period 2 element on:
[Wikipedia]
[Google]
[Amazon]
A period 2 element is one of the
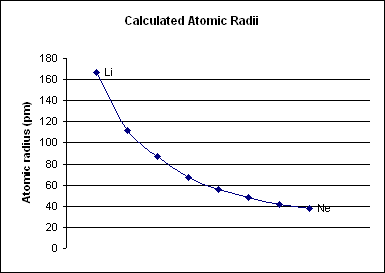 Period 2 is the first period in the periodic table from which
Period 2 is the first period in the periodic table from which
 Lithium (Li) is an
Lithium (Li) is an
at WebElements. Lithium is one of the few elements synthesized in the
 Beryllium (Be) is the chemical element with atomic number 4, occurring in the form of 9Be. At standard temperature and pressure, beryllium is a strong, steel-grey, light-weight,
Beryllium (Be) is the chemical element with atomic number 4, occurring in the form of 9Be. At standard temperature and pressure, beryllium is a strong, steel-grey, light-weight,
at WebElements. It also has one of the highest
 Boron (B) is the chemical element with atomic number 5, occurring as 10B and 11B. At standard temperature and pressure, boron is a
Boron (B) is the chemical element with atomic number 5, occurring as 10B and 11B. At standard temperature and pressure, boron is a
at WebElements. Boron's most common
of boron. These are the only stable isotopes of boron; however other isotopes have been synthesised. Boron forms covalent bonds with other
 Carbon is the chemical element with atomic number 6, occurring as 12C, 13C and 14C.Carbon
Carbon is the chemical element with atomic number 6, occurring as 12C, 13C and 14C.Carbon
at WebElements. At standard temperature and pressure, carbon is a solid, occurring in many different allotropes, the most common of which are
by Mahananda Dasgupta of the Department of Nuclear Physics at Australian National University. 13C is also stable, with six protons and seven neutrons, at 1.1%. Trace amounts of 14C also occur naturally but this isotope is radioactive and decays with a half life of 5730 years; it is used for
 Nitrogen is the chemical element with atomic number 7, the symbol N and
Nitrogen is the chemical element with atomic number 7, the symbol N and
at WebElements. Many industrially important compounds, such as
 Fluorine is the chemical element with atomic number 9. It occurs naturally in its only stable form 19F.
Fluorine is a pale-yellow, diatomic gas under normal conditions and down to very low temperatures. Short one electron of the highly stable octet in each atom, fluorine molecules are unstable enough that they easily snap, with loose fluorine atoms tending to grab single electrons from just about any other element. Fluorine is the most reactive of all elements, and it even attacks many oxides to replace oxygen with fluorine. Fluorine even attacks silica, one of the favored materials for transporting strong acids, and burns asbestos. It attacks common salt, one of the most stable compounds, with the release of chlorine. It never appears uncombined in nature and almost never stays uncombined for long. It burns hydrogen simultaneously if either is liquid or gaseous—even at temperatures close to absolute zero. It is extremely difficult to isolate from any compounds, let alone keep uncombined.
Fluorine gas is extremely dangerous because it attacks almost all organic material, including live flesh. Many of the binary compounds that it forms (called fluorides) are themselves highly toxic, including soluble fluorides and especially
Fluorine is the chemical element with atomic number 9. It occurs naturally in its only stable form 19F.
Fluorine is a pale-yellow, diatomic gas under normal conditions and down to very low temperatures. Short one electron of the highly stable octet in each atom, fluorine molecules are unstable enough that they easily snap, with loose fluorine atoms tending to grab single electrons from just about any other element. Fluorine is the most reactive of all elements, and it even attacks many oxides to replace oxygen with fluorine. Fluorine even attacks silica, one of the favored materials for transporting strong acids, and burns asbestos. It attacks common salt, one of the most stable compounds, with the release of chlorine. It never appears uncombined in nature and almost never stays uncombined for long. It burns hydrogen simultaneously if either is liquid or gaseous—even at temperatures close to absolute zero. It is extremely difficult to isolate from any compounds, let alone keep uncombined.
Fluorine gas is extremely dangerous because it attacks almost all organic material, including live flesh. Many of the binary compounds that it forms (called fluorides) are themselves highly toxic, including soluble fluorides and especially
 Neon is the chemical element with atomic number 10, occurring as 20Ne, 21Ne and 22Ne.
Neon is a monatomic gas. With a complete octet of outer electrons it is highly resistant to removal of any electron, and it cannot accept an electron from anything. Neon has no tendency to form any normal compounds under normal temperatures and pressures; it is effectively inert. It is one of the so-called "noble gases".
Neon is a trace component of the atmosphere without any biological role.
Neon is the chemical element with atomic number 10, occurring as 20Ne, 21Ne and 22Ne.
Neon is a monatomic gas. With a complete octet of outer electrons it is highly resistant to removal of any electron, and it cannot accept an electron from anything. Neon has no tendency to form any normal compounds under normal temperatures and pressures; it is effectively inert. It is one of the so-called "noble gases".
Neon is a trace component of the atmosphere without any biological role.
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
s in the second row (or period) of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
increases; a new row is started when chemical behavior begins to repeat, creating columns
A column or pillar in architecture and structural engineering is a structural element that transmits, through compression, the weight of the structure above to other structural elements below. In other words, a column is a compression member ...
of elements with similar properties.
The second period contains the elements lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
, beryllium
Beryllium is a chemical element; it has Symbol (chemistry), symbol Be and atomic number 4. It is a steel-gray, hard, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with ...
, boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
, carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
, nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
, oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
, and neon
Neon is a chemical element; it has symbol Ne and atomic number 10. It is the second noble gas in the periodic table. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with approximately two-thirds the density of ...
. In a quantum mechanical
Quantum mechanics is the fundamental physical theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is the foundation of a ...
description of atomic structure, this period corresponds to the filling of the second () shell, more specifically its 2s and 2p subshells. Period 2 elements (carbon, nitrogen, oxygen, fluorine and neon) obey the octet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The ru ...
in that they need eight electrons to complete their valence shell
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with b ...
(lithium and beryllium obey duet rule, boron is electron deficient.), where at most eight electrons can be accommodated: two in the 2s orbital and six in the 2p subshell.
Periodic trends
 Period 2 is the first period in the periodic table from which
Period 2 is the first period in the periodic table from which periodic trends
In chemistry, periodic trends are specific patterns present in the periodic table that illustrate different aspects of certain Chemical element, elements when grouped by period (periodic table), period and/or Group (periodic table), group. They w ...
can be drawn. Period 1, which only contains two elements (hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
and helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
), is too small to draw any conclusive trends from it, especially because the two elements behave nothing like other s-block elements. Period 2 has much more conclusive trends. For all elements in period 2, as the atomic number increases, the atomic radius
The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Since the boundary is not a well-defined physical entity, there ...
of the elements decreases, the electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
increases, and the ionization energy
In physics and chemistry, ionization energy (IE) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom, Ion, positive ion, or molecule. The first ionization energy is quantitatively expressed as
: ...
increases.
Period 2 only has two metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated wit ...
s (lithium and beryllium) of eight elements, less than for any subsequent period both by number and by proportion. It also has the most number of nonmetals, namely five, among all periods. The elements in period 2 often have the most extreme properties in their respective groups; for example, fluorine is the most reactive halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
, neon is the most inert noble gas
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of Group (periodic table), group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some ...
, and lithium is the least reactive alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
.
All period 2 elements completely obey the Madelung rule; in period 2, lithium and beryllium fill the 2s subshell, and boron, carbon, nitrogen, oxygen, fluorine, and neon fill the 2p subshell. The period shares this trait with periods 1 and 3, none of which contain transition element
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinide ...
s or inner transition elements, which often vary from the rule.
:
Lithium
 Lithium (Li) is an
Lithium (Li) is an alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
with atomic number 3, occurring naturally in two isotopes: 6Li and 7Li. The two make up all natural occurrence of lithium on Earth, although further isotopes have been synthesized. In ionic compound
In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions (Cation, cations) and negatively charged ions (Anion, anions), which results in a compound with no net electric charge (electrica ...
s, lithium loses an electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
to become positively charged, forming the cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
Li+. Lithium is the first alkali metal in the periodic table,Hydrogen is occasionally referred to as an alkali metal, although this is rare. and the first metal of any kind in the periodic table.See note 1. At standard temperature and pressure
Standard temperature and pressure (STP) or standard conditions for temperature and pressure are various standard sets of conditions for experimental measurements used to allow comparisons to be made between different sets of data. The most used ...
, lithium is a soft, silver-white, highly reactive metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated wit ...
. With a density
Density (volumetric mass density or specific mass) is the ratio of a substance's mass to its volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' (or ''d'') can also be u ...
of 0.564 g⋅cm−3, lithium is the lightest metal and the least dense solid element.Lithiumat WebElements. Lithium is one of the few elements synthesized in the
Big Bang
The Big Bang is a physical theory that describes how the universe expanded from an initial state of high density and temperature. Various cosmological models based on the Big Bang concept explain a broad range of phenomena, including th ...
.
Lithium is the 31st most abundant element on earth, occurring in concentrations of between 20 and 70 ppm by weight, but due to its high reactivity it is only found naturally in compounds.Kamienski et al. "Lithium and lithium compounds". ''Kirk-Othmer Encyclopedia of Chemical Technology''. John Wiley & Sons, Inc. Published online 2004.
Lithium salts
In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions ( cations) and negatively charged ions (anions), which results in a compound with no net electric charge (electrically neutral). ...
are used in the pharmacology industry as mood stabilising drugs
A drug is any chemical substance other than a nutrient or an essential dietary ingredient, which, when administered to a living organism, produces a biological effect. Consumption of drugs can be via inhalation, injection, smoking, ingestio ...
. They are used in the treatment of bipolar disorder
Bipolar disorder (BD), previously known as manic depression, is a mental disorder characterized by periods of Depression (mood), depression and periods of abnormally elevated Mood (psychology), mood that each last from days to weeks, and in ...
, where they have a role in treating depression and mania
Mania, also known as manic syndrome, is a Psychiatry, psychiatric Abnormality (behavior), behavioral syndrome defined as a state of Abnormality (behavior), abnormally elevated arousal, affect (psychology), affect, and energy level. During a mani ...
and may reduce the chances of suicide
Suicide is the act of intentionally causing one's own death.
Risk factors for suicide include mental disorders, physical disorders, and substance abuse. Some suicides are impulsive acts driven by stress (such as from financial or ac ...
. The most common compounds used are lithium carbonate
Lithium carbonate is an inorganic compound, the lithium salt of carbonic acid with the chemical formula, formula . This white Salt (chemistry), salt is widely used in processing metal oxides. It is on the WHO Model List of Essential Medicines, Wor ...
, Li2CO3, lithium citrate, Li3C6H5O7, lithium sulphate, Li2SO4, and lithium orotate, LiC5H3N2O4·H2O. Lithium is also used in batteries as an anode
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the devic ...
and its alloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have prop ...
s with aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
, cadmium
Cadmium is a chemical element; it has chemical symbol, symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12 element, group 12, zinc and mercury (element), mercury. Like z ...
, copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
and manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
are used to make high performance parts for aircraft
An aircraft ( aircraft) is a vehicle that is able to flight, fly by gaining support from the Atmosphere of Earth, air. It counters the force of gravity by using either Buoyancy, static lift or the Lift (force), dynamic lift of an airfoil, or, i ...
, most notably the external tank of the Space Shuttle
The Space Shuttle is a retired, partially reusable launch system, reusable low Earth orbital spacecraft system operated from 1981 to 2011 by the U.S. National Aeronautics and Space Administration (NASA) as part of the Space Shuttle program. ...
.
Beryllium
 Beryllium (Be) is the chemical element with atomic number 4, occurring in the form of 9Be. At standard temperature and pressure, beryllium is a strong, steel-grey, light-weight,
Beryllium (Be) is the chemical element with atomic number 4, occurring in the form of 9Be. At standard temperature and pressure, beryllium is a strong, steel-grey, light-weight, brittle
A material is brittle if, when subjected to stress, it fractures with little elastic deformation and without significant plastic deformation. Brittle materials absorb relatively little energy prior to fracture, even those of high strength. ...
, bivalent alkaline earth metal
The alkaline earth metals are six chemical elements in group (periodic table), group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar p ...
, with a density of 1.85 g⋅cm−3.Berylliumat WebElements. It also has one of the highest
melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
s of all the light metals. Beryllium's most common isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
is 9Be, which contains 4 protons and 5 neutrons. It makes up almost 100% of all naturally occurring beryllium and is its only stable isotope; however other isotopes have been synthesised. In ionic compounds, beryllium loses its two valence electron
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with b ...
s to form the cation, Be2+.
Small amounts of beryllium were synthesised during the Big Bang
The Big Bang is a physical theory that describes how the universe expanded from an initial state of high density and temperature. Various cosmological models based on the Big Bang concept explain a broad range of phenomena, including th ...
, although most of it decayed or reacted further to create larger nuclei, like carbon, nitrogen or oxygen. Beryllium is a component of 100 out of 4000 known mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Mi ...
s, such as bertrandite
Bertrandite is a beryllium sorosilicate hydroxide mineral with composition: Be4Si2O7(OH)2. Bertrandite is a colorless to pale yellow orthorhombic mineral with a hardness of 6–7.
It is commonly found in beryllium rich pegmatites and is in part ...
, Be4Si2O7(OH)2, beryl
Beryl ( ) is a mineral composed of beryllium aluminium Silicate minerals#Cyclosilicates, silicate with the chemical formula Be3Al2(SiO3)6. Well-known varieties of beryl include emerald and Aquamarine (gem), aquamarine. Naturally occurring Hex ...
, Al2Be3Si6O18, chrysoberyl
The mineral or gemstone chrysoberyl is an aluminate of beryllium with the formula Be Al2 O4. The name chrysoberyl is derived from the Greek words χρυσός ''chrysos'' and βήρυλλος ''beryllos'', meaning "a gold-white spar". Despit ...
, Al2BeO4, and phenakite, Be2SiO4. Precious forms of beryl are aquamarine, red beryl and emerald
Emerald is a gemstone and a variety of the mineral beryl (Be3Al2(SiO3)6) colored green by trace amounts of chromium or sometimes vanadium.Hurlbut, Cornelius S. Jr., and Kammerling, Robert C. (1991). ''Gemology'', John Wiley & Sons, New York ...
. The most common sources of beryllium used commercially are beryl and bertrandite and production of it involves the reduction of beryllium fluoride
Beryllium fluoride is the inorganic compound with the formula Be F2. This white solid is the principal precursor for the manufacture of beryllium metal. Its structure resembles that of quartz, but BeF2 is highly soluble in water.
Properties
Ber ...
with magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
metal or the electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of c ...
of molten beryllium chloride
Beryllium chloride is an inorganic compound with the formula BeCl2. It is a colourless, hygroscopic solid that dissolves well in many polar solvents. Its properties are similar to those of aluminium chloride, due to beryllium's diagonal relations ...
, containing some sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
as beryllium chloride is a poor conductor of electricity.
Due to its stiffness, light weight, and dimensional stability over a wide temperature range, beryllium metal is used in as a structural material in aircraft, missiles and communication satellite
A communications satellite is an artificial satellite that relays and amplifies radio telecommunication signals via a transponder; it creates a communication channel between a source transmitter and a receiver at different locations on Earth. ...
s. It is used as an alloying agent in beryllium copper
Beryllium copper (BeCu), also known as copper beryllium (CuBe), beryllium bronze, and spring copper, is a copper alloy with 0.5–3% beryllium. Copper beryllium alloys are often used because of their high strength and good conductivity of both ...
, which is used to make electrical components due to its high electrical and heat conductivity. Sheets of beryllium are used in X-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ran ...
detectors to filter out visible light
Light, visible light, or visible radiation is electromagnetic radiation that can be perceived by the human eye. Visible light spans the visible spectrum and is usually defined as having wavelengths in the range of 400–700 nanometres (nm ...
and let only X-rays through. It is used as a neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, ideally without capturing any, leaving them as thermal neutrons with only minimal (thermal) kinetic energy. These thermal neutrons are immensely ...
in nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
s because light nuclei are more effective at slowing down neutrons than heavy nuclei. Beryllium's low weight and high rigidity also make it useful in the construction of tweeter
A tweeter or treble speaker is a special type of loudspeaker (usually dome, inverse dome or horn-type) that is designed to produce high audio frequencies, typically from 2,000 to 20,000 Hertz, Hz. The name is derived from the high pitched sound ...
s in loudspeaker
A loudspeaker (commonly referred to as a speaker or, more fully, a speaker system) is a combination of one or more speaker drivers, an enclosure, and electrical connections (possibly including a crossover network). The speaker driver is an ...
s.
Beryllium and beryllium compounds are classified by the International Agency for Research on Cancer
The International Agency for Research on Cancer (IARC; ) is an intergovernmental agency forming part of the World Health Organization of the United Nations.
Its role is to conduct and coordinate research into the causes of cancer. It also cance ...
as Group 1 carcinogens; they are carcinogenic to both animals and humans. Chronic berylliosis is a pulmonary
The lungs are the primary organs of the respiratory system in many animals, including humans. In mammals and most other tetrapods, two lungs are located near the backbone on either side of the heart. Their function in the respiratory syste ...
and systemic granulomatous disease caused by exposure to beryllium. Between 1% – 15% of people are sensitive to beryllium and may develop an inflammatory reaction in their respiratory system
The respiratory system (also respiratory apparatus, ventilatory system) is a biological system consisting of specific organs and structures used for gas exchange in animals and plants. The anatomy and physiology that make this happen varies grea ...
and skin
Skin is the layer of usually soft, flexible outer tissue covering the body of a vertebrate animal, with three main functions: protection, regulation, and sensation.
Other animal coverings, such as the arthropod exoskeleton, have different ...
, called chronic beryllium disease or berylliosis. The body's immune system
The immune system is a network of biological systems that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to bacteria, as well as Tumor immunology, cancer cells, Parasitic worm, parasitic ...
recognises the beryllium as foreign particles and mounts an attack against them, usually in the lungs where they are breathed in. This can cause fever, fatigue, weakness, night sweats and difficulty in breathing.
Boron
 Boron (B) is the chemical element with atomic number 5, occurring as 10B and 11B. At standard temperature and pressure, boron is a
Boron (B) is the chemical element with atomic number 5, occurring as 10B and 11B. At standard temperature and pressure, boron is a trivalent
In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Valence is generally understood to be the number of chemica ...
metalloid
A metalloid is a chemical element which has a preponderance of material property, properties in between, or that are a mixture of, those of metals and Nonmetal (chemistry), nonmetals. The word metalloid comes from the Latin language, Latin ''meta ...
that has several different allotropes
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: th ...
. Amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymousl ...
boron is a brown powder formed as a product of many chemical reactions. Crystalline
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macrosc ...
boron is a very hard, black material with a high melting point and exists in many polymorphs: Two rhombohedral
In geometry, a rhombohedron (also called a rhombic hexahedron or, inaccurately, a rhomboid) is a special case of a parallelepiped in which all six faces are congruent rhombus, rhombi. It can be used to define the rhombohedral lattice system, a Ho ...
forms, α-boron and β-boron containing 12 and 106.7 atoms in the rhombohedral unit cell respectively, and 50-atom tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the Cube (geometry), cube becomes a rectangular Pri ...
boron are the most common. Boron has a density of 2.34−3. Boronat WebElements. Boron's most common
isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
is 11B at 80.22%, which contains 5 protons and 6 neutrons. The other common isotope is 10B at 19.78%, which contains 5 protons and 5 neutrons.Propertiesof boron. These are the only stable isotopes of boron; however other isotopes have been synthesised. Boron forms covalent bonds with other
nonmetal
In the context of the periodic table, a nonmetal is a chemical element that mostly lacks distinctive metallic properties. They range from colorless gases like hydrogen to shiny crystals like iodine. Physically, they are usually lighter (less ...
s and has oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
s of 1, 2, 3 and 4.
Boron does not occur naturally as a free element, but in compounds such as borate
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt of such anions, such as sodium metaborate, and borax . The name also refers to esters of su ...
s. The most common sources of boron are tourmaline
Tourmaline ( ) is a crystalline silicate mineral, silicate mineral group in which boron is chemical compound, compounded with chemical element, elements such as aluminium, iron, magnesium, sodium, lithium, or potassium. This gemstone comes in a ...
, borax
The BORAX Experiments were a series of safety experiments on boiling water nuclear reactors conducted by Argonne National Laboratory in the 1950s and 1960s at the National Reactor Testing Station in eastern Idaho.
, Na2B4O5(OH)4·8H2O, and kernite
Kernite, also known as rasorite, is a hydrated sodium borate hydroxide mineral with formula . It is a colorless to white mineral crystallizing in the monoclinic crystal system typically occurring as prismatic to acicular (crystal habit), acicular ...
, Na2B4O5(OH)4·2H2O. it is difficult to obtain pure boron. It can be made through the magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
reduction of boron trioxide
Boron trioxide or diboron trioxide is the oxide of boron with the formula . It is a colorless transparent solid, almost always glassy (amorphous), which can be crystallized only with great difficulty. It is also called boric oxide or boria. It h ...
, B2O3. This oxide is made by melting boric acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen orthoborate, trihydroxidoboron or boracic acid. It is usually encountered as colorless crystals or a white ...
, B(OH)3, which in turn is obtained from borax. Small amounts of pure boron can be made by the thermal decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition of a substance caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic ...
of boron bromide, BBr3, in hydrogen gas over hot tantalum
Tantalum is a chemical element; it has Symbol (chemistry), symbol Ta and atomic number 73. It is named after Tantalus, a figure in Greek mythology. Tantalum is a very hard, ductility, ductile, lustre (mineralogy), lustrous, blue-gray transition ...
wire, which acts as a catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
. The most commercially important sources of boron are: sodium tetraborate
The BORAX Experiments were a series of safety experiments on boiling water reactor, boiling water nuclear reactors conducted by Argonne National Laboratory in the 1950s and 1960s at the National Reactor Testing Station in eastern Idaho.
pentahydrate, Na2B4O7 · 5H2O, which is used in large amounts in making insulating fiberglass
Fiberglass (American English) or fibreglass (English in the Commonwealth of Nations, Commonwealth English) is a common type of fibre-reinforced plastic, fiber-reinforced plastic using glass fiber. The fibers may be randomly arranged, flattened i ...
and sodium perborate
Sodium perborate are chemical compounds with chemical formula (H2O)x. Commonly encountered salts are the anhydrous form (x = 0) and as a hydrate, hexahydrate (x = 6). These two species are sometimes called, respectively, "monohydrate" or PBS-1 a ...
bleach
Bleach is the generic name for any chemical product that is used industrially or domestically to remove color from (i.e. to whiten) fabric or fiber (in a process called bleaching) or to disinfect after cleaning. It often refers specifically t ...
; boron carbide
Boron carbide (chemical formula approximately B4C) is an extremely hard boron–carbon ceramic, a covalent material used in tank armor, bulletproof vests, engine sabotage powders,
as well as numerous industrial applications. With a Vickers har ...
, a ceramic
A ceramic is any of the various hard, brittle, heat-resistant, and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcela ...
material, is used to make armour materials, especially in bulletproof vest
A bulletproof vest, also known as a ballistic vest or bullet-resistant vest, is a type of body armor designed to absorb impact and prevent the penetration of firearm projectiles and explosion fragments to the torso. The vest can be either soft ...
s for soldiers and police officers; orthoboric acid, H3BO3 or boric acid, used in the production of textile fiberglass
Fiberglass (American English) or fibreglass (English in the Commonwealth of Nations, Commonwealth English) is a common type of fibre-reinforced plastic, fiber-reinforced plastic using glass fiber. The fibers may be randomly arranged, flattened i ...
and flat panel display
A flat-panel display (FPD) is an electronic display used to display visual content such as text or images. It is present in consumer, medical, transportation, and industrial equipment.
Flat-panel displays are thin, lightweight, provide better ...
s; sodium tetraborate decahydrate, Na2B4O7 · 10H2O or borax, used in the production of adhesives; and the isotope boron-10 is used as a control for nuclear reactors, as a shield for nuclear radiation, and in instruments used for detecting neutrons.
Boron is an essential plant micronutrient
Micronutrients are essential chemicals required by organisms in small quantities to perform various biogeochemical processes and regulate physiological functions of cells and organs. By enabling these processes, micronutrients support the heal ...
, required for cell wall strength and development, cell division, seed and fruit development, sugar transport and hormone development. However, high soil concentrations of over 1.0 ppm can cause necrosis in leaves and poor growth. Levels as low as 0.8 ppm can cause these symptoms to appear in plants particularly boron-sensitive. Most plants, even those tolerant of boron in the soil, will show symptoms of boron toxicity when boron levels are higher than 1.8 ppm. In animals, boron is an ultratrace element; in human diets, daily intake ranges from 2.1 to 4.3 mg boron/kg body weight (bw)/day. It is also used as a supplement for the prevention and treatment of osteoporosis and arthritis.
Carbon
 Carbon is the chemical element with atomic number 6, occurring as 12C, 13C and 14C.Carbon
Carbon is the chemical element with atomic number 6, occurring as 12C, 13C and 14C.Carbonat WebElements. At standard temperature and pressure, carbon is a solid, occurring in many different allotropes, the most common of which are
graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
, diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of e ...
, the fullerenes
A fullerene is an allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may have hollow sphere- ...
and amorphous carbon
Amorphous carbon is free, reactive carbon that has no crystalline structure. Amorphous carbon materials may be stabilized by terminating dangling-π bonds with hydrogen. As with other amorphous solids, some short-range order can be observed. Amo ...
. Graphite is a soft, hexagonal crystalline, opaque black semimetal
A semimetal is a material with a small energy overlap between the bottom of the Electrical conduction, conduction Electronic band structure, band and the top of the valence band, but they do not overlap in momentum space. According to Band theory ...
with very good conductive
In physics and electrical engineering, a conductor is an object or type of material that allows the flow of Electric charge, charge (electric current) in one or more directions. Materials made of metal are common electrical conductors. The flow ...
and thermodynamically stable properties. Diamond however is a highly transparent colourless cubic crystal
In crystallography, the cubic (or isometric) crystal system is a crystal system where the Crystal structure#Unit cell, unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There ...
with poor conductive properties, is the hardest known naturally occurring mineral and has the highest refractive index
In optics, the refractive index (or refraction index) of an optical medium is the ratio of the apparent speed of light in the air or vacuum to the speed in the medium. The refractive index determines how much the path of light is bent, or refrac ...
of all gemstones
A gemstone (also called a fine gem, jewel, precious stone, semiprecious stone, or simply gem) is a piece of mineral crystal which, when cut or polished, is used to make jewelry or other adornments. Certain rocks (such as lapis lazuli, opal, a ...
. In contrast to the crystal lattice
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystal, crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that ...
structure of diamond and graphite, the fullerenes
A fullerene is an allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may have hollow sphere- ...
are molecules
A molecule is a group of two or more atoms that are held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry ...
, named after Richard Buckminster Fuller
Richard Buckminster Fuller (; July 12, 1895 – July 1, 1983) was an American architect, systems theorist, writer, designer, inventor, philosopher, and futurist. He styled his name as R. Buckminster Fuller in his writings, publishing more th ...
whose architecture the molecules resemble. There are several different fullerenes, the most widely known being the "buckeyball" C60. Little is known about the fullerenes and they are a current subject of research. There is also amorphous carbon, which is carbon without any crystalline structure. In mineralogy
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical mineralogy, optical) properties of minerals and mineralized artifact (archaeology), artifacts. Specific s ...
, the term is used to refer to soot
Soot ( ) is a mass of impure carbon particles resulting from the incomplete combustion of hydrocarbons. Soot is considered a hazardous substance with carcinogenic properties. Most broadly, the term includes all the particulate matter produced b ...
and coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other Chemical element, elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal i ...
, although these are not truly amorphous as they contain small amounts of graphite or diamond. Carbon's most common isotope at 98.9% is 12C, with six protons and six neutrons.Presentation about isotopesby Mahananda Dasgupta of the Department of Nuclear Physics at Australian National University. 13C is also stable, with six protons and seven neutrons, at 1.1%. Trace amounts of 14C also occur naturally but this isotope is radioactive and decays with a half life of 5730 years; it is used for
radiocarbon dating
Radiocarbon dating (also referred to as carbon dating or carbon-14 dating) is a method for Chronological dating, determining the age of an object containing organic material by using the properties of carbon-14, radiocarbon, a radioactive Isotop ...
. Other isotopes of carbon
Carbon (6C) has 14 known isotopes, from to as well as , of which only and are stable. The longest-lived radioisotope is , with a half-life of years. This is also the only carbon radioisotope found in nature, as trace quantities are formed ...
have also been synthesised. Carbon forms covalent bonds with other non-metals with an oxidation state of −4, −2, +2 or +4.
Carbon is the fourth most abundant element in the universe by mass after hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
, helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
and oxygen and is the second most abundant element in the human body by mass after oxygen, the third most abundant by number of atoms. There are an almost infinite number of compounds that contain carbon due to carbon's ability to form long stable chains of C — C bonds. The simplest carbon-containing molecules are the hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
s, which contain carbon and hydrogen, although they sometimes contain other elements in functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s. Hydrocarbons are used as fossil fuels
A fossil fuel is a flammable carbon compound- or hydrocarbon-containing material formed naturally in the Earth's crust from the buried remains of prehistoric organisms (animals, plants or microplanktons), a process that occurs within geologica ...
and to manufacture plastics
Plastics are a wide range of synthetic or semisynthetic materials composed primarily of polymers. Their defining characteristic, plasticity, allows them to be molded, extruded, or pressed into a diverse range of solid forms. This adaptab ...
and petrochemicals
Petrochemicals (sometimes abbreviated as petchems) are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable so ...
. All organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s, those essential for life, contain at least one atom of carbon. When combined with oxygen and hydrogen, carbon can form many groups of important biological compounds including sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecul ...
s, lignan
The lignans are a large group of low molecular weight polyphenols found in plants, particularly seeds, whole grains, and vegetables. The name derives from the Latin word for "wood". Lignans are precursors to phytoestrogens. They may play a rol ...
s, chitin
Chitin (carbon, C8hydrogen, H13oxygen, O5nitrogen, N)n ( ) is a long-chain polymer of N-Acetylglucosamine, ''N''-acetylglucosamine, an amide derivative of glucose. Chitin is the second most abundant polysaccharide in nature (behind only cell ...
s, alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s, fat
In nutrition science, nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such chemical compound, compounds, most commonly those that occur in living beings or in food.
The term often refers specif ...
s, and aromatic ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s, carotenoids
Carotenoids () are yellow, orange, and red organic compound, organic pigments that are produced by plants and algae, as well as several bacteria, archaea, and Fungus, fungi. Carotenoids give the characteristic color to pumpkins, carrots, parsnips ...
and terpenes
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predomi ...
. With nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
it forms alkaloid
Alkaloids are a broad class of natural product, naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids.
Alkaloids are produced by a large varie ...
s, and with the addition of sulfur also it forms antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy ...
s, amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s, and rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds.
Types of polyisoprene ...
products. With the addition of phosphorus to these other elements, it forms DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
and RNA
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins (messenger RNA). RNA and deoxyrib ...
, the chemical-code carriers of life, and adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate that provides energy to drive and support many processes in living cell (biology), cells, such as muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all known ...
(ATP), the most important energy-transfer molecule in all living cells.
Nitrogen
 Nitrogen is the chemical element with atomic number 7, the symbol N and
Nitrogen is the chemical element with atomic number 7, the symbol N and atomic mass
Atomic mass ( or ) is the mass of a single atom. The atomic mass mostly comes from the combined mass of the protons and neutrons in the nucleus, with minor contributions from the electrons and nuclear binding energy. The atomic mass of atoms, ...
14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless and mostly inert diatomic
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear mol ...
gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere
The atmosphere of Earth is composed of a layer of gas mixture that surrounds the Earth's planetary surface (both lands and oceans), known collectively as air, with variable quantities of suspended aerosols and particulates (which create weathe ...
. The element nitrogen was discovered as a separable component of air, by Scottish physician Daniel Rutherford, in 1772. It occurs naturally in form of two isotopes: nitrogen-14 and nitrogen-15.Nitrogenat WebElements. Many industrially important compounds, such as
ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
, nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
, organic nitrates (propellant
A propellant (or propellent) is a mass that is expelled or expanded in such a way as to create a thrust or another motive force in accordance with Newton's third law of motion, and "propel" a vehicle, projectile, or fluid payload. In vehicle ...
s and explosive
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An ex ...
s), and cyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Ionic cyanides contain the cyanide anion . This a ...
s, contain nitrogen. The extremely strong bond in elemental nitrogen dominates nitrogen chemistry, causing difficulty for both organisms and industry in breaking the bond to convert the molecule into useful compounds, but at the same time causing release of large amounts of often useful energy when the compounds burn, explode, or decay back into nitrogen gas.
Nitrogen occurs in all living organisms, and the nitrogen cycle
The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmosphere, atmospheric, terrestrial ecosystem, terrestrial, and marine ecosystems. The conversion of nitrogen can ...
describes movement of the element from air into the biosphere
The biosphere (), also called the ecosphere (), is the worldwide sum of all ecosystems. It can also be termed the zone of life on the Earth. The biosphere (which is technically a spherical shell) is virtually a closed system with regard to mat ...
and organic compounds, then back into the atmosphere. Synthetically produced nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in wa ...
s are key ingredients of industrial fertilizer
A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Man ...
s, and also key pollutants in causing the eutrophication
Eutrophication is a general term describing a process in which nutrients accumulate in a body of water, resulting in an increased growth of organisms that may deplete the oxygen in the water; ie. the process of too many plants growing on the s ...
of water systems. Nitrogen is a constituent element of amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s and thus of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s, and of nucleic acid
Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a pentose, 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nuclei ...
s (DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
and RNA
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins (messenger RNA). RNA and deoxyrib ...
). It resides in the chemical structure
A chemical structure of a molecule is a spatial arrangement of its atoms and their chemical bonds. Its determination includes a chemist's specifying the molecular geometry and, when feasible and necessary, the electronic structure of the target m ...
of almost all neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a Chemical synapse, synapse. The cell receiving the signal, or target cell, may be another neuron, but could also be a gland or muscle cell.
Neurotra ...
s, and is a defining component of alkaloid
Alkaloids are a broad class of natural product, naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids.
Alkaloids are produced by a large varie ...
s, biological molecules produced by many organisms.
Oxygen
Oxygen is the chemical element with atomic number 8, occurring mostly as 16O, but also 17O and 18O. Oxygen is the third-most common element by mass in the universe (although there are more carbon atoms, each carbon atom is lighter). It is highly electronegative and non-metallic, usually diatomic, gas down to very low temperatures. Only fluorine is more reactive among non-metallic elements. It is two electrons short of a full octet and readily takes electrons from other elements. It reacts violently withalkali metals
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
and white phosphorus
White phosphorus, yellow phosphorus, or simply tetraphosphorus (P4) is an allotrope of phosphorus. It is a translucent waxy solid that quickly yellows in light (due to its photochemical conversion into red phosphorus), and impure white phospho ...
at room temperature and less violently with alkali earth metals heavier than magnesium. At higher temperatures it burns most other metals and many non-metals (including hydrogen, carbon, and sulfur). Many oxides are extremely stable substances difficult to decompose—like water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
, carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
, alumina
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula . It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly ...
, silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
, and iron oxides (the latter often appearing as rust
Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture. Rust consists of hydrous iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO(OH) ...
). Oxygen is part of substances best described as some salts of metals and oxygen-containing acids (thus nitrates, sulfates, phosphates, silicates, and carbonates.
Oxygen is essential to all life. Plants and phytoplankton
Phytoplankton () are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater Aquatic ecosystem, ecosystems. The name comes from the Greek language, Greek words (), meaning 'plant', and (), mea ...
photosynthesize water and carbon dioxide and water, both oxides, in the presence of sunlight to form sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecul ...
s with the release of oxygen. The sugars are then turned into such substances as cellulose and (with nitrogen and often sulfur) proteins and other essential substances of life. Animals especially but also fungi and bacteria ultimately depend upon photosynthesizing plants and phytoplankton for food and oxygen.
Fire
Fire is the rapid oxidation of a fuel in the exothermic chemical process of combustion, releasing heat, light, and various reaction Product (chemistry), products.
Flames, the most visible portion of the fire, are produced in the combustion re ...
uses oxygen to oxidize compounds typically of carbon and hydrogen to water and carbon dioxide (although other elements may be involved) whether in uncontrolled conflagrations that destroy buildings and forests or the controlled fire within engines or that supply electrical energy from turbines, heat for keeping buildings warm, or the motive force that drives vehicles.
Oxygen forms roughly 21% of the Earth's atmosphere; all of this oxygen is the result of photosynthesis. Pure oxygen has use in medical treatment of people who have respiratory difficulties. Excess oxygen is toxic.
Oxygen was originally associated with the formation of acids—until some acids were shown to not have oxygen in them. Oxygen is named for its formation of acids, especially with non-metals. Some oxides of some non-metals are extremely acidic, like sulfur trioxide
Sulfur trioxide (alternative spelling sulphur trioxide) is the chemical compound with the formula SO3. It has been described as "unquestionably the most conomicallyimportant sulfur oxide". It is prepared on an industrial scale as a precursor to ...
, which forms sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
on contact with water. Most oxides with metals are alkaline, some extremely so, like potassium oxide
Potassium oxide ( K O) is an ionic compound of potassium and oxygen. It is a base. This pale yellow solid is the simplest oxide of potassium. It is a highly reactive compound that is rarely encountered. Some industrial materials, such as fertil ...
. Some metallic oxides are amphoteric, like aluminum oxide, which means that they can react with both acids and bases.
Although oxygen is normally a diatomic gas, oxygen can form an allotrope known as ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
. Ozone is a triatomic gas even more reactive than oxygen. Unlike regular diatomic oxygen, ozone is a toxic material generally considered a pollutant. In the upper atmosphere, some oxygen forms ozone which has the property of absorbing dangerous ultraviolet rays within the ozone layer
The ozone layer or ozone shield is a region of Earth's stratosphere that absorption (electromagnetic radiation), absorbs most of the Sun's ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the a ...
. Land life was impossible before the formation of an ozone layer.
Fluorine
 Fluorine is the chemical element with atomic number 9. It occurs naturally in its only stable form 19F.
Fluorine is a pale-yellow, diatomic gas under normal conditions and down to very low temperatures. Short one electron of the highly stable octet in each atom, fluorine molecules are unstable enough that they easily snap, with loose fluorine atoms tending to grab single electrons from just about any other element. Fluorine is the most reactive of all elements, and it even attacks many oxides to replace oxygen with fluorine. Fluorine even attacks silica, one of the favored materials for transporting strong acids, and burns asbestos. It attacks common salt, one of the most stable compounds, with the release of chlorine. It never appears uncombined in nature and almost never stays uncombined for long. It burns hydrogen simultaneously if either is liquid or gaseous—even at temperatures close to absolute zero. It is extremely difficult to isolate from any compounds, let alone keep uncombined.
Fluorine gas is extremely dangerous because it attacks almost all organic material, including live flesh. Many of the binary compounds that it forms (called fluorides) are themselves highly toxic, including soluble fluorides and especially
Fluorine is the chemical element with atomic number 9. It occurs naturally in its only stable form 19F.
Fluorine is a pale-yellow, diatomic gas under normal conditions and down to very low temperatures. Short one electron of the highly stable octet in each atom, fluorine molecules are unstable enough that they easily snap, with loose fluorine atoms tending to grab single electrons from just about any other element. Fluorine is the most reactive of all elements, and it even attacks many oxides to replace oxygen with fluorine. Fluorine even attacks silica, one of the favored materials for transporting strong acids, and burns asbestos. It attacks common salt, one of the most stable compounds, with the release of chlorine. It never appears uncombined in nature and almost never stays uncombined for long. It burns hydrogen simultaneously if either is liquid or gaseous—even at temperatures close to absolute zero. It is extremely difficult to isolate from any compounds, let alone keep uncombined.
Fluorine gas is extremely dangerous because it attacks almost all organic material, including live flesh. Many of the binary compounds that it forms (called fluorides) are themselves highly toxic, including soluble fluorides and especially hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
. Fluorine forms very strong bonds with many elements. With sulfur it can form the extremely stable and chemically inert sulfur hexafluoride
Sulfur hexafluoride or sulphur hexafluoride ( British spelling) is an inorganic compound with the formula SF6. It is a colorless, odorless, non-flammable, and non-toxic gas. has an octahedral geometry, consisting of six fluorine atoms attache ...
; with carbon it can form the remarkable material Teflon
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off from ...
that is a stable and non-combustible solid with a high melting point and a very low coefficient of friction that makes it an excellent liner for cooking pans and raincoats. Fluorine-carbon compounds include some unique plastics.
it is also used as a reactant in the making of toothpaste.
Neon
 Neon is the chemical element with atomic number 10, occurring as 20Ne, 21Ne and 22Ne.
Neon is a monatomic gas. With a complete octet of outer electrons it is highly resistant to removal of any electron, and it cannot accept an electron from anything. Neon has no tendency to form any normal compounds under normal temperatures and pressures; it is effectively inert. It is one of the so-called "noble gases".
Neon is a trace component of the atmosphere without any biological role.
Neon is the chemical element with atomic number 10, occurring as 20Ne, 21Ne and 22Ne.
Neon is a monatomic gas. With a complete octet of outer electrons it is highly resistant to removal of any electron, and it cannot accept an electron from anything. Neon has no tendency to form any normal compounds under normal temperatures and pressures; it is effectively inert. It is one of the so-called "noble gases".
Neon is a trace component of the atmosphere without any biological role.
Notes
References
External links
* {{DEFAULTSORT:Period 02 Periods (periodic table) Pages containing element color directly