|
Silicate
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for any salt of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate. The name "silicate" is sometimes extended to any anions containing silicon, even if they do not fit the general formula or contain other atoms besides oxygen; such as hexafluorosilicate .Most commonly, silicates are encountered as silicate minerals. For diverse manufacturing, technological, and artistic needs, silicates are versatile materials, both natural (such as granite, gravel, and garnet) and artificial (such as Portland cement, ceramics, glass, and waterglass). Structural principles In all silicates, silicon atom occupies the center of an idealized tetrahedro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicate Minerals
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust. In mineralogy, silica (silicon dioxide, ) is usually considered a silicate mineral. Silica is found in nature as the mineral quartz, and its polymorphs. On Earth, a wide variety of silicate minerals occur in an even wider range of combinations as a result of the processes that have been forming and re-working the crust for billions of years. These processes include partial melting, crystallization, fractionation, metamorphism, weathering, and diagenesis. Living organisms also contribute to this geologic cycle. For example, a type of plankton known as diatoms construct their exoskeletons ("frustules") from silica extracted from seawater. The frustules of dead diatoms are a major constituent of deep ocean sediment, and of diatomaceous earth. General structure A silicate mineral is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inosilicate
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust. In mineralogy, silica (silicon dioxide, ) is usually considered a silicate mineral. Silica is found in nature as the mineral quartz, and its polymorphs. On Earth, a wide variety of silicate minerals occur in an even wider range of combinations as a result of the processes that have been forming and re-working the crust for billions of years. These processes include partial melting, crystallization, fractionation, metamorphism, weathering, and diagenesis. Living organisms also contribute to this geologic cycle. For example, a type of plankton known as diatoms construct their exoskeletons ("frustules") from silica extracted from seawater. The frustules of dead diatoms are a major constituent of deep ocean sediment, and of diatomaceous earth. General structure A silicate mineral is general ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Because of its high chemical affinity for oxygen, it was not until 1823 that Jöns Jakob Berzelius was first able to prepare it and characterize it in pure form. Its oxides form a family of anions known as silicates. Its melting and boiling points of 1414 °C and 3265 °C, respectively, are the second highest among all the metalloids and nonmetals, being surpassed only by boron. Silicon is the eighth most common element in the universe by mass, but very rarely occurs as the pure element in the Earth's crust. It is widely distributed in space in cosmic dusts, planetoids, and planets as various forms of silicon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrosilicate
A pyrosilicate is a type of chemical compound; either an ionic compound that contains the pyrosilicate anion , or an organic compound with the hexavalent ≡-O-≡ group. The anion is also called disilicateViktor Renman (2017): "Structural and Electrochemical Relations in Electrode Materials for Rechargeable Batteries", Doctoral Thesis, Uppsala University, Department of Chemistry. ORCID: 0000-0001-8739-4054 Structure The pyrosilicate anion can be described as two tetrahedra that share a vertex (an oxygen atom). The vertices that are not shared carry a negative charge each. The structure of solid sodium pyrosilicate was described by Volker Kahlenberg and others in 2010.Volker Kahlenberg, Thomas Langreiter, and Erik Arroyabe (2010): " – The Missing Structural Link among Alkali Pyrosilicates". ''Zeitschrift für anorganishe und allgemeine Chemie'' (''Journal for Inorganic and General Chemistry''), volume 636, issue 11, pages 1974-1979. Yuri Smolin and Yuri Shepelev determin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluorosilicic Acid
Hexafluorosilicic acid is an inorganic compound with the chemical formula . Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless. Hexafluorosilicic acid is produced naturally on a large scale in volcanoes.Palache, C., Berman, H., and Frondel, C. (1951) Dana’s System of Mineralogy, Volume II: Halides, Nitrates, Borates, Carbonates, Sulfates, Phosphates, Arsenates, Tungstates, Molybdates, etc. John Wiley and Sons, Inc., New York, 7th edition.Anthony, J.W., Bideaux, R.A., Bladh, K.W., and Nichols, M.C. (1997) Handbook of Mineralogy, Volume III: Halides, Hydroxides, Oxides. Mineral Data Publishing, Tucson.link to bararite It is manufactured as a coproduct in the production of phosphate fertilizers. The resulting hexafluorosilicic acid is almost exclusively consumed as a precursor to aluminum trifluoride and synthetic cryolite, which are used in aluminium processing. Salts d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Metasilicate
Sodium metasilicate is the chemical substance with formula , which is the main component of commercial sodium silicate solutions. It is an ionic compound consisting of sodium cations and the polymeric metasilicate anions ��–sub>''n''. It is a colorless crystalline hygroscopic and deliquescent solid, soluble in water (giving an alkaline solution) but not in alcohols.Chemical Book"Sodium metasilicate" Accessed on 2018-05-13. Preparation and properties The anhydrous compound can be prepared by fusing silicon dioxide (silica, quartz) with sodium oxide in 1:1 molar ratio.J. F. Schairer and N. L. Bowen (1956): "The system ——". ''American Journal of Science'', volume 254, issue 3, pages 129-195 The compound crystallizes from solution as various hydrates, such as * pentahydrate ·5 (CAS 10213-79-3, EC 229-912-9, PubChem 57652358) * nonahydrate ·9 (CAS 13517-24-3, EC 229-912-9, PubChem 57654617)M. F. Bechtold (1955): "Polymerization and Properties of Dilute Aqueous Silicic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
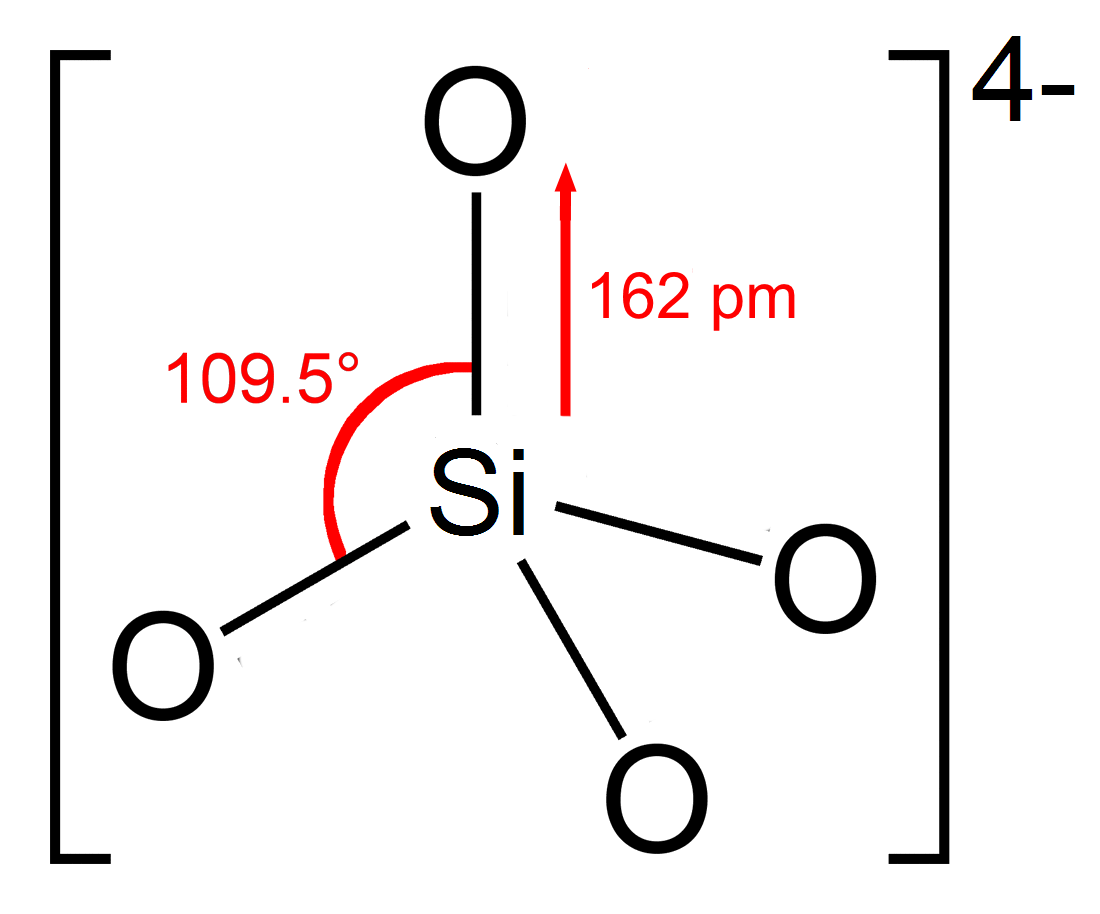
Orthosilicate
In chemistry, orthosilicate is the anion , or any of its salts and esters. It is one of the silicate anions. It is occasionally called the silicon tetroxide anion or group.C. A. Kumins, and A. E. Gessler (1953), "Short-Cycle Syntheses of Ultramarine Blue". ''Indunstrial & Engineering Chemistry'', volume 45, issue 3, pages 567–572. Orthosilicate salts, like sodium orthosilicate, are stable, and occur widely in nature as silicate minerals, being the defining feature of the nesosilicates. Olivine, a magnesium or iron(II) orthosilicate, is the most abundant mineral in the upper mantle. The orthosilicate anion is a strong base, the conjugate base of the extremely weak orthosilicic acid (p''K''a2 = 13.2 at 25 °C). This equilibrium is difficult to study since the acid tends to decompose into a hydrated silica condensate. Structure The orthosilicate ion or group has tetrahedral shape, with one silicon atom surrounded by four oxygen atoms. In the anion, each oxygen ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass
Glass is a non-Crystallinity, crystalline, often transparency and translucency, transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenching) of the Melting, molten form; some glasses such as volcanic glass are naturally occurring. The most familiar, and historically the oldest, types of manufactured glass are "silicate glasses" based on the chemical compound silicon dioxide, silica (silicon dioxide, or quartz), the primary constituent of sand. Soda–lime glass, containing around 70% silica, accounts for around 90% of manufactured glass. The term ''glass'', in popular usage, is often used to refer only to this type of material, although silica-free glasses often have desirable properties for applications in modern communications technology. Some objects, such as drinking glasses and glasses, eyeglasses, are so commonly made of silicate- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Olivine
The mineral olivine () is a magnesium iron silicate with the chemical formula . It is a type of nesosilicate or orthosilicate. The primary component of the Earth's upper mantle, it is a common mineral in Earth's subsurface, but weathers quickly on the surface. For this reason, olivine has been proposed as a good candidate for accelerated weathering to sequester carbon dioxide from the Earth's oceans and atmosphere, as part of climate change mitigation. Olivine also has many other historical uses, such as the gemstone peridot (or chrysolite), as well as industrial applications like metalworking processes. The ratio of magnesium to iron varies between the two endmembers of the solid solution series: forsterite (Mg-endmember: ) and fayalite (Fe-endmember: ). Compositions of olivine are commonly expressed as molar percentages of forsterite (Fo) and fayalite (Fa) (''e.g.'', Fo70Fa30). Forsterite's melting temperature is unusually high at atmospheric pressure, almost , whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyroxene
The pyroxenes (commonly abbreviated to ''Px'') are a group of important rock-forming inosilicate minerals found in many igneous and metamorphic rocks. Pyroxenes have the general formula , where X represents calcium (Ca), sodium (Na), iron (Fe II) or magnesium (Mg) and more rarely zinc, manganese or lithium, and Y represents ions of smaller size, such as chromium (Cr), aluminium (Al), magnesium (Mg), cobalt (Co), manganese (Mn), scandium (Sc), titanium (Ti), vanadium (V) or even iron (Fe II) or (Fe III). Although aluminium substitutes extensively for silicon in silicates such as feldspars and amphiboles, the substitution occurs only to a limited extent in most pyroxenes. They share a common structure consisting of single chains of silica tetrahedra. Pyroxenes that crystallize in the monoclinic system are known as clinopyroxenes and those that crystallize in the orthorhombic system are known as orthopyroxenes. The name ''pyroxene'' is derived from the Ancient Greek words for 'fire' ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metasilicate
320 px, Idealized structure of sodium metasilicate. Metasilicates are silicates containing ions of empirical formula . Common stoichiometries include MSiO3 and MIISiO3. Metasilicates can be cyclic, usually the hexamer or chains . Common compounds containing metasilicate anion are: * Metasilicic acid (hydrogen metasilicate) * Sodium metasilicate * Calcium metasilicate Wollastonite is a calcium inosilicate mineral ( Ca Si O3) that may contain small amounts of iron, magnesium, and manganese substituting for calcium. It is usually white. It forms when impure limestone or dolomite is subjected to high temperature a ... References {{Inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramethyl Orthosilicate
Tetramethyl orthosilicate (TMOS) is the chemical compound with the formula Si(OCH3)4. This molecule consists of four methoxy groups bonded to a silicon atom. The basic properties are similar to the more popular tetraethyl orthosilicate, which is usually preferred because the product of hydrolysis, ethanol, is less toxic than methanol. Tetramethyl orthosilicate hydrolyzes to SiO2: :Si(OCH3)4 + 2 H2O → SiO2 + 4 CH3OH In organic synthesis, Si(OCH3)4 has been used to convert ketones and aldehydes to the corresponding ketals and acetal In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragment ...s, respectively.Sakurai, H. "Silicon(IV) Methoxide" in Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley & Sons. Safety The hydrolysis of Si(OCH3)4 produces insoluble SiO2 an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



2SiF6%2C_ICSDcode_40388full.png)


