|
Isotopes Of Boron
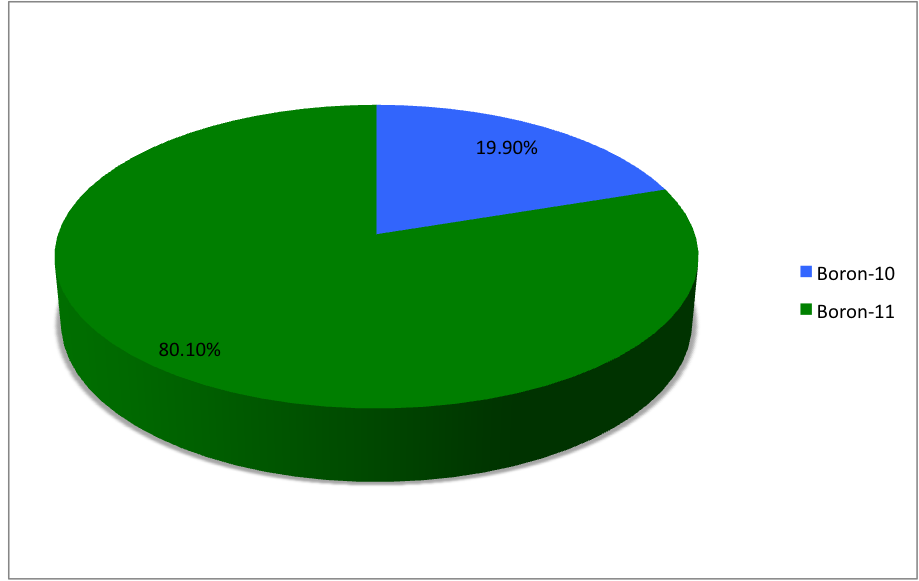
Boron (5B) naturally occurs as Isotope, isotopes and , the latter of which makes up about 80% of natural boron. There are 13 Radionuclide, radioisotopes that have been discovered, with mass numbers from 7 to 21, all with short half-life, half-lives, the longest being that of , with a half-life of only and with a half-life of . All other isotopes have half-lives shorter than . Those isotopes with mass below 10 decay into helium (via short-lived isotopes of beryllium for and ) while those with mass above 11 mostly become carbon. List of isotopes , -id=Boron-7 , , style="text-align:center" , 5 , style="text-align:center" , 2 , , [] , proton emission, p , Subsequently decays by double proton emission to for a net reaction of → + 3 , (3/2−) , , , - , Has 1 halo nucleus, halo protonIntermediate product of Proton–proton chain#The p–p III branch, a branch of proton–proton chain in stellar nucleosynthesis as part of the process converting hydrogen to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride. Boron is synthesized entirely by cosmic ray spallation and supernovas and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. It is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known deposits are in Turkey, the largest producer of boron minerals. Elemental boron is found in smal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Isomer
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have Half-life, half-lives of 10−9 seconds or longer, 100 to 1000 times longer than the half-lives of the excited nuclear states that decay with a "prompt" half life (ordinarily on the order of 10−12 seconds). Some references recommend seconds to distinguish the metastable half life from the normal "prompt" Induced gamma emission, gamma-emission half-life. Occasionally the half-lives are far longer than this and can last minutes, hours, or years. For example, the Isotopes of tantalum#Tantalum-180m, nuclear isomer survives so long (at least years) that it has never been observed to decay spontaneously. The half-life of a nuclear isomer can even exceed that of the ground state of the same nuclide, as shown by as well as isotopes of rhenium, , isotopes of iridium, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Helium
Helium (He) (standard atomic weight: ) has nine known isotopes, but only helium-3 (He) and helium-4 (He) are stable. All radioisotopes are short-lived; the longest-lived is He with half-life . The least stable is He, with half-life (), though He may have an even shorter half-life. In Earth's atmosphere, the ratio of He to He is . However, the isotopic abundance of helium varies greatly depending on its origin. In the Local Interstellar Cloud, the proportion of He to He is , which is ~121 times higher than in Earth's atmosphere. Rocks from Earth's crust have isotope ratios varying by as much as a factor of ten; this is used in geology to investigate the origin of rocks and the composition of the Earth's mantle. The different formation processes of the two stable isotopes of helium produce the differing isotope abundances. Equal mixtures of liquid He and He below separate into two immiscible phases due to differences in quantum statistics: He atoms are bosons while He atoms are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Beryllium
Beryllium (4Be) has 11 known Isotope, isotopes and 3 known nuclear isomer, isomers, but only one of these isotopes () is stable and a primordial nuclide. As such, beryllium is considered a monoisotopic element. It is also a mononuclidic element, because its other isotopes have such short half-lives that none are primordial and their abundance is very low (standard atomic weight is ). Beryllium is unique as being the only monoisotopic element with both an even number of protons and an odd number of neutrons. There are 25 other monoisotopic elements but all have odd atomic numbers, and even numbers of neutrons. Of the 10 radionuclide, radioisotopes of beryllium, the most stable are with a half-life of million years and with a half-life of . All other radioisotopes have half-lives under , most under . The least stable isotope is , with a half-life of . The 1:1 neutron–proton ratio seen in stable isotopes of many light elements (up to oxygen, and in elements with even atomic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Carbon
Carbon (6C) has 14 known isotopes, from to as well as , of which only and are stable. The longest-lived radioisotope is , with a half-life of years. This is also the only carbon radioisotope found in nature, as trace quantities are formed cosmogenically by the reaction + → + . The most stable artificial radioisotope is , which has a half-life of . All other radioisotopes have half-lives under 20 seconds, most less than 200 milliseconds. The least stable isotope is , with a half-life of . Light isotopes tend to decay into isotopes of boron and heavy ones tend to decay into isotopes of nitrogen. List of isotopes , -id=Carbon-8 , , style="text-align:right" , 6 , style="text-align:right" , 2 , , [] , proton emission, 2p , Also immediately emits two protons for the net reaction of → + 4 , 0+ , , , -id=Carbon-9 , rowspan=3, , rowspan=3 style="text-align:right" , 6 , rowspan=3 style="text-align:right" , 3 , rowspan=3, , rowspan=3, , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Capture Therapy Of Cancer
Neutron capture therapy (NCT) is a type of radiotherapy for treating locally invasive malignant tumors such as primary brain tumors, recurrent cancers of the head and neck region, and cutaneous and extracutaneous melanomas. It is a two-step process: ''first'', the patient is injected with a tumor-localizing drug containing the stable isotope boron-10 (B), which has a high propensity to capture low energy "thermal" neutrons. The neutron cross section of B (3,837 barns) is 1,000 times more than that of other elements, such as nitrogen, hydrogen, or oxygen, that occur in tissue. In the ''second'' step, the patient is radiated with epithermal neutrons, the sources of which in the past have been nuclear reactors and now are accelerators that produce higher energy epithermal neutrons. After losing energy as they penetrate tissue, the resultant low energy "thermal" neutrons are captured by the B atoms. The resulting decay reaction yields high-energy alpha particles that kill the canc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Weakly Interacting Massive Particles
Weakly interacting massive particles (WIMPs) are hypothetical particles that are one of the proposed candidates for dark matter. There exists no formal definition of a WIMP, but broadly, it is an elementary particle which interacts via gravity and any other force (or forces) which is as weak as or weaker than the weak nuclear force, but also non-vanishing in strength. Many WIMP candidates are expected to have been produced thermally in the early Universe, similarly to the particles of the Standard Model according to Big Bang cosmology, and usually will constitute cold dark matter. Obtaining the correct abundance of dark matter today via thermal production requires a self-annihilation Cross section (physics), cross section of \langle \sigma v \rangle ≃ , which is roughly what is expected for a new particle in the 100 GeV/''c''2 mass range that interacts via the electroweak force. Experimental efforts to detect WIMPs include the search for products of WIMP annihilation, inclu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar Neutrino Flux
Solar may refer to: Astronomy * Of or relating to the Sun ** Solar telescope, a special purpose telescope used to observe the Sun ** A device that utilizes solar energy (e.g. "solar panels") ** Solar calendar, a calendar whose dates indicate the position of the Earth on its revolution around the Sun ** Solar eclipse, an eclipse of a sun in which it is obstructed by the moon ** Solar System, the planetary system made up by the Sun and the objects orbiting it * Solar Maximum Mission, a satellite * SOLAR (ISS), an observatory on International Space Station Music * "Solar" (composition), attributed to Miles Davis * ''Solar'' (Red Garland album), 1962 * ''Solar'' (Taeyang album), 2010 * ''Solar'', a 2011 album by Rubik * "Solar", a song by Northlane from ''Mesmer'', 2017 * "Solar", a song by Sault from ''Air'', 2022 * ”Solar”, a song by Stam1na from '' Taival'', 2018 * SOLAR Records, a record label Geography * Solar (Spanish term), a type of urban site * Solar, County ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutrino
A neutrino ( ; denoted by the Greek letter ) is an elementary particle that interacts via the weak interaction and gravity. The neutrino is so named because it is electrically neutral and because its rest mass is so small ('' -ino'') that it was long thought to be zero. The rest mass of the neutrino is much smaller than that of the other known elementary particles (excluding massless particles). The weak force has a very short range, the gravitational interaction is extremely weak due to the very small mass of the neutrino, and neutrinos do not participate in the electromagnetic interaction or the strong interaction. Consequently, neutrinos typically pass through normal matter unimpeded and with no detectable effect. Weak interactions create neutrinos in one of three leptonic flavors: # electron neutrino, # muon neutrino, # tau neutrino, Each flavor is associated with the correspondingly named charged lepton. Although neutrinos were long believed to be mas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium-11
Naturally occurring lithium (3Li) is composed of two stable isotopes, lithium-6 (6Li) and lithium-7 (7Li), with the latter being far more abundant on Earth. Both of the natural isotopes have an unexpectedly low nuclear binding energy per nucleon ( for 6Li and for 7Li) when compared with the adjacent lighter and heavier elements, helium ( for helium-4) and beryllium ( for beryllium-9). The longest-lived radioisotope of lithium is 8Li, which has a half-life of just . 9Li has a half-life of , and 11Li has a half-life of . All of the remaining isotopes of lithium have half-lives that are shorter than 10 nanoseconds. The shortest-lived known isotope of lithium is 4Li, which decays by proton emission with a half-life of about (), although the half-life of 3Li is yet to be determined, and is likely to be much shorter, like 2He (helium-2, diproton) which undergoes proton emission within s. Both 7Li and 6Li are two of the primordial nuclides that were produced in the Big Bang, with 7 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beryllium-8
Beryllium-8 (8Be, Be-8) is a radionuclide with 4 neutrons and 4 protons. It is an unbound resonance and nominally an isotope of beryllium. It has a half-life on the order of 8.19 seconds, decaying into two alpha particles. This has important ramifications in stellar nucleosynthesis as it creates a bottleneck in the creation of heavier chemical elements. The properties of 8Be have also led to speculation on the fine tuning of the universe, and theoretical investigations on cosmological evolution had 8Be been stable. Discovery The discovery of beryllium-8 occurred shortly after the construction of the first particle accelerator in 1932. Physicists John Douglas Cockcroft and Ernest Walton performed their first experiment with their accelerator at the Cavendish Laboratory in Cambridge, in which they irradiated lithium-7 with protons. They reported that this populated a nucleus with ''A'' = 8 that near-instantaneously decays into two alpha particles. This activi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Emission
Neutron emission is a mode of radioactive decay in which one or more neutrons are ejected from a Atomic nucleus, nucleus. It occurs in the most neutron-rich/proton-deficient nuclides, and also from excited states of other nuclides as in photodisintegration, photoneutron emission and beta-delayed neutron emission. As only a neutron is lost by this process the number of protons remains unchanged, and an atom does not become an atom of a different element, but a different isotope of the same element. Neutrons are also produced in the spontaneous fission, spontaneous and nuclear fission, induced fission of certain heavy nuclides. Spontaneous neutron emission As a consequence of the Pauli exclusion principle, nuclei with an excess of protons or neutrons have a higher average energy per nucleon. Nuclei with a sufficient excess of neutrons have a greater energy than the combination of a free neutron and a nucleus with one less neutron, and therefore can decay by neutron emission. Nuclei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |