rhombohedral lattice system which is designated the alpha polymorph of ...
(, density = 5.3). The reason is their high density favorable to gamma attenuation. Both types of aggregates need to be checked for ASR as they may contain reactive silica impurities under a form or another.
As elevated temperature in the range of may be reached in the concrete of the primary confinement wall around nuclear reactors, particular attention has to be paid to the selection of aggregates and heavy aggregates to avoid alkali-silica reaction promoted by reactive silica impurities and accelerated by the high temperature to which concrete is exposed.
In some hydrothermal deposits, baryte is associated with silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is o ...
mineralization and can also contain reactive cristobalite
Cristobalite is a mineral polymorph of silica that is formed at very high temperatures. It has the same chemical formula as quartz, SiO2, but a distinct crystal structure. Both quartz and cristobalite are polymorphs with all the members of the q ...
while oxy-hydroxides of Fe(III), in particular ferrihydrite
Ferrihydrite (Fh) is a widespread hydrous ferric oxyhydroxide mineral at the Earth's surface, and a likely constituent in extraterrestrial materials. It forms in several types of environments, from freshwater to marine systems, aquifers to hyd ...
, exhibit a strong affinity for dissolved silica present in water and may constitute an ultimate sink for .
This explains how microcrystalline silica can progressively accumulate in the mineral gangue
In mining, gangue () is the commercially worthless material that surrounds, or is closely mixed with, a wanted mineral in an ore deposit. It is thus distinct from overburden, which is the waste rock or materials overlying an ore or mineral body ...
of iron oxides.
Dissolved silica (), and its corresponding silicate anion (), strongly sorbs onto hydrous ferric oxides (HFO) and ferric oxides hydrated surface (>Fe–OH) by ligand exchange:
: + >Fe–OH +
In this ligand exchange reaction, a silicate anion (also often more simply written as ) is making a nucleophilic substitution
In chemistry, a nucleophilic substitution is a class of chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass c ...
onto a >Fe–OH ferrol surface group of HFO and ejects a hydroxide anion while taking its place onto the ferrol group. This mechanism explains the formation of strong inner sphere complexes of silica at the surface of iron oxy-hydroxides and iron oxides. The surface of iron oxides becomes progressively coated with silica and a silica gangue
In mining, gangue () is the commercially worthless material that surrounds, or is closely mixed with, a wanted mineral in an ore deposit. It is thus distinct from overburden, which is the waste rock or materials overlying an ore or mineral body ...
forms at the surface of iron oxide ores. This explains why some iron ores are rich in silica and may therefore be sensitive to the alkali-silica reaction. Very low level of reactive silica in heavy aggregates are sufficient to induce ASR. This is why heavy aggregates must be systematically tested for ASR before nuclear applications such as radiation shielding or immobilization of strongly irradiating radioactive waste
Radioactive waste is a type of hazardous waste that contains radioactive material. Radioactive waste is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, rare-earth mining, and nuclear weap ...
.
Another reason of concern for the possible accelerated development of ASR in the concrete of nuclear structures is the progressive amorphization of the silica contained in aggregates exposed to high neutron fluence
The neutron flux, φ, is a scalar quantity used in nuclear physics and nuclear reactor physics. It is the total length travelled by all free neutrons per unit time and volume. Equivalently, it can be defined as the number of neutrons travelli ...
. This process is also known as metamictization and is known to create amorphous halo's in minerals like zircon
Zircon () is a mineral belonging to the group of nesosilicates and is a source of the metal zirconium. Its chemical name is zirconium(IV) silicate, and its corresponding chemical formula is Zr SiO4. An empirical formula showing some of th ...
rich in uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weakly ...
and thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
when their crystal structure is submitted to intense alpha-particles internal bombardment and becomes amorph ( metamict state).
The loss of mechanical properties of heavily neutron-irradiated concrete component such as the biological shield of a reactor at the end of the service life of a nuclear power plant is expected to be due to radiation-induced swelling of aggregates, which leads to volumetric expansion of the concrete.
Prevention of the risk
The only way to prevent, or to limit, the risk of ASR is to avoid one or several of the three elements in the critical triangle aggregate reactivity – cement alkali content – water: * by selecting non-reactive aggregates after testing them according to an appropriate standard test method (see next section); * by using a low-alkali (LA) cement: with a maximum alkali content expressed in < 0.60% of the cement mass, according to EN 197-1 European standard for cement,EN 197-1 European Standard. Cement – Part 1: Composition, specifications and conformity criteria for common cements. or by limiting the total alkali content in concrete (''e.g.'', less than 3 kg /m3 of concrete for a CEM I cement (OPC)). Example of standard for concrete in Belgium: NBN EN 206 and its national supplement NBN B 15-001;NBN EN 206:2013+A1:2016 Concrete – Specification, performance, production and conformity. Publication date: 11/2016.NBN B 15-001:2018. Concrete – Specification, performance, production and conformity – National supplement for Belgium to NBN EN 206:2013+A1:2016. Publication date: 07/2018. * by limiting the contact of underground or meteoritic water infiltrations with the concrete structure (water tight membrane, roofing, sufficient water drainage, ...). This last precaution is always advisable when possible and the only one also sometimes applicable for existing ASR-affected concrete structures.Methods for testing potential alkali reactivity
The American Society for Testing and Materials (ASTM International
ASTM International, formerly known as American Society for Testing and Materials, is an international standards organization that develops and publishes voluntary consensus technical standards for a wide range of materials, products, systems, a ...
) has developed different standardized test methods for screening aggregates for their susceptibility to ASR:
* ASTM C227: "Test Method for Potential Alkali Reactivity of Cement-Aggregate Combinations (Mortar-Bar Method)"
* ASTM C289: "Standard Test Method for Potential Alkali-Silica Reactivity of Aggregates (Chemical Method)"
* ASTM C295: "Guide for Petrographic Examination of Aggregate for Concrete"
* ASTM C1260: "Test Method for Potential Reactivity of Aggregates (Mortar-Bar-Test)". It is a rapid test of aggregates: immersion of mortar bars in NaOH 1 M at 80 °C for 14 days used to quickly identify highly reactive aggregates or quasi non-reactive aggregates. Beside an elevated temperature, the C1260 method also involves the use of a large quantity/inventory of NaOH in the solution in which the mortar bar is immersed. A large pool of OH– anions is thus available to diffuse inside the mortar bar to dissolve silica present in aggregates. Consequently, this test is very severe and may exclude valuable aggregates. In case of non-decisive results, the long-term ASTM C1293 test method has to be used for a final screening. The main advantage of the ASTM C1260 test is that it allows to quickly identify extreme cases: very insensitive or very reactive aggregates.
* ASTM C1293: "Test Method for Concrete Aggregates by Determination of Length Change of Concrete Due to Alkali-Silica Reaction". It is a long-term confirmation test (1 or 2 years) at 38 °C in a water-saturated moist atmosphere (inside a thermostated oven) with concrete prisms containing the aggregates to be characterised mixed with a high-alkali cement specially selected to induce ASR. The concrete prisms are not directly immersed in an alkaline solution, but wrapped with moist tissues and tightly packed inside a water-tight plastic foils.
* ASTM C1567: "Standard Test Method for Determining the Potential Alkali-Silica Reactivity of Combinations of Cementitious Materials and Aggregate (Accelerated Mortar-Bar Method)"
Other concrete prism methods have also been internationally developed to detect potential alkali-reactivity of aggregates or sometimes hardened concrete cores, ''e.g.'':
* The Oberholster method on which the ASTM C1260 test is based. It is a severe short duration test with immersion of the mortar prism or concrete core in a solution of NaOH 1 M at 80 °C for 14 days.
* The Duggan method starts with a first immersion of several concrete cores in distilled water at 22 °C for rehydration during 3 days. It is then followed by heating for one day in a dry oven at 82 °C and then with a succession of cycles of one day hydration followed by one day drying at 82 °C. The expansion of the concrete cores is measured till 14 or 20 days.Scott, J.F., Duggan, C.R., (1986). Potential new test for alkali aggregate reactivity, Roe. 7th Intl. Conf. on Alkali Aggregate Reactions, Ottawa Canada, ed. P.E. Grattan-Bellew, Noyes publ., N.J., USA, 319-323.Duggan C.R., Scott J.F. (1987). Proposed new test for alkali-aggregate reactivity, Canadian National Railways, Technical Research Report, Montreal, Canada, April 13, 1987, revised Oct. 31,1989.Duggan C.R. and Scott J.F. (1989a). Establishment of new acceptance rejection limits for proposed test method for detection of potentially deleterious expansion of concrete, presented to ASTM Subcommittee C09.02.02, sept 1989.Duggan C.R. and Scott J.F. (1989b). New test for deleterious expansion in concrete, 8th Intl. Conf. on Alkali-Aggregate Reaction Kyoto, Japan, 403408. It is a short duration test for ASR/AAR but much softer than the Oberholster test. It can also be used to measure the expansion of concrete due to delayed ettringite formation (DEF). The mechanical stresses induced by the thermal cycles create micro-cracks in the concrete matrix and so facilitate the accessibility to water of the reactive mineral phases in the treated samples.Day, R. L. (1992)The effect of secondary ettringite formation on the durability of concrete: A literature analysis (No. RD108T).
See mainly Chapter 7: Rapid test method for secondary ettringite formation. pp. 81-95 of the PDF file (pp. 69-83 of the hard copy)
Available in open access on the site of Cement.org
/ref> * The concrete microbar test was proposed by Grattan-Bellew ''et al.'' (2003) as a universal accelerated test for alkali-aggregate reaction. * CSA A23.1-14A and CSA A23.2-14A: Canadian CSA standard concrete prism tests for potential expansivity of cement/aggregate combinations.A23.1-14/A23.2-14 Concrete materials and methods of concrete construction / Test methods and standard practices for concrete. Published by CSA Group in 2014, 690 pages.
/ref> CSA A23.2-14A is a long-term test in which concrete prisms are stored under saturated moist conditions at a temperature of 38 °C, for a minimum of 365 days. It is the Canadian standard equivalent to ASTM C1293. * LCPC/IFSTTAR (1997) LPC-44. Alkali reaction in concrete. Residual expansion tests on hardened concrete.LCPC/IFSTTAR (1997) Alcali-réaction du béton. Essai d'expansion résiduelle sur béton durci. Projet de méthode d'essai LCP 44. Février 1997. 15 pp. MethodeDEssai-LCPC-ME44.pdf. https://www.ifsttar.fr/fileadmin/user_upload/editions/lcpc/MethodeDEssai/MethodeDEssai-LCPC-ME44.pdf * RILEM AAR-3 concrete prism method (storage at 38 °C). * RILEM AAR-4 concrete prism method (storage at 60 °C). * RILEM AAR-4 alternative method (storage at 60 °C). * German concrete test method (storage at 40 °C). * Norwegian concrete prism method (storage at 38 °C).
Known affected structures

Australia
*Adelaide Festival Centre
Adelaide Festival Centre, Australia's first multi-purpose arts centre and the home of South Australia's performing arts, was built in the 1970s, designed by Hassell Architects. The Festival Theatre opened in June 1973 with the rest of the cent ...
car park, demolished in 2017
* Centennial Hall, Adelaide (1936-2007)
* Dee Why ocean pool, Dee Why
Dee Why is a coastal suburb of northern Sydney, in the state of New South Wales, Australia, 18 kilometres north-east of the Sydney central business district. It is the administrative centre of the local government area of Northern Beach ...
, Australia.
* King St Bridge, demolished and replaced in 2011 (crossing the Patawalonga River, Glenelg North, South Australia).
* Manly Surf Pavilion, Manly, Australia (1939–1981).
* The MCG's old Southern Stand, demolished in 1990 and replaced with the Great Southern Stand which was completed in 1992
* Westpoint Blacktown car park
Belgium
* Many bridges and civil engineering works of motorways because the improper use of highly reactive siliceousTournaisian
The Tournaisian is in the ICS geologic timescale the lowest stage or oldest age of the Mississippian, the oldest subsystem of the Carboniferous. The Tournaisian age lasted from Ma to Ma. It is preceded by the Famennian (the uppermost stag ...
limestone
Limestone ( calcium carbonate ) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of . Limestone forms wh ...
( lower carboniferous Dinantian) during the years 1960 – 1970 when most of the motorways were constructed in Belgium. ASR damages started to be recognised only in the 1980s. The Tournaisian limestone
Limestone ( calcium carbonate ) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of . Limestone forms wh ...
may contain up to 25–30 wt. % of reactive biogenic silica
Biogenic silica (bSi), also referred to as opal, biogenic opal, or amorphous opaline silica, forms one of the most widespread biogenic minerals. For example, microscopic particles of silica called phytoliths can be found in grasses and other plant ...
originating from the spicule
Spicules are any of various small needle-like anatomical structures occurring in organisms
Spicule may also refer to:
* Spicule (sponge), small skeletal elements of sea sponges
*Spicule (nematode), reproductive structures found in male nematodes ...
s of siliceous sponges deposited with calcium carbonate in the marine sediments.
* Pommeroeul lock
Lock(s) may refer to:
Common meanings
*Lock and key, a mechanical device used to secure items of importance
*Lock (water navigation), a device for boats to transit between different levels of water, as in a canal
Arts and entertainment
* ''Lock ...
in Hainaut on the canal Hensies – Pommeroeul – Condé.
* Tour & Taxis
Tour & Taxis (french: Tour et Taxis, nl, Thurn en Taxis) is a large former industrial site in Brussels, Belgium. It is situated on the Brussels Canal in the City of Brussels, just north-west of the city centre, immediately adjacent to Laeken ...
car access ramp in Brussels with liquid exudations of amber alkali gel evidenced on concrete cores by SPW experts (Public Services of Wallonia).
* External containment building of the Tihange 2 nuclear power plant.
* Poorly conditioned radioactive waste from the Doel nuclear power plant: evaporator concentrates and spent ion-exchange resins (SIER) exuding out of the concrete immobilization matrix very large quantities of liquid sodium silicagel.
Canada
Alkali-aggregate reactions (AAR), both alkali-silica (ASR) and alkali-carbonate (ACR, involving dolomite) reactions, were identified in Canada since the years 1950's.Rogers, C., Grattan-Bellew, P. E., Hooton, R. D., Ryell, J., & Thomas, M. D. (2000). Alkali-aggregate reactions in Ontario. Canadian Journal of Civil Engineering, 27(2), 246-260.Fournier, B., & Bérubé, M. A. (2000). Alkali-aggregate reaction in concrete: a review of basic concepts and engineering implications. Canadian Journal of Civil Engineering, 27(2), 167-191.Bérubé, M. A., Smaoui, N., Bissonnette, B., & Fournier, B. (2005). Outil d'évaluation et de gestion des ouvrages d'art affectés de réactions alcalis-silice (RAS). Études et Recherches en Transport, Ministère des Transports du Québec. * Many hydraulic dams are affected by ASR in Canada because of the wide use of reactive aggregates. Indeed, reactive frost-sensitivechert
Chert () is a hard, fine-grained sedimentary rock composed of microcrystalline or cryptocrystalline quartz, the mineral form of silicon dioxide (SiO2). Chert is characteristically of biological origin, but may also occur inorganically as a ...
is very often found in glacio-fluvial environments from which gravel
Gravel is a loose aggregation of rock fragments. Gravel occurs naturally throughout the world as a result of sedimentary and erosive geologic processes; it is also produced in large quantities commercially as crushed stone.
Gravel is classif ...
s are commonly extracted in Canada. Another reason is also the presence of reactive silica in Paleozoic
The Paleozoic (or Palaeozoic) Era is the earliest of three geologic eras of the Phanerozoic Eon.
The name ''Paleozoic'' ( ;) was coined by the British geologist Adam Sedgwick in 1838
by combining the Greek words ''palaiós'' (, "old") and ' ...
limestone
Limestone ( calcium carbonate ) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of . Limestone forms wh ...
s like the siliceous Ordovician
The Ordovician ( ) is a geologic period and system, the second of six periods of the Paleozoic Era. The Ordovician spans 41.6 million years from the end of the Cambrian Period million years ago (Mya) to the start of the Silurian Period Mya. ...
limestone (Bobcaygeon Formation
The Bobcaygeon Formation is a geologic formation in Ontario. It preserves fossils dating back to the Ordovician period.
See also
* List of fossiliferous stratigraphic units in Ontario
This is a list of stratigraphic units in Ontario bearin ...
) from the Spratt's quarry near Ottawa
Ottawa (, ; Canadian French: ) is the capital city of Canada. It is located at the confluence of the Ottawa River and the Rideau River in the southern portion of the province of Ontario. Ottawa borders Gatineau, Quebec, and forms the core ...
in Ontario
Ontario ( ; ) is one of the thirteen provinces and territories of Canada.Ontario is located in the geographic eastern half of Canada, but it has historically and politically been considered to be part of Central Canada. Located in Central C ...
.Limestone Industries of Ontario, Volume 2 Ontario Geological Survey. Engineering and Terrain Geology Section Ontario, Ministry of Natural Resources, 1989 – Limestone – 196 pages The Spratt's limestone aggregates (from the company ''"Spratt Sand and Gravel Limited"'') are widely used for ASR studies in Canada and worldwide as described by Rogers ''et al.'' (2000) and also recommended by RILEM (International Union of Laboratories and Experts in Construction Materials, Systems, and Structures).Nixon, J. P., & Sims, I. (Eds.). (2016). RILEM recommendation of the prevention of damage by alkali-aggregate reactions in new concrete structures. Dordrecht: Springer.
* Many bridges and civil engineering works of motorways.
* Interchange Robert Bourassa – Charest (Québec city: interchange autoroutes 740 – 440) demolished in 2010.Fournier, B., Sanchez, L., & Beauchemin, S. (2015). Outils d'investigation de la réactivité alcalis-granulats dans les infrastructures en béton. Rapport Final, Ministère des transports du Québec, Service des matériaux d'infrastructure, Secteur béton de ciment, août (Vol. 2015, p. 293).
* Gentilly 2 nuclear power plant.
* Building of the National Gallery of Canada
The National Gallery of Canada (french: Musée des beaux-arts du Canada), located in the capital city of Ottawa, Ontario, is Canada's national art museum. The museum's building takes up , with of space used for exhibiting art. It is one of the ...
at Ottawa
Ottawa (, ; Canadian French: ) is the capital city of Canada. It is located at the confluence of the Ottawa River and the Rideau River in the southern portion of the province of Ontario. Ottawa borders Gatineau, Quebec, and forms the core ...
.
France
* FormerTérénez bridge
The Térénez bridge is a cable-stayed bridge, located between Landévennec and Rosnoën, Finistère, France.
It is 515 meters long, and connects Brittany with the Crozon peninsula.
It is a fan cable arrangement, with a curved deck.
Construct ...
in Brittany, built in 1951 and replaced in 2011.
Germany
* East GermanDeutsche Reichsbahn
The ''Deutsche Reichsbahn'', also known as the German National Railway, the German State Railway, German Reich Railway, and the German Imperial Railway, was the German national railway system created after the end of World War I from the regi ...
used numerous concrete ties in the 1970s to replace previous wooden ties. However, the gravel from the Baltic Sea
The Baltic Sea is an arm of the Atlantic Ocean that is enclosed by Denmark, Estonia, Finland, Germany, Latvia, Lithuania, Poland, Russia, Sweden and the North and Central European Plain.
The sea stretches from 53°N to 66°N latitude and fr ...
caused ASR and the ties had to be replaced earlier than planned, lasting well into the 1990s.
* After reunification, many Autobahn
The (; German plural ) is the federal controlled-access highway system in Germany. The official German term is (abbreviated ''BAB''), which translates as 'federal motorway'. The literal meaning of the word is 'Federal Auto(mobile) Track'. ...
s in East Germany were refurbished with concrete that turned out to have been defective and affected by ASR, necessitating expensive replacement work.
New Zealand
* Fairfield Bridge in Hamilton, New Zealand. Repaired in 1991 at a cost of NZ$1.1 million.United Kingdom
* Keybridge House, South Lambeth Road,Vauxhall
Vauxhall ( ) is a district in South West London, part of the London Borough of Lambeth, England. Vauxhall was part of Surrey until 1889 when the County of London was created. Named after a medieval manor, "Fox Hall", it became well known for ...
, London, England
England is a country that is part of the United Kingdom. It shares land borders with Wales to its west and Scotland to its north. The Irish Sea lies northwest and the Celtic Sea to the southwest. It is separated from continental Europe ...
.
* Millennium Stadium
The Millennium Stadium ( cy, Stadiwm y Mileniwm), known since 2016 as the Principality Stadium ( cy, Stadiwm Principality) for sponsorship reasons, is the national stadium of Wales. Located in Cardiff, it is the home of the Wales national ru ...
North Stand (part of the old National Stadium
Many countries have a national sport stadium, which typically serves as the primary or exclusive home for one or more of a country's national representative sports teams. The term is most often used in reference to an association football stadiu ...
), Cardiff
Cardiff (; cy, Caerdydd ) is the capital city, capital and List of urban areas in the United Kingdom, largest city of Wales. It forms a Principal areas of Wales, principal area, officially known as the City and County of Cardiff ( cy, Dinas a ...
, Wales
Wales ( cy, Cymru ) is a Countries of the United Kingdom, country that is part of the United Kingdom. It is bordered by England to the Wales–England border, east, the Irish Sea to the north and west, the Celtic Sea to the south west and the ...
.
* Merafield Bridge, A38, England
England is a country that is part of the United Kingdom. It shares land borders with Wales to its west and Scotland to its north. The Irish Sea lies northwest and the Celtic Sea to the southwest. It is separated from continental Europe ...
. Demolished manually in 2016.
*Pebble Mill Studios
Pebble Mill Studios was the BBC's television studio complex located in Edgbaston, Birmingham, England, United Kingdom, which served as the headquarters for BBC Birmingham from 1971 until 2004. The nine-acre site was opened by Princess Anne on ...
, Birmingham. Demolished in 2005
*Royal Devon and Exeter Hospital
The Royal Devon and Exeter Hospital (commonly referred to as RD&E), and with a main site sometimes known as Wonford Hospital, is a large teaching hospital situated in Exeter, Devon, England, and is run by the Royal Devon University Healthcare NH ...
, Wonford
Wonford is a former village, manor and ecclesiastical parish in Devon, England, now a part of the City of Exeter. The 13th century St Loye's Chapel situated within the parish now gives its name to the surrounding location. Wonford is situated n ...
. Demolished and replaced in the mid-1990s.
* Steve Bull Stand, Molineux Stadium
Molineux Stadium ( ) in Wolverhampton, West Midlands, England, has been the home ground of Premier League club Wolverhampton Wanderers since 1889. The first stadium built for use by a Football League club, it was one of the first British groun ...
, Wolverhampton
Wolverhampton () is a City status in the United Kingdom, city, metropolitan borough and administrative centre in the West Midlands (county), West Midlands, England. The population size has increased by 5.7%, from around 249,500 in 2011 United ...
United States
*Seabrook Station Nuclear Power Plant
The Seabrook Nuclear Power Plant, more commonly known as Seabrook Station, is a nuclear power plant located in Seabrook, New Hampshire, United States, approximately north of Boston and south of Portsmouth. It has operated since 1990. With i ...
in Seabrook, New Hampshire
Seabrook is a town in Rockingham County, New Hampshire, United States. The population was 8,401 at the 2020 census. Located at the southern end of the coast of New Hampshire on the border with Massachusetts, Seabrook is noted as the location of th ...
.
* Seminoe Dam in Wyoming
Wyoming () is a state in the Mountain West subregion of the Western United States. It is bordered by Montana to the north and northwest, South Dakota and Nebraska to the east, Idaho to the west, Utah to the southwest, and Colorado to the sou ...
.
* Chickamauga Dam in Tennessee
Tennessee ( , ), officially the State of Tennessee, is a landlocked state in the Southeastern region of the United States. Tennessee is the 36th-largest by area and the 15th-most populous of the 50 states. It is bordered by Kentucky to ...
.
* Sixth Street Viaduct
The Sixth Street Viaduct, also known as the Sixth Street Bridge, is a viaduct bridge that connects the Arts District in Downtown Los Angeles with the Boyle Heights neighborhood.
The Sixth Street Viaduct spans the Los Angeles River, the San ...
in Los Angeles. Demolished in 2016.
See also
* Alkali-carbonate reaction * Alkali–aggregate reaction *Calthemite
Calthemite is a secondary deposit, derived from concrete, lime, mortar or other calcareous material outside the cave environment.Smith, G.K. (2016). "Calcite straw stalactites growing from concrete structures", Cave and Karst Science 43(1), 4 ...
: Secondary calcium carbonate deposit growing under man-made structures
* Carbonatation
Carbonatation is a chemical reaction in which calcium hydroxide reacts with carbon dioxide and forms insoluble calcium carbonate:
:Ca(OH)2CO2->CaCO3H_2O
The process of forming a carbonate is sometimes referred to as "carbonation", although th ...
* Colloidal silica
* Construction aggregate
Construction aggregate, or simply aggregate, is a broad category of coarse- to medium-grained particulate material used in construction, including sand, gravel, crushed stone, slag, recycled concrete and geosynthetic aggregates. Aggregate ...
* Cracking pattern
* Crocodile cracking
Crocodile cracking, also called alligator cracking and perhaps misleadingly fatigue cracking, is a common type of distress in asphalt pavement. The following is more closely related to fatigue cracking which is characterized by interconnecting ...
: distress in asphalt pavement characterized by interconnecting or interlaced cracking in the asphalt layer
* Energetically modified cement (EMC)
* Gyrolite
Gyrolite, NaCa16(Si23Al)O60(OH)8·14H2O, is a rare silicate mineral (basic sodium calcium silicate hydrate: N-C-S-H, in cement chemist notation) belonging to the class of phyllosilicates. Gyrolite is also often associated with zeolites. It is mos ...
, a product of slag hydration and ASR gel ageing
* Hydrated silica
* Pozzolanic reaction
The pozzolanic activity is a measure for the degree of reaction over time or the reaction rate between a pozzolan and Ca2+ or calcium hydroxide (Ca(OH)2) in the presence of water. The rate of the pozzolanic reaction is dependent on the intrinsic c ...
* Silicate: see solid SiO2 hydrolysis/dissolution and Si–OH deprotonation reactions at high pH
* Siliceous sponge
* Soda lime
Soda lime is a mixture of NaOH and CaO chemicals, used in granular form in closed breathing environments, such as general anaesthesia, submarines, rebreathers and recompression chambers, to remove carbon dioxide from breathing gases to preven ...
: the mechanism of ASR catalysed by NaOH is analogous to the trapping mechanism of CO2 by Ca(OH)2 impregnated with NaOH
References
Further reading
*External links
* {{DEFAULTSORT:Alkali-silica reaction Building materials Catalysis Cement Chemical reactions Concrete Fracture mechanics Materials degradation Mechanical failure modes Patterns Pavements Silicates