|
Lactate Racemase
The lactate racemase enzyme (Lar) () interconverts the D- and L-enantiomers of lactic acid. It is classified under the isomerase, racemase, epimerase, and enzyme acting on hydroxyl acids and derivatives classes of enzymes. It is found in certain halophilic archaea, such as ''Haloarcula marismortui'', and in a few species of bacteria, such as several ''Lactobacillus'' species (which produce D- and L- lactate) including ''Lactobacillus sakei'', ''Lactobacillus curvatus'', and ''Lactobacillus plantarum'', as well as in non-lactic acid bacteria such as ''Clostridium beijerinckii''. The gene encoding lactate racemase in ''L. plantarum'' was identified as ''larA'' and shown to be associated with a widespread maturation system involving ''larB'', ''larC1'', ''larC2'', and ''larE''. The optimal pH for its activity is 5.8-6.2 in ''L. sakei''. Structure and properties The molecular weight of lactate racemase differs in the various organisms in which it has been found, ranging from 25,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Weight
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not consi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pincer Ligand
In chemistry, a transition metal pincer complex is a type of coordination complex with a pincer ligand. Pincer ligands are chelating agents that binds tightly to three adjacent coplanar sites in a meridional configuration. The inflexibility of the pincer-metal interaction confers high thermal stability to the resulting complexes. This stability is in part ascribed to the constrained geometry of the pincer, which inhibits cyclometallation of the organic substituents on the donor sites at each end. In the absence of this effect, cyclometallation is often a significant deactivation process for complexes, in particular limiting their ability to effect C-H bond activation. The organic substituents also define a hydrophobic pocket around the reactive coordination site. Stoichiometric and catalytic applications of pincer complexes have been studied at an accelerating pace since the mid-1970s. Most pincer ligands contain phosphines.Jensen, C. M., "Iridium PCP pincer complexes: hig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometimes the symbol Cyz is used. The deprotonated form can generally be described by the symbol Cym as well. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. When used as a food additive, it has the E number E920. Cysteine is encoded by the codons UGU and UGC. The sulfur-containing amino acids cysteine and methionine are more easily oxidized than the other amino acids. Structure Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has chirality in the older / notation based on homology to - and -glyceraldehyde. In the newer ''R''/''S'' system of designating chi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Monophosphate
Adenosine monophosphate (AMP), also known as 5'-adenylic acid, is a nucleotide. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine; it is an ester of phosphoric acid and the nucleoside adenosine. As a substituent it takes the form of the prefix adenylyl-. AMP plays an important role in many cellular metabolic processes, being interconverted to Adenosine diphosphate, ADP and/or Adenosine triphosphate, ATP. AMP is also a component in the synthesis of RNA. AMP is present in all known forms of life. Production and degradation AMP does not have the high energy phosphoanhydride bond associated with ADP and ATP. AMP can be produced from Adenosine diphosphate, ADP: : 2 ADP → ATP + AMP Or AMP may be produced by the hydrolysis of one high energy phosphate bond of ADP: : ADP + H2O → AMP + phosphate, Pi AMP can also be formed by hydrolysis of Adenosine triphosphate, ATP into AMP and pyrophosphate: : ATP + H2O → AMP + pyrophosphate, PPi When RNA i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotinamide Adenine Dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine nucleobase and the other nicotinamide. NAD exists in two forms: an oxidized and reduced form, abbreviated as NAD and NADH (H for hydrogen), respectively. In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The cofactor is, therefore, found in two forms in cells: NAD is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction, also with H+, forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, most notably as a substrate of enzymes in adding or removing chemical groups to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michigan State University
Michigan State University (Michigan State, MSU) is a public university, public Land-grant university, land-grant research university in East Lansing, Michigan. It was founded in 1855 as the Agricultural College of the State of Michigan, the first of its kind in the United States. It is considered a Public Ivy, or a public institution which offers an academic experience similar to that of an Ivy League university. After the introduction of the Morrill Land-Grant Acts, Morrill Act in 1862, the state designated the college a land-grant institution in 1863, making it the first of the land-grant colleges in the United States. The college became coeducational in 1870. In 1955, the state officially made the college a university, and the current name, Michigan State University, was adopted in 1964. Today, Michigan State has the largest undergraduate enrollment among Michigan's colleges and universities and approximately 634,300 living alums worldwide. The university is a member of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactic Acid
Lactic acid is an organic acid. It has a molecular formula . It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as well as natural sources. Lactic acid is an alpha-hydroxy acid (AHA) due to the presence of a hydroxyl group adjacent to the carboxyl group. It is used as a synthetic intermediate in many organic synthesis industries and in various biochemical industries. The conjugate base of lactic acid is called lactate (or the lactate anion). The name of the derived acyl group is lactoyl. In solution, it can ionize by loss of a proton to produce the lactate ion . Compared to acetic acid, its p''K'' is 1 unit less, meaning lactic acid is ten times more acidic than acetic acid. This higher acidity is the consequence of the intramolecular hydrogen bonding between the α-hydroxyl and the carboxylate group. Lactic acid is chiral, consisting of two enantiomers. One ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomerase
Isomerases are a general class of enzymes that convert a molecule from one isomer to another. Isomerases facilitate intramolecular rearrangements in which bonds are broken and formed. The general form of such a reaction is as follows: A–B → B–A There is only one substrate yielding one product. This product has the same molecular formula as the substrate but differs in bond connectivity or spatial arrangement. Isomerases catalyze reactions across many biological processes, such as in glycolysis and carbohydrate metabolism. Isomerization Isomerases catalyze changes within one molecule. They convert one isomer to another, meaning that the end product has the same molecular formula but a different physical structure. Isomers themselves exist in many varieties but can generally be classified as structural isomers or stereoisomers. Structural isomers have a different ordering of bonds and/or different bond connectivity from one another, as in the case of hexane and it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
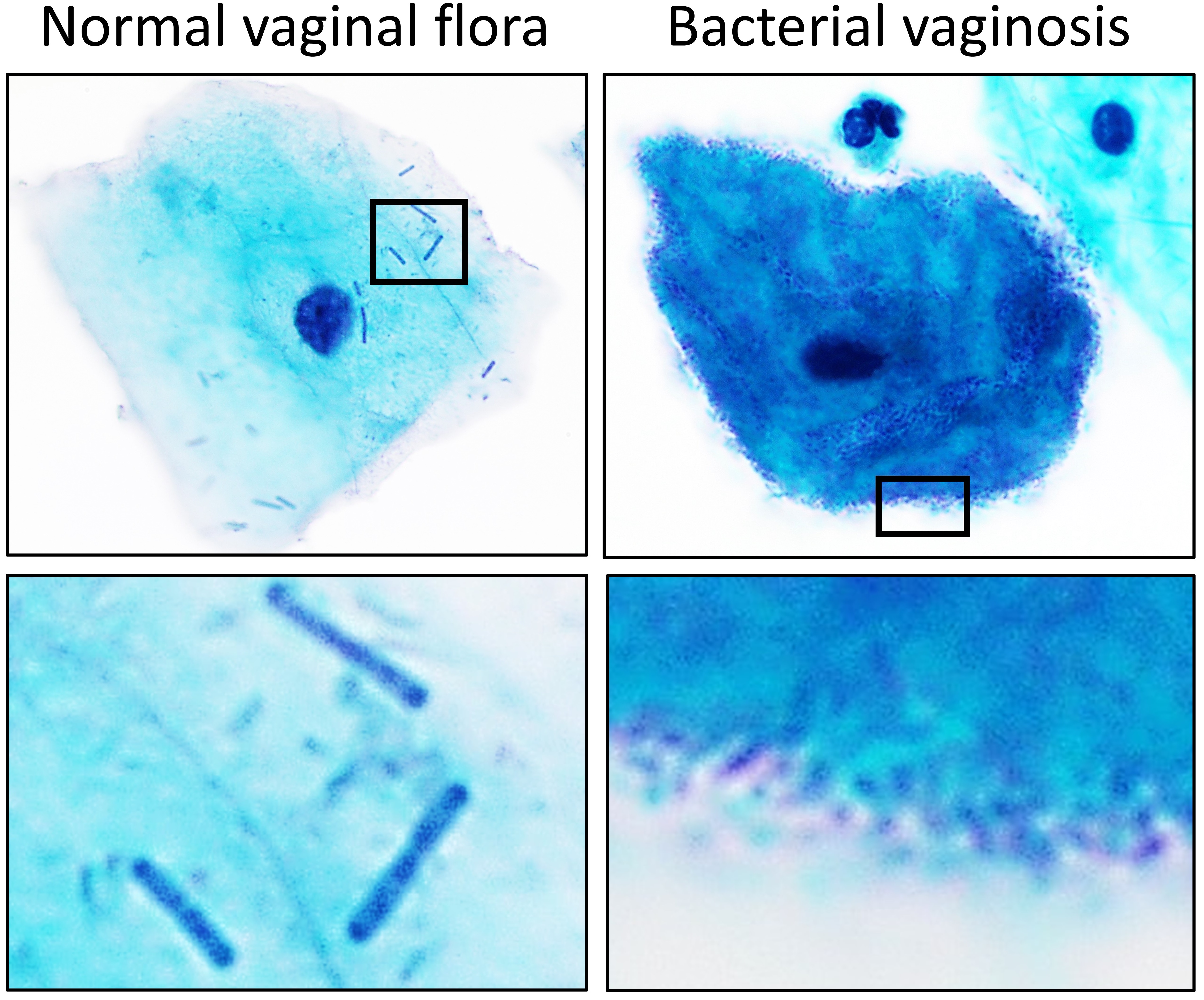
Lactobacillus
''Lactobacillus'' is a genus of Gram-positive, aerotolerant anaerobes or microaerophilic, rod-shaped, non-spore-forming bacteria. Until 2020, the genus ''Lactobacillus'' comprised over 260 phylogenetically, ecologically, and metabolically diverse species; a taxonomic revision of the genus assigned lactobacilli to 25 genera (see below). ''Lactobacillus'' species constitute a significant component of the human and animal microbiota at a number of body sites, such as the digestive system, and the female genital system. In women of European ancestry, ''Lactobacillus'' species are normally a major part of the vaginal microbiota. ''Lactobacillus'' forms biofilms in the vaginal and gut microbiota, allowing them to persist during harsh environmental conditions and maintain ample populations. ''Lactobacillus'' exhibits a mutualistic relationship with the human body, as it protects the host against potential invasions by pathogens, and in turn, the host provides a source of nutrien ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among the first life forms to appear on Earth, and are present in most of its habitats. Bacteria inhabit soil, water, acidic hot springs, radioactive waste, and the deep biosphere of Earth's crust. Bacteria are vital in many stages of the nutrient cycle by recycling nutrients such as the fixation of nitrogen from the atmosphere. The nutrient cycle includes the decomposition of dead bodies; bacteria are responsible for the putrefaction stage in this process. In the biological communities surrounding hydrothermal vents and cold seeps, extremophile bacteria provide the nutrients needed to sustain life by converting dissolved compounds, such as hydrogen sulphide and methane, to energy. Bacteria also live in symbiotic and parasitic relationsh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



4-3D-balls.png)