|
Alkali–aggregate Reaction
Surface of a concrete pillar with crack pattern of alkali–silica reaction Alkali–aggregate reaction is a term mainly referring to a reaction which occurs over time in concrete between the highly alkaline cement paste and non-crystalline silicon dioxide, which is found in many common aggregates. This reaction can cause the expansion of the altered aggregate, leading to spalling and loss of strength of concrete. More accurate terminology The alkali–aggregate reaction is a general, but relatively vague, expression which can lead to confusion. More precise definitions include the following: # Alkali–silica reaction (ASR, the most common reaction of this type); # Alkali–silicate reaction, and; # Alkali–carbonate reaction. The alkali–silica reaction is the most common form of alkali–aggregate reaction. Two other types are: * the alkali–silicate reaction, in which layer silicate minerals (clay minerals), sometimes present as impurities, are attacked, and; * the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ASR Concrete Pillar National Gallery Of Canada 02
The Asr prayer ( ar, صلاة العصر ', "afternoon prayer") is one of the five mandatory salah (Islamic prayer). As an Islamic day starts at sunset, the Asr prayer is technically the fifth prayer of the day. If counted from midnight, it is the third prayer of the day.see 'Glossary' Retrieved 12 July 2020Significance of Offering The Isha Prayer and Its Benefits QuranReading website, Published 29 January 2015, Retrieved 14 May 2017 The Asr prayer consists of four obligatory rakat. An additional four rakat are [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Slaked Lime
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed or slaked with water. It has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide. Properties Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. With a solubility product ''K''sp of 5.02 at 25 °C, its dissociation in water is large enough that its solutions are basic according to the following dissolution reaction: : Ca(OH)2 → Ca2+ + 2 OH− At ambient temperature, calcium hydroxide ( portlandite) di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reactions
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (chemistry), products, which usually have properties different from the reactants. Reactions often consist of a sequence o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concrete
Concrete is a composite material composed of fine and coarse aggregate bonded together with a fluid cement (cement paste) that hardens (cures) over time. Concrete is the second-most-used substance in the world after water, and is the most widely used building material. Its usage worldwide, ton for ton, is twice that of steel, wood, plastics, and aluminum combined. Globally, the ready-mix concrete industry, the largest segment of the concrete market, is projected to exceed $600 billion in revenue by 2025. This widespread use results in a number of environmental impacts. Most notably, the production process for cement produces large volumes of greenhouse gas emissions, leading to net 8% of global emissions. Other environmental concerns include widespread illegal sand mining, impacts on the surrounding environment such as increased surface runoff or urban heat island effect, and potential public health implications from toxic ingredients. Significant research and developmen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cement
A cement is a binder, a chemical substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel (aggregate) together. Cement mixed with fine aggregate produces mortar for masonry, or with sand and gravel, produces concrete. Concrete is the most widely used material in existence and is behind only water as the planet's most-consumed resource. Cements used in construction are usually inorganic, often lime or calcium silicate based, which can be characterized as hydraulic or the less common non-hydraulic, depending on the ability of the cement to set in the presence of water (see hydraulic and non-hydraulic lime plaster). Hydraulic cements (e.g., Portland cement) set and become adhesive through a chemical reaction between the dry ingredients and water. The chemical reaction results in mineral hydrates that are not very water-soluble and so are quite durable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pozzolanic Reaction
The pozzolanic activity is a measure for the degree of reaction over time or the reaction rate between a pozzolan and Ca2+ or calcium hydroxide (Ca(OH)2) in the presence of water. The rate of the pozzolanic reaction is dependent on the intrinsic characteristics of the pozzolan such as the specific surface area, the chemical composition and the active phase content. Physical surface adsorption is not considered as being part of the pozzolanic activity, because no irreversible molecular bonds are formed in the process. Reaction The pozzolanic reaction is the chemical reaction that occurs in portland cement upon the addition of pozzolans. It is the main reaction involved in the Roman concrete invented in Ancient Rome and used to build, for example, the Pantheon. The pozzolanic reaction converts a silica-rich precursor with no cementing properties, to a calcium silicate, with good cementing properties. In chemical terms, the pozzolanic reaction occurs between calcium hydroxide, als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calthemite
Calthemite is a secondary deposit, derived from concrete, lime, mortar or other calcareous material outside the cave environment.Smith, G.K. (2016). "Calcite straw stalactites growing from concrete structures", Cave and Karst Science 43(1), 4–10. http://bcra.org.uk/pub/candks/index.html?j=127Smith, G K., (2015). "Calcite Straw Stalactites Growing From Concrete Structures". Proceedings of the 30th 'Australian Speleological Federation' conference, Exmouth, Western Australia, edited by Moulds, T. pp 93 -108 Calthemites grow on or under, man-made structures and mimic the shapes and forms of cave speleothems, such as stalactites, stalagmites, flowstone etc.Hill, C A and Forti, P, (1997). Cave Minerals of the World, Second Edition. untsville, Alabama: National Speleological Society Inc. Calthemite is derived from the Latin ''calx'' (genitive ''calcis'') "lime" + Latin < Greek ''théma'', "deposit" meaning ‘something laid down’, (also Mediaeval Latin ''thema'', "deposit") and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energetically Modified Cement
Energetically modified cements (EMCs) are a class of cements made from pozzolans (e.g. fly ash, volcanic ash, pozzolana), silica sand, blast furnace slag, or Portland cement (or blends of these ingredients). The term "energetically modified" arises by virtue of the mechanochemistry process applied to the raw material, more accurately classified as "high energy ball milling" (HEBM). This causes, amongst others, a thermodynamic transformation in the material to increase its chemical reactivity. For EMCs, the HEBM process used is a unique form of specialised vibratory milling discovered in Sweden and applied only to cementitious materials, here called "EMC Activation". By improving the reactivity of pozzolans, their strength-development ''rate'' is increased. This allows for compliance with modern product-performance requirements ("technical standards") for concretes and mortars. In turn, this allows for the replacement of Portland cement in the concrete and mortar mixes. This h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
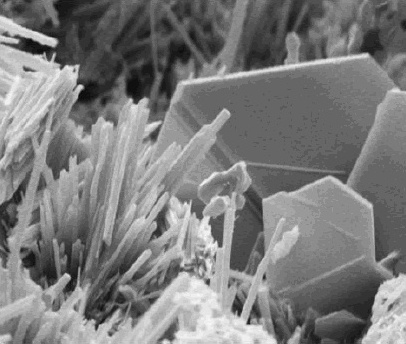
Calcium Silicate Hydrate
Calcium silicate hydrate (or C-S-H) is the main product of the hydration of Portland cement and is primarily responsible for the strength in cement based materials (e.g. concrete). Preparation When water is added to cement, each of the compounds undergoes hydration and contributes to the final state of the concrete. Only calcium silicates contribute to the strength. Tricalcium silicate is responsible for most of the early strength (first 7 days). Dicalcium silicate, which reacts more slowly, only contributes to late strength. Calcium silicate hydrate (also shown as C-S-H) is a result of the reaction between the silicate phases of Portland cement and water. This reaction typically is expressed as: : Tricalcium silicate + water -> Calcium silicate hydrate + Calcium hydroxide + heat The stoichiometry of C-S-H in cement paste is variable and the state of chemically and physically bound water in its structure is not transparent, which is why "-" is used between C, S, and H. Synthet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pozzolan
Pozzolans are a broad class of siliceous and aluminous materials which, in themselves, possess little or no cementitious value but which will, in finely divided form and in the presence of water, react chemically with calcium hydroxide (Ca(OH)2) at ordinary temperature to form compounds possessing cementitious properties. The quantification of the capacity of a pozzolan to react with calcium hydroxide and water is given by measuring its pozzolanic activity. ''Pozzolana'' are naturally occurring pozzolans of volcanic origin. History Mixtures of calcined lime and finely ground, active aluminosilicate materials were pioneered and developed as inorganic binders in the Ancient world. Architectural remains of the Minoan civilization on Crete have shown evidence of the combined use of slaked lime and additions of finely ground potsherds for waterproof renderings in baths, cisterns and aqueducts. Evidence of the deliberate use of volcanic materials such as volcanic ashes or tuffs by t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




_Luleå_Sweden_08_2020.jpg)
.jpg)