|
Cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometimes the symbol Cyz is used. The deprotonated form can generally be described by the symbol Cym as well. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. When used as a food additive, it has the E number E920. Cysteine is encoded by the codons UGU and UGC. The sulfur-containing amino acids cysteine and methionine are more easily oxidized than the other amino acids. Structure Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has chirality in the older / notation based on homology to - and -glyceraldehyde. In the newer ''R''/''S'' system of de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenocysteine
Selenocysteine (symbol Sec or U, in older publications also as Se-Cys) is the 21st proteinogenic amino acid. Selenoproteins contain selenocysteine residues. Selenocysteine is an analogue of the more common cysteine with selenium in place of the sulfur. Selenocysteine is present in several enzymes (for example glutathione peroxidases, tetraiodothyronine 5′ deiodinases, thioredoxin reductases, formate dehydrogenases, glycine reductases, selenophosphate synthetase 2, methionine-''R''-sulfoxide reductase B1 ( SEPX1), and some hydrogenases). It occurs in all three domains of life, including important enzymes (listed above) present in humans. Selenocysteine was discovered by biochemist Thressa Stadtman at the National Institutes of Health. Chemistry Selenocysteine is the Se-analogue of cysteine. It is rarely encountered outside of living tissue (and is not available commercially) because it is very susceptible to air-oxidation. More common is the oxidized derivative sel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical role in the metabolism and health of many species, including humans. It is encoded by the codon AUG. Methionine is also an important part of angiogenesis, the growth of new blood vessels. Supplementation may benefit those suffering from copper poisoning. Overconsumption of methionine, the methyl group donor in DNA methylation, is related to cancer growth in a number of studies. Methionine was first isolated in 1921 by John Howard Mueller. Biochemical details Methionine (abbreviated as Met or M; encoded by the codon AUG) is an α-amino acid that is used in the biosynthesis of proteins. It contains a carboxyl group (which is in the deprotonated −COO− form under biological pH conditions), an amino group (which is in the prot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteinogenic Amino Acid
Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino acids, 20 in the standard genetic code and an additional 2 (selenocysteine and pyrrolysine) that can be incorporated by special translation mechanisms. In contrast, non-proteinogenic amino acids are amino acids that are either not incorporated into proteins (like GABA, L-DOPA, or triiodothyronine), misincorporated in place of a genetically encoded amino acid, or not produced directly and in isolation by standard cellular machinery (like hydroxyproline). The latter often results from post-translational modification of proteins. Some non-proteinogenic amino acids are incorporated into nonribosomal peptides which are synthesized by non-ribosomal peptide synthetases. Both eukaryotes and prokaryotes can incorporate selenocysteine into their p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystine
Cystine is the oxidized derivative of the amino acid cysteine and has the formula (SCH2CH(NH2)CO2H)2. It is a white solid that is poorly soluble in water. As a residue in proteins, cystine serves two functions: a site of redox reactions and a mechanical linkage that allows proteins to retain their three-dimensional structure. Formation and reactions Structure Cystine is the disulfide derived from the amino acid cysteine. The conversion can be viewed as an oxidation: : Cystine contains a disulfide bond, two amine groups, and two carboxylic acid groups. As for other amino acids, the amine and carboxylic acid groups exist is rapid equilibrium with the ammonium-carboxylate tautomer. The great majority of the literature concerns the ''l,l-''cystine, derived from ''l''-cysteine. Other isomers include ''d,d''-cystine and the meso isomer d,l-cystine, neither of which is biologically significant. Occurrence Cystine is common in many foods such as eggs, meat, dairy products, and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfide Bond
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. '' Persulfide'' usually refers to compounds. In inorganic chemistry disulfide usually refers to the corresponding anion (−S−S−). Organic disulfides Symmetrical disulfides are compounds of the formula . Most disulfides encountered in organo sulfur chemistry are symmetrical disulfides. Unsymmetrical disulfides (also called heterodisulfides) are compounds of the formula . They are less common in organic chemistry, but most disulfides in nature are unsymmetrical. Properties The disulfide bonds are strong, with a typical bond dissociation energy of 60 kcal/mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in the Earth's crust. Selenium – from Greek ( 'Moon') – was discovered in 1817 by , who noted the similarity of the new element to the previously discovered tellurium (named for the Earth). Selenium is found in metal sulfide ores, where it partially replaces the sulfur. Commercially, selenium is produced as a byproduct in the refining of these ores, most often during production. Minerals that are pure selenide or selenate compounds are known but rare. The chief commercial uses for selenium today are glassmaking and pigments. Selenium is a semiconductor and is used in photocells. Applications in electronics, once important, h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Essential Amino Acid
An essential amino acid, or indispensable amino acid, is an amino acid that cannot be synthesized from scratch by the organism fast enough to supply its demand, and must therefore come from the diet. Of the 21 amino acids common to all life forms, the nine amino acids humans cannot synthesize are phenylalanine, valine, threonine, tryptophan, methionine, leucine, isoleucine, lysine, and histidine. Six other amino acids are considered conditionally essential in the human diet, meaning their synthesis can be limited under special pathophysiological conditions, such as prematurity in the infant or individuals in severe catabolic distress. These six are arginine, cysteine, glycine, glutamine, proline, and tyrosine. Six amino acids are non-essential (dispensable) in humans, meaning they can be synthesized in sufficient quantities in the body. These six are alanine, aspartic acid, asparagine, glutamic acid, serine, and selenocysteine (considered the 21st amino acid). Py ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant. Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' ( Mac OS X Leopard). because the thiolate group () bonds very str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Codon
The genetic code is the set of rules used by living cells to translate information encoded within genetic material ( DNA or RNA sequences of nucleotide triplets, or codons) into proteins. Translation is accomplished by the ribosome, which links proteinogenic amino acids in an order specified by messenger RNA (mRNA), using transfer RNA (tRNA) molecules to carry amino acids and to read the mRNA three nucleotides at a time. The genetic code is highly similar among all organisms and can be expressed in a simple table with 64 entries. The codons specify which amino acid will be added next during protein biosynthesis. With some exceptions, a three-nucleotide codon in a nucleic acid sequence specifies a single amino acid. The vast majority of genes are encoded with a single scheme (see the RNA codon table). That scheme is often referred to as the canonical or standard genetic code, or simply ''the'' genetic code, though variant codes (such as in mitochondria) exist. History Ef ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
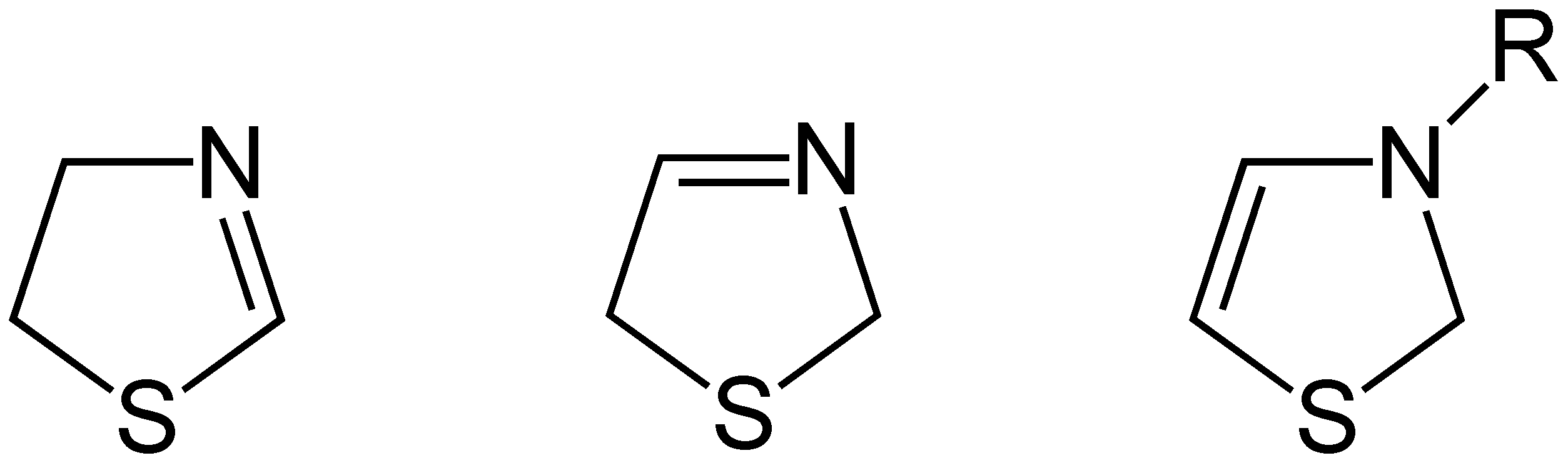
Thiazoline
Thiazolines (or dihydrothiazoles) are a group of isomeric 5-membered heterocyclic compounds containing both sulfur and nitrogen in the ring. Although unsubstituted thiazolines are rarely encountered themselves, their derivatives are more common and some are bioactive. For example, in a common post-translational modification, cysteine residues are converted into thiazolines. The name thiazoline originates from the Hantzsch–Widman nomenclature. Isomers Three structural isomers of thiazoline exist depending on the position of the double bond. These forms do not readily interconvert and hence are not tautomers. Of these 2-thiazoline is the most common. A fourth structure exists in which the N and S atoms are adjacent; this known as isothiazoline. Synthesis Thiazolines were first prepared by dialkylation of thioamides by Richard Willstatter in 1909. 2-Thiazolines are commonly prepared from 2-aminoethanethiols (e.g. cysteamine). They may also be synthesized via the Asinge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E Number
E numbers ("E" stands for "Europe") are codes for substances used as food additives, including those found naturally in many foods such as vitamin C, for use within the European Union (EU) and European Free Trade Association (EFTA). Commonly found on food labels, their safety assessment and approval are the responsibility of the European Food Safety Authority (EFSA). The fact that an additive has an E number implies that its use was at one time permitted in products for sale in the European Single Market; some of these additives are no longer allowed today. Having a single unified list for food additives was first agreed upon in 1962 with food colouring. In 1964, the directives for preservatives were added, in 1970 antioxidants were added, in 1974 emulsifiers, stabilisers, thickeners and gelling agents were added as well. Numbering schemes The numbering scheme follows that of the International Numbering System for Food Additives, International Numbering System (INS) as determ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |