|
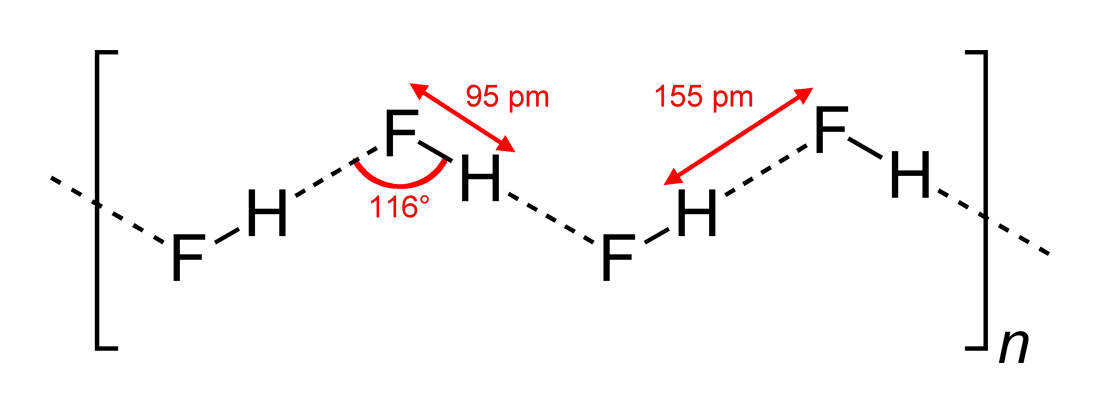
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hafnium
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869, though it was not identified until 1922, by Dirk Coster and George de Hevesy. Hafnium is named after , the Latin name for Copenhagen, where it was discovered. Hafnium is used in filaments and electrodes. Some semiconductor fabrication processes use its oxide for integrated circuits at 45 nanometers and smaller feature lengths. Some superalloys used for special applications contain hafnium in combination with niobium, titanium, or tungsten. Hafnium's large neutron capture cross section makes it a good material for neutron absorption in control rods in nuclear power plants, but at the same time requires that it be removed from the neutron-transparent corrosion-resistant zirconium alloys used in nuclear r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hydrofluoric Acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling point near room temperature. It is used to make most organofluorine compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to etch glass and silicon wafers. Uses Production of organofluorine compounds The principal use of hydrofluoric acid is in organofluorine chemistry. Many organofluorine compounds are prepared using HF as the fluorine source, including Teflon, fluoropolymers, fluorocarbons, and refrigerants such as freon. Many pharmaceuticals contain fluorine. Production of inorganic fluorides Most high-volume inorganic fluoride compounds are prepared from hydrofluoric acid. Foremost ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish Pomerania, German-Swedish pharmaceutical chemist. Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified the elements molybdenum, tungsten, barium, nitrogen, and chlorine, among others. Scheele discovered organic acids Tartaric acid, tartaric, Oxalic acid, oxalic, Uric acid, uric, Lactic acid, lactic, and Citric acid, citric, as well as Hydrofluoric acid, hydrofluoric, Hydrocyanic acid, hydrocyanic, and Arsenic acid, arsenic acids. He preferred speaking German to Swedish his whole life, as German was commonly spoken among Swedish pharmacists.Fors, Hjalmar 2008. "Stepping through Science’s Door: C. W. Scheele, from Pharmacist's Apprentice to Man of Science". Ambix 55: 29–49 Biography Scheele was born in Stralsund, in western Pomerania, which at the time was a Dominions of Sweden, Swedish Dominion inside the Holy Roman Empire. Scheele's father, Joachim (or Jo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Cornea
The cornea is the transparency (optics), transparent front part of the eyeball which covers the Iris (anatomy), iris, pupil, and Anterior chamber of eyeball, anterior chamber. Along with the anterior chamber and Lens (anatomy), lens, the cornea Refraction, refracts light, accounting for approximately two-thirds of the eye's total optical power. In humans, the refractive power of the cornea is approximately 43 dioptres. The cornea can be reshaped by surgical procedures such as LASIK. While the cornea contributes most of the eye's focusing power, its Focus (optics), focus is fixed. Accommodation (eye), Accommodation (the refocusing of light to better view near objects) is accomplished by changing the geometry of the lens. Medical terms related to the cornea often start with the prefix "''wikt:kerat-, kerat-''" from the Ancient Greek, Greek word κέρας, ''horn''. Structure The cornea has myelinated, unmyelinated nerve endings sensitive to touch, temperature and chemicals; a to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Blindness
Visual or vision impairment (VI or VIP) is the partial or total inability of visual perception. In the absence of treatment such as corrective eyewear, assistive devices, and medical treatment, visual impairment may cause the individual difficulties with normal daily tasks, including reading and walking. The terms ''low vision'' and ''blindness'' are often used for levels of impairment which are difficult or impossible to correct and significantly impact daily life. In addition to the various permanent conditions, fleeting temporary vision impairment, amaurosis fugax, may occur, and may indicate serious medical problems. The most common causes of visual impairment globally are uncorrected refractive errors (43%), cataracts (33%), and glaucoma (2%). Refractive errors include near-sightedness, far-sightedness, presbyopia, and astigmatism (eye), astigmatism. Cataracts are the most common cause of blindness. Other disorders that may cause visual problems include age-related macular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Moisture
Moisture is the presence of a liquid, especially water, often in trace amounts. Moisture is defined as water in the adsorbed or absorbed phase. Small amounts of water may be found, for example, in the air (humidity), in foods, and in some commercial products. Moisture also refers to the amount of water vapor present in the air. The soil also includes moisture. Moisture control in products Control of moisture in products can be a vital part of the process of the product. There is a substantial amount of moisture in what seems to be dry matter. Ranging in products from cornflake cereals to laundry detergent, washing powders, moisture can play an important role in the final quality of the product. There are two main aspects of concern in moisture control in products: allowing too much moisture or too little of it. For example, adding some water to cornflake cereal, which is sold by weight, reduces costs and prevents it from tasting too dry, but adding too much water can affect th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Corrosive
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion. In the most common use of the word, this means electrochemical oxidation of metal in reaction with an oxidant such as oxygen, hydrogen, or hydroxide. Rusting, the formation of red-orange iron oxides, is a well-known example of electrochemical corrosion. This type of corrosion typically produces oxides or salts of the original metal and results in a distinctive coloration. Corrosion can also occur in materials other than metals, such as ceramics or polymers, although in this context, the term "degradation" is more common. Corrosion degrades the useful properties of materials and structures including mechanical strength, appearance, and permeability to liquids and gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hydrogen Halide
In chemistry, hydrogen halides (hydrohalic acids when in the aqueous phase) are diatomic, inorganic compounds that function as Arrhenius acids. The formula is HX where X is one of the halogens: fluorine, chlorine, bromine, iodine, astatine, or tennessine. All known hydrogen halides are gases at standard temperature and pressure.The Acidity of the Hydrogen Halides. (2020, August 21). Retrieved May 5, 2021, from https://chem.libretexts.org/@go/page/3699 Comparison to hydrohalic acids The hydrogen halides are diatomic molecules with no tendency to ionize in the gas phase (although liquified hydrogen fluoride is a polar solvent somewhat similar to water). Thus, chemists distinguish hydrogen chloride from hydrochloric acid. The former is a gas at room temperature that reacts with water to give the acid. Once the acid has formed, the diatomic molecule can be regenerated only with difficulty, but not by normal distillation. Commonly the names of the acid and the molecules are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hydrogen Bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently bonded to a more Electronegativity, electronegative donor atom or group (Dn), interacts with another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Unlike simple Dipole–dipole attraction, dipole–dipole interactions, hydrogen bonding arises from charge transfer (nB → σ*AH), Atomic orbital, orbital interactions, and quantum mechanical Delocalized electron, delocalization, making it a resonance-assisted interaction rather than a mere electrostatic attraction. The general notation for hydrogen bonding is Dn−H···Ac, where the solid line represents a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are nitrogen (N), oxyg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Superacid
In chemistry, a superacid (according to the original definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (), which has a Hammett acidity function (''H''0) of −12. According to the modern definition, a superacid is a medium in which the chemical potential of the proton is higher than in pure sulfuric acid. Commercially available superacids include trifluoromethanesulfonic acid (), also known as triflic acid, and fluorosulfuric acid (), both of which are about a thousand times stronger (i.e. have more negative ''H''0 values) than sulfuric acid. Most strong superacids are prepared by the combination of a strong Lewis acid and a strong Brønsted acid. A strong superacid of this kind is fluoroantimonic acid. Another group of superacids, the carborane acid group, contains some of the strongest known acids. Finally, when treated with anhydrous acid, zeolites (microporous aluminosilicate minerals) will contain superacidic sites within their pores. These m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Petrochemical Industry
file:Jampilen Petrochemical Co. 02.jpg, 300px, Jampilen Petrochemical co., Asaluyeh, Iran The petrochemical industry is concerned with the production and trade of petrochemicals. A major part is constituted by the plastics industry, plastics (polymer) industry. It directly interfaces with the petroleum industry, especially the downstream (petroleum industry), downstream sector. Companies The top global petrochemical companies based on different KPIs: Countries and sites *Marun petrochemical complex Technology Conferences *Asia Petrochemical Industry Conference *International Petrochemical Conference by the American Fuel and Petrochemical Manufacturers, AFPM Associations *American Fuel and Petrochemical Manufacturers (AFPM) *European Petrochemical Association (EPCA) *Gulf Petrochemicals and Chemicals Association (GPCA) *Petrochemicals Europe (industry sector of Cefic) Awards *Medal "For the Tapping of the Subsoil and Expansion of the Petrochemical Complex of Western Siberia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Polytetrafluoroethylene
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a corporate spin-off, spin-off from DuPont (1802–2017), DuPont, which originally invented the compound in 1938. Polytetrafluoroethylene is a fluorocarbon solid, as it is a high-molecular-weight polymer consisting wholly of carbon and fluorine. PTFE is hydrophobic: neither water nor water-containing substances Wetting, wet PTFE, as fluorocarbons exhibit only small London dispersion forces due to the low polarizability, electric polarizability of fluorine. PTFE has one of the lowest Friction#Coefficient of friction, coefficients of friction of any solid. Polytetrafluoroethylene is used as a non-stick coating for Cookware and bakeware, pans and other Cookware and bakeware, cookware. It is Chemically inert, non-reactive, partly because of the strength of car ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |