Displacement reaction on:
[Wikipedia]
[Google]
[Amazon]
 A chemical reaction is a process that leads to the
A chemical reaction is a process that leads to the
 Chemical reactions such as combustion in fire,
Chemical reactions such as combustion in fire,


 The most important elementary reactions are unimolecular and bimolecular reactions. Only one molecule is involved in a unimolecular reaction; it is transformed by isomerization or a
The most important elementary reactions are unimolecular and bimolecular reactions. Only one molecule is involved in a unimolecular reaction; it is transformed by isomerization or a AB -> A + B
: Dissociation of a molecule AB into fragments A and B
For A + B -> AB
Another possibility is that only a portion of one molecule is transferred to the other molecule. This type of reaction occurs, for example, in HA + B -> A + HB
for example
:NaCl + AgNO3 -> NaNO3 + AgCl(v)
2CO(g) + MoO2(s) -> 2CO2(g) + Mo(s) ;
This reaction to form
/ref> Δ''H''° is zero at , and the reaction becomes exothermic above that temperature. Changes in temperature can also reverse the direction tendency of a reaction. For example, theCO(g) + H2O() <=> CO2(g) + H2(g)
is favored by low temperatures, but its reverse is favored by high temperatures. The shift in reaction direction tendency occurs at .
Reactions can also be characterized by their

A + B->AB
Two or more reactants yielding one product is another way to identify a synthesis reaction. One example of a synthesis reaction is the combination of 8Fe + S8->8FeS
Another example is simple hydrogen gas combined with simple oxygen gas to produce a more complex substance, such as water.To react or not to react?
Utah State Office of Education. Retrieved 4 June 2011.
AB->A + B
One example of a decomposition reaction is the 2H2O->2H2 + O2
A + BC->AC + B
One example of a single displacement reaction is when Mg + 2H2O->Mg(OH)2 + H2 (^)
AB + CD->AD + CB
For example, when Pb(NO3)2 + 2KI->PbI2(v) + 2KNO3
C8H18(l) + 25/2 O2(g)->8CO2 + 9H2O(l)
releases 5500 kJ. A combustion reaction can also result from 2Mg(s) + O2->2MgO(s)
S(s) + O2(g)->SO2(g)
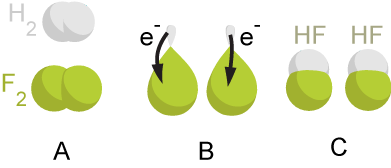

2Na(s) + Cl2(g)->2NaCl(s)
In the reaction, sodium metal goes from an oxidation state of 0 (as it is a pure element) to +1: in other words, the sodium lost one electron and is said to have been oxidized. On the other hand, the chlorine gas goes from an oxidation of 0 (it is also a pure element) to −1: the chlorine gains one electron and is said to have been reduced. Because the chlorine is the one reduced, it is considered the electron acceptor, or in other words, induces oxidation in the sodium – thus the chlorine gas is considered the oxidizing agent. Conversely, the sodium is oxidized or is the electron donor, and thus induces a reduction in the other species and is considered the ''reducing agent''.
Which of the involved reactants would be a reducing or oxidizing agent can be predicted from the
 In complexation reactions, several
In complexation reactions, several
\underset + \underset <=> \underset + \underset
The reverse reaction is possible, and thus the acid/base and conjugated base/acid are always in equilibrium. The equilibrium is determined by the acid and base dissociation constants (''K''a and ''K''b) of the involved substances. A special case of the acid-base reaction is the neutralization where an acid and a base, taken at the exact same amounts, form a neutral

 In
In
 In
In
 In the third type of substitution reaction, radical substitution, the attacking particle is a
In the third type of substitution reaction, radical substitution, the attacking particle is a X. + R-H -> X-H + R.
:;R. + X2 -> R-X + X.
:: Reactions during the chain reaction of radical substitution
 The E2 mechanism also requires a base, but there the attack of the base and the elimination of the leaving group proceed simultaneously and produce no ionic intermediate. In contrast to the E1 eliminations, different stereochemical configurations are possible for the reaction product in the E2 mechanism, because the attack of the base preferentially occurs in the anti-position with respect to the leaving group. Because of the similar conditions and reagents, the E2 elimination is always in competition with the SN2-substitution.
The E2 mechanism also requires a base, but there the attack of the base and the elimination of the leaving group proceed simultaneously and produce no ionic intermediate. In contrast to the E1 eliminations, different stereochemical configurations are possible for the reaction product in the E2 mechanism, because the attack of the base preferentially occurs in the anti-position with respect to the leaving group. Because of the similar conditions and reagents, the E2 elimination is always in competition with the SN2-substitution.
 The counterpart of elimination is an addition where double or triple bonds are converted into single bonds. Similar to substitution reactions, there are several types of additions distinguished by the type of the attacking particle. For example, in the
The counterpart of elimination is an addition where double or triple bonds are converted into single bonds. Similar to substitution reactions, there are several types of additions distinguished by the type of the attacking particle. For example, in the 
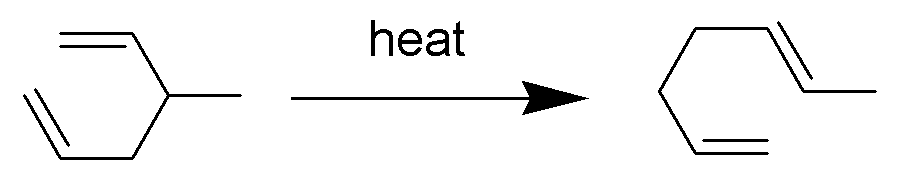 In a rearrangement reaction, the carbon skeleton of a
In a rearrangement reaction, the carbon skeleton of a
 Biochemical reactions are mainly controlled by
Biochemical reactions are mainly controlled by
 Chemical reactions are central to
Chemical reactions are central to
 A chemical reaction is a process that leads to the
A chemical reaction is a process that leads to the chemical transformation
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., wi ...
of one set of chemical substance
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., wi ...
s to another. Classically, chemical
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., wi ...
reactions encompass changes that only involve the positions of electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s in the forming and breaking of chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
s between atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between ...
. Nuclear chemistry
Nuclear chemistry is the sub-field of chemistry dealing with radioactivity, nuclear processes, and transformations in the nuclei of atoms, such as nuclear transmutation and nuclear properties.
It is the chemistry of radioactive elements such as t ...
is a sub-discipline of chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
that involves the chemical reactions of unstable
In numerous fields of study, the component of instability within a system is generally characterized by some of the outputs or internal states growing without bounds. Not all systems that are not stable are unstable; systems can also be mar ...
and radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
elements where both electronic and nuclear changes can occur.
The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change
Chemical changes occur when a substance combines with another to form a new substance, called chemical synthesis or, alternatively, chemical decomposition into two or more different substances. These processes are called chemical reactions and, ...
, and they yield one or more products
Product may refer to:
Business
* Product (business), an item that serves as a solution to a specific consumer problem.
* Product (project management), a deliverable or set of deliverables that contribute to a business solution
Mathematics
* Produ ...
, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reaction
An elementary reaction is a chemical reaction in which one or more chemical species react directly to form products in a single reaction step and with a single transition state. In practice, a reaction is assumed to be elementary if no reaction in ...
s, and the information on the precise course of action is part of the reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
. Chemical reactions are described with chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between ...
s, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.
Chemical reactions happen at a characteristic reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit ...
at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy
The term "thermal energy" is used loosely in various contexts in physics and engineering. It can refer to several different well-defined physical concepts. These include the internal energy or enthalpy of a body of matter and radiation; heat, d ...
available to reach the activation energy necessary for breaking bonds between atoms.
Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous and reduce the free energy if they occur at constant temperature and pressure. Non-spontaneous reactions require an input of energy to go forward (examples include charging a battery driven by an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation
In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic field, electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy. It includes radio waves, microwaves, inf ...
usually in the form of sunlight).
A reaction may be classified as redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction ...
in which oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
and reduction occur or nonredox in which there is no oxidation and reduction occurring. Most simple redox reactions may be classified as a combination, decomposition, or single displacement reaction.
Different chemical reactions are used during chemical synthesis
As a topic of chemistry, chemical synthesis (or combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In moder ...
in order to obtain the desired product. In biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathway
In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell. The reactants, products, and intermediates of an enzymatic reaction are known as metabolites, which are modified by a sequence of chemical reac ...
s. These reactions are often catalyzed
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
by protein enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
s. Enzymes increase the rates of biochemical reactions, so that metabolic
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cell ...
syntheses and decompositions impossible under ordinary conditions can occur at the temperature and concentrations present within a cell
Cell most often refers to:
* Cell (biology), the functional basic unit of life
Cell may also refer to:
Locations
* Monastic cell, a small room, hut, or cave in which a religious recluse lives, alternatively the small precursor of a monastery ...
.
The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a transformation o ...
, radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
s and reactions between elementary particle
In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles. Particles currently thought to be elementary include electrons, the fundamental fermions ( quarks, leptons, an ...
s, as described by quantum field theory
In theoretical physics, quantum field theory (QFT) is a theoretical framework that combines classical field theory, special relativity, and quantum mechanics. QFT is used in particle physics to construct physical models of subatomic particles and ...
.
History
 Chemical reactions such as combustion in fire,
Chemical reactions such as combustion in fire, fermentation
Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food ...
and the reduction of ores to metals were known since antiquity. Initial theories of transformation of materials were developed by Greek philosophers, such as the Four-Element Theory of Empedocles
Empedocles (; grc-gre, Ἐμπεδοκλῆς; , 444–443 BC) was a Greek pre-Socratic philosopher and a native citizen of Akragas, a Greek city in Sicily. Empedocles' philosophy is best known for originating the cosmogonic theory of the fo ...
stating that any substance is composed of the four basic elements – fire, water, air and earth. In the Middle Ages
In the history of Europe, the Middle Ages or medieval period lasted approximately from the late 5th to the late 15th centuries, similar to the post-classical period of global history. It began with the fall of the Western Roman Empire a ...
, chemical transformations were studied by alchemists
Alchemy (from Arabic: ''al-kīmiyā''; from Ancient Greek: χυμεία, ''khumeía'') is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practiced in China, India, the Muslim world, ...
. They attempted, in particular, to convert lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
into gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile met ...
, for which purpose they used reactions of lead and lead-copper alloys with sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
.
The artificial production of chemical substances already was a central goal for medieval alchemists. Examples include the synthesis of ammonium chloride from organic substances as described in the works (c. 850–950) attributed to Jābir ibn Ḥayyān, or the production of mineral acids
A mineral acid (or inorganic acid) is an acid derived from one or more inorganic compounds, as opposed to organic acids which are acidic, organic compounds. All mineral acids form hydrogen ions and the conjugate base when dissolved in water.
Ch ...
such as sulfuric and nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
s by later alchemists, starting from c. 1300. The production of mineral acids involved the heating of sulfate and nitrate minerals such as copper sulfate Copper sulfate may refer to:
* Copper(II) sulfate, CuSO4, a common compound used as a fungicide and herbicide
* Copper(I) sulfate
Copper(I) sulfate, also known as cuprous sulfate, is an inorganic compound with the chemical formula Cu2 SO4. It ...
, alum
An alum () is a type of chemical compound, usually a hydrated double salt, double sulfate salt (chemistry), salt of aluminium with the general chemical formula, formula , where is a valence (chemistry), monovalent cation such as potassium or a ...
and saltpeter
Potassium nitrate is a chemical compound with the chemical formula . This alkali metal nitrate salt is also known as Indian saltpetre (large deposits of which were historically mined in India). It is an ionic salt of potassium ions K+ and nitrat ...
. In the 17th century, Johann Rudolph Glauber
Johann Rudolf Glauber (10 March 1604 – 16 March 1670) was a German-Dutch alchemist and chemist. Some historians of science have described him as one of the first chemical engineers. His discovery of sodium sulfate in 1625 led to the com ...
produced hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
and sodium sulfate
Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 milli ...
by reacting sulfuric acid and sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
. With the development of the lead chamber process
The lead chamber process was an industrial method used to produce sulfuric acid in large quantities. It has been largely supplanted by the contact process.
In 1746 in Birmingham, England, John Roebuck began producing sulfuric acid in lead-lined ...
in 1746 and the Leblanc process, allowing large-scale production of sulfuric acid and sodium carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions ...
, respectively, chemical reactions became implemented into the industry. Further optimization of sulfuric acid technology resulted in the contact process
The contact process is the current method of producing sulfuric acid in the high concentrations needed for industrial processes. Platinum was originally used as the catalyst for this reaction; however, as it is susceptible to reacting with arsenic ...
in the 1880s, and the Haber process
The Haber process, also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. It is named after its inventors, the German chemists Fritz Haber and C ...
was developed in 1909–1910 for ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
synthesis.
From the 16th century, researchers including Jan Baptist van Helmont
Jan Baptist van Helmont (; ; 12 January 1580 – 30 December 1644) was a chemist, physiologist, and physician from Brussels. He worked during the years just after Paracelsus and the rise of iatrochemistry, and is sometimes considered to ...
, Robert Boyle
Robert Boyle (; 25 January 1627 – 31 December 1691) was an Anglo-Irish natural philosopher, chemist, physicist, alchemist and inventor. Boyle is largely regarded today as the first modern chemist, and therefore one of the founders of ...
, and Isaac Newton
Sir Isaac Newton (25 December 1642 – 20 March 1726/27) was an English mathematician, physicist, astronomer, alchemist, theologian, and author (described in his time as a "natural philosopher"), widely recognised as one of the grea ...
tried to establish theories of experimentally observed chemical transformations. The phlogiston theory
The phlogiston theory is a superseded scientific theory that postulated the existence of a fire-like element called phlogiston () contained within combustible bodies and released during combustion. The name comes from the Ancient Greek (''burni ...
was proposed in 1667 by Johann Joachim Becher
Johann Joachim Becher (; 6 May 1635 – October 1682) was a German physician, alchemist, precursor of chemistry, scholar and adventurer, best known for his development of the phlogiston theory of combustion, and his advancement of Austrian cameral ...
. It postulated the existence of a fire-like element called "phlogiston", which was contained within combustible bodies and released during combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
. This proved to be false in 1785 by Antoine Lavoisier
Antoine-Laurent de Lavoisier ( , ; ; 26 August 17438 May 1794), When reduced without charcoal, it gave off an air which supported respiration and combustion in an enhanced way. He concluded that this was just a pure form of common air and th ...
who found the correct explanation of the combustion as a reaction with oxygen from the air.
Joseph Louis Gay-Lussac
Joseph Louis Gay-Lussac (, , ; 6 December 1778 – 9 May 1850) was a French chemist and physicist. He is known mostly for his discovery that water is made of two parts hydrogen and one part oxygen (with Alexander von Humboldt), for two laws ...
recognized in 1808 that gases always react in a certain relationship with each other. Based on this idea and the atomic theory of John Dalton
John Dalton (; 5 or 6 September 1766 – 27 July 1844) was an English chemist, physicist and meteorologist. He is best known for introducing the atomic theory into chemistry, and for his research into colour blindness, which he had. Colour b ...
, Joseph Proust
Joseph Louis Proust (26 September 1754 – 5 July 1826) was a French chemist. He was best known for his discovery of the law of definite proportions in 1794, stating that chemical compounds always combine in constant proportions.
Life
Joseph L. ...
had developed the law of definite proportions
In chemistry, the law of definite proportions, sometimes called Proust's law, or law of constant composition states that a given
chemical compound always contains its component elements in fixed ratio (by mass) and does not depend on its source an ...
, which later resulted in the concepts of stoichiometry
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equal ...
and chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between ...
s.
Regarding the organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
, it was long believed that compounds obtained from living organisms were too complex to be obtained synthetically. According to the concept of vitalism
Vitalism is a belief that starts from the premise that "living organisms are fundamentally different from non-living entities because they contain some non-physical element or are governed by different principles than are inanimate things." Wher ...
, organic matter was endowed with a "vital force" and distinguished from inorganic materials. This separation was ended however by the synthesis of urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important r ...
from inorganic precursors by Friedrich Wöhler
Friedrich Wöhler () FRS(For) HonFRSE (31 July 180023 September 1882) was a German chemist known for his work in inorganic chemistry, being the first to isolate the chemical elements beryllium and yttrium in pure metallic form. He was the firs ...
in 1828. Other chemists who brought major contributions to organic chemistry include Alexander William Williamson
Prof Alexander William Williamson FRS FRSE PCS MRIA (1 May 18246 May 1904) was an English chemist. He is best known today for the Williamson ether synthesis.
Life
Williamson was born in 1824 in Wandsworth, London, the second of three child ...
with his synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
** Organic synthesis, the chemical synthesis of organ ...
of ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be c ...
s and Christopher Kelk Ingold
Sir Christopher Kelk Ingold (28 October 1893 – 8 December 1970) was a British chemist based in Leeds and London. His groundbreaking work in the 1920s and 1930s on reaction mechanisms and the electronic structure of organic compounds was resp ...
, who, among many discoveries, established the mechanisms of substitution reaction
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions ar ...
s.
Characteristics
The general characteristics of chemical reactions are: * Evolution of a gas * Formation of aprecipitate
In an aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a super-saturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading ...
* Change in temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
* Change in state
State may refer to:
Arts, entertainment, and media Literature
* ''State Magazine'', a monthly magazine published by the U.S. Department of State
* ''The State'' (newspaper), a daily newspaper in Columbia, South Carolina, United States
* ''Our S ...
Equations

Chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between ...
s are used to graphically illustrate chemical reactions. They consist of chemical
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., wi ...
or structural formula
The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are possibly arranged in the real three-dimensional space. The chemical bondi ...
s of the reactants on the left and those of the products on the right. They are separated by an arrow (→) which indicates the direction and type of the reaction; the arrow is read as the word "yields". The tip of the arrow points in the direction in which the reaction proceeds. A double arrow () pointing in opposite directions is used for equilibrium reactions. Equations should be balanced according to the stoichiometry
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equal ...
, the number of atoms of each species should be the same on both sides of the equation. This is achieved by scaling the number of involved molecules (A, B, C and D in a schematic example below) by the appropriate integers ''a, b, c'' and ''d''.
:
More elaborate reactions are represented by reaction schemes, which in addition to starting materials and products show important intermediates or transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked wi ...
s. Also, some relatively minor additions to the reaction can be indicated above the reaction arrow; examples of such additions are water, heat, illumination, a catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
, etc. Similarly, some minor products can be placed below the arrow, often with a minus sign.
Retrosynthetic analysis
Retrosynthetic analysis is a technique for solving problems in the planning of organic syntheses. This is achieved by transforming a target molecule into simpler precursor structures regardless of any potential reactivity/interaction with reagents. ...
can be applied to design a complex synthesis reaction. Here the analysis starts from the products, for example by splitting selected chemical bonds, to arrive at plausible initial reagents. A special arrow (⇒) is used in retro reactions.
Elementary reactions
Theelementary reaction
An elementary reaction is a chemical reaction in which one or more chemical species react directly to form products in a single reaction step and with a single transition state. In practice, a reaction is assumed to be elementary if no reaction in ...
is the smallest division into which a chemical reaction can be decomposed, it has no intermediate products. Most experimentally observed reactions are built up from many elementary reactions that occur in parallel or sequentially. The actual sequence of the individual elementary reactions is known as reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
. An elementary reaction involves a few molecules, usually one or two, because of the low probability for several molecules to meet at a certain time.
dissociation
Dissociation, in the wide sense of the word, is an act of disuniting or separating a complex object into parts. Dissociation may also refer to:
* Dissociation (chemistry), general process in which molecules or ionic compounds (complexes, or salts ...
into one or more other molecules. Such reactions require the addition of energy in the form of heat or light. A typical example of a unimolecular reaction is the cis–trans isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
, in which the cis-form of a compound converts to the trans-form or vice versa.
In a typical dissociation
Dissociation, in the wide sense of the word, is an act of disuniting or separating a complex object into parts. Dissociation may also refer to:
* Dissociation (chemistry), general process in which molecules or ionic compounds (complexes, or salts ...
reaction, a bond in a molecule splits (ruptures) resulting in two molecular fragments. The splitting can be homolytic or heterolytic. In the first case, the bond is divided so that each product retains an electron and becomes a neutral radical
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
. In the second case, both electrons of the chemical bond remain with one of the products, resulting in charged ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
s. Dissociation plays an important role in triggering chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
Chain reactions are one way that syst ...
s, such as hydrogen–oxygen or polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer, monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are ...
reactions.
:bimolecular
In chemistry, molecularity is the number of molecules that come together to react in an elementary (single-step) reactionAtkins, P.; de Paula, J. Physical Chemistry. Oxford University Press, 2014 and is equal to the sum of stoichiometric coeffici ...
reactions, two molecules collide and react with each other. Their merger is called chemical synthesis
As a topic of chemistry, chemical synthesis (or combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In moder ...
or an addition reaction
In organic chemistry, an addition reaction is, in simplest terms, an organic reaction where two or more molecules combine to form a larger one (the adduct)..
Addition reactions are limited to chemical compounds that have multiple bonds, such as ...
.
:redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction ...
and acid-base reactions. In redox reactions, the transferred particle is an electron, whereas in acid-base reactions it is a proton. This type of reaction is also called metathesis.
:Chemical equilibrium
Most chemical reactions are reversible; that is, they can and do run in both directions. The forward and reverse reactions are competing with each other and differ inreaction rates
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit ...
. These rates depend on the concentration and therefore change with the time of the reaction: the reverse rate gradually increases and becomes equal to the rate of the forward reaction, establishing the so-called chemical equilibrium. The time to reach equilibrium depends on parameters such as temperature, pressure, and the materials involved, and is determined by the minimum free energy. In equilibrium, the Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and pr ...
must be zero. The pressure dependence can be explained with the Le Chatelier's principle
Le Chatelier's principle (pronounced or ), also called Chatelier's principle (or the Equilibrium Law), is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibria. The principle is named after French c ...
. For example, an increase in pressure due to decreasing volume causes the reaction to shift to the side with fewer moles of gas.
The reaction yield stabilizes at equilibrium but can be increased by removing the product from the reaction mixture or changed by increasing the temperature or pressure. A change in the concentrations of the reactants does not affect the equilibrium constant but does affect the equilibrium position.
Thermodynamics
Chemical reactions are determined by the laws ofthermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of the ...
. Reactions can proceed by themselves if they are exergonic
An exergonic process is one which there is a positive flow of energy from the system to the surroundings. This is in contrast with an endergonic process. Constant pressure, constant temperature reactions are exergonic if and only if the Gibbs fr ...
, that is if they release free energy. The associated free energy change of the reaction is composed of the changes of two different thermodynamic quantities, enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
and entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
:
:; .
:: : free energy, : enthalpy, : temperature, : entropy, : difference (change between original and product)
Reactions can be exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
, where Δ''H'' is negative and energy is released. Typical examples of exothermic reactions are combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
, precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls under gravitational pull from clouds. The main forms of precipitation include drizzle, rain, sleet, snow, ice pellets, graupel and hail. ...
and crystallization
Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposi ...
, in which ordered solids are formed from disordered gaseous or liquid phases. In contrast, in endothermic
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. p. ...
reactions, heat is consumed from the environment. This can occur by increasing the entropy of the system, often through the formation of gaseous or dissolved reaction products, which have higher entropy. Since the entropy term in the free-energy change increases with temperature, many endothermic reactions preferably take place at high temperatures. On the contrary, many exothermic reactions such as crystallization occur preferably at lower temperatures. A change in temperature can sometimes reverse the sign of the enthalpy of a reaction, as for the carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
reduction of molybdenum dioxide
Molybdenum dioxide is the chemical compound with the formula MoO. It is a violet-colored solid and is a metallic conductor. The mineralogical form of this compound is called tugarinovite, and is only very rarely found.
Structure
It crystallize ...
:
:carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
and molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lea ...
is endothermic at low temperatures, becoming less so with increasing temperature.Reaction Web/ref> Δ''H''° is zero at , and the reaction becomes exothermic above that temperature. Changes in temperature can also reverse the direction tendency of a reaction. For example, the
water gas shift reaction
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a s ...
:internal energy
The internal energy of a thermodynamic system is the total energy contained within it. It is the energy necessary to create or prepare the system in its given internal state, and includes the contributions of potential energy and internal kinet ...
change, which takes into account changes in the entropy, volume and chemical potential
In thermodynamics, the chemical potential of a species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potential of a species ...
s. The latter depends, among other things, on the activities of the involved substances.
:;
:: : internal energy, : entropy, : pressure, : chemical potential, : number of molecules, : small change sign
Kinetics
The speed at which reactions take place is studied byreaction kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in w ...
. The rate depends on various parameters, such as:
* Reactant
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
concentrations, which usually make the reaction happen at a faster rate if raised through increased collisions per unit of time. Some reactions, however, have rates that are ''independent'' of reactant concentrations, due to a limited number of catalytic sites. These are called zero order reactions.
* Surface area
The surface area of a solid object is a measure of the total area that the surface of the object occupies. The mathematical definition of surface area in the presence of curved surfaces is considerably more involved than the definition of arc ...
available for contact between the reactants, in particular solid ones in heterogeneous systems. Larger surface areas lead to higher reaction rates.
* Pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
– increasing the pressure decreases the volume between molecules and therefore increases the frequency of collisions between the molecules.
* Activation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
, which is defined as the amount of energy required to make the reaction start and carry on spontaneously. Higher activation energy implies that the reactants need more energy to start than a reaction with lower activation energy.
* Temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
, which hastens reactions if raised, since higher temperature increases the energy of the molecules, creating more collisions per unit of time,
* The presence or absence of a catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
. Catalysts are substances that make weak bonds with reactants or intermediates and change the pathway (mechanism) of a reaction which in turn increases the speed of a reaction by lowering the activation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
needed for the reaction to take place. A catalyst is not destroyed or changed during a reaction, so it can be used again.
* For some reactions, the presence of electromagnetic radiation
In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic field, electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy. It includes radio waves, microwaves, inf ...
, most notably ultraviolet light
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
, is needed to promote the breaking of bonds to start the reaction. This is particularly true for reactions involving radicals.
Several theories allow calculating the reaction rates at the molecular level. This field is referred to as reaction dynamics. The rate ''v'' of a first-order reaction
In chemistry, the rate law or rate equation for a reaction is an equation that links the initial or forward reaction rate with the concentrations or pressures of the reactants and constant parameters (normally rate coefficients and partial react ...
, which could be the disintegration of a substance A, is given by:
:
Its integration yields:
:
Here ''k'' is the first-order rate constant, having dimension 1/time, ''t'') is the concentration at a time ''t'' and sub>0 is the initial concentration. The rate of a first-order reaction depends only on the concentration and the properties of the involved substance, and the reaction itself can be described with a characteristic half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ato ...
. More than one time constant is needed when describing reactions of higher order. The temperature dependence of the rate constant usually follows the Arrhenius equation
In physical chemistry, the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based on the work of Dutch chemist Jacobus Henricus van 't Hoff who had noted in 18 ...
:
:
where ''E''a is the activation energy and ''k''B is the Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constant, ...
. One of the simplest models of reaction rate is the collision theory
Collision theory is a principle of chemistry used to predict the rates of chemical reactions. It states that when suitable particles of the reactant hit each other with correct orientation, only a certain amount of collisions result in a percept ...
. More realistic models are tailored to a specific problem and include the transition state theory
In chemistry, transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.
T ...
, the calculation of the potential energy surface
A potential energy surface (PES) describes the energy of a system, especially a collection of atoms, in terms of certain parameters, normally the positions of the atoms. The surface might define the energy as a function of one or more coordinates; ...
, the Marcus theory
In theoretical chemistry, Marcus theory is a theory originally developed by Rudolph A. Marcus, starting in 1956, to explain the rates of electron transfer reactions – the rate at which an electron can move or jump from one chemical species ( ...
and the Rice–Ramsperger–Kassel–Marcus (RRKM) theory.
Reaction types
Four basic types
Synthesis
In a synthesis reaction, two or more simple substances combine to form a more complex substance. These reactions are in the general form:iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
and sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
to form iron(II) sulfide
Iron(II) sulfide or ferrous sulfide (Br.E. sulphide) is one of a family chemical compounds and minerals with the approximate formula . Iron sulfides are often iron-deficient non-stoichiometric. All are black, water-insoluble solids.
Preparatio ...
:
Utah State Office of Education. Retrieved 4 June 2011.
Decomposition
A decomposition reaction is when a more complex substance breaks down into its more simple parts. It is thus the opposite of a synthesis reaction and can be written aselectrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
of water to make oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
and hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
gas:
Single displacement
In asingle displacement reaction
A single-displacement reaction, also known as single replacement reaction or exchange reaction, is a chemical reaction in which one element is replaced by another in a compound.
It can be represented generically as:
:A + BC -> AC + B
where eithe ...
, a single uncombined element replaces another in a compound; in other words, one element trades places with another element in a compound These reactions come in the general form of:
magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
replaces hydrogen in water to make magnesium hydroxide
Magnesium hydroxide is the inorganic compound with the chemical formula Mg(OH)2. It occurs in nature as the mineral brucite. It is a white solid with low solubility in water (). Magnesium hydroxide is a common component of antacids, such as milk ...
and hydrogen gas:
Double displacement
In adouble displacement reaction
A salt metathesis reaction, sometimes called a double displacement reaction, is a chemical process involving the exchange of bonds between two reacting chemical species which results in the creation of products with similar or identical bonding a ...
, the anions and cations of two compounds switch places and form two entirely different compounds. These reactions are in the general form:
barium chloride
Barium chloride is an inorganic compound with the formula Ba Cl2. It is one of the most common water-soluble salts of barium. Like most other water-soluble barium salts, it is white, highly toxic, and imparts a yellow-green coloration to a flame. ...
(BaCl2) and magnesium sulfate
Magnesium sulfate or magnesium sulphate (in English-speaking countries other than the US) is a chemical compound, a salt with the formula , consisting of magnesium cations (20.19% by mass) and sulfate anions . It is a white crystalline solid, ...
(MgSO4) react, the SO42− anion switches places with the 2Cl− anion, giving the compounds BaSO4 and MgCl2.
Another example of a double displacement reaction is the reaction of lead(II) nitrate
Lead(II) nitrate is an inorganic compound with the chemical formula Pb( NO3)2. It commonly occurs as a colourless crystal or white powder and, unlike most other lead(II) salts, is soluble in water.
Known since the Middle Ages by the name plumbum ...
with potassium iodide
Potassium iodide is a chemical compound, medication, and dietary supplement. It is a medication used for treating hyperthyroidism, in radiation emergencies, and for protecting the thyroid gland when certain types of radiopharmaceuticals are u ...
to form lead(II) iodide
Lead(II) iodide or lead iodide is a chemical compound with the formula . At room temperature, it is a bright yellow odorless crystalline solid, that becomes orange and red when heated. It was formerly called plumbous iodide.
The compound current ...
and potassium nitrate
Potassium nitrate is a chemical compound with the chemical formula . This alkali metal nitrate salt is also known as Indian saltpetre (large deposits of which were historically mined in India). It is an ionic salt of potassium ions K+ and nitrat ...
:
Combustion
In acombustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
reaction, an element or compound reacts with an oxidant, usually oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
, often producing energy in the form of heat
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is al ...
or light
Light or visible light is electromagnetic radiation that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 tera ...
. Combustion reactions frequently involve a hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ex ...
. For instance, the combustion of 1 mole (114 g) of octane in oxygen
carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
, magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
or sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
reacting with oxygen.
Oxidation and reduction


Redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction ...
reactions can be understood in terms of the transfer of electrons from one involved species (reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are commonly reducing agents include the Earth meta ...
) to another (oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ). In ot ...
). In this process, the former species is ''oxidized'' and the latter is ''reduced''. Though sufficient for many purposes, these descriptions are not precisely correct. Oxidation is better defined as an increase in oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
of atoms and reduction as a decrease in oxidation state. In practice, the transfer of electrons will always change the oxidation state, but there are many reactions that are classed as "redox" even though no electron transfer occurs (such as those involving covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
bonds).
In the following redox reaction, hazardous sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
metal reacts with toxic chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
gas to form the ionic compound sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
, or common table salt:
electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
of their elements. Elements with low electronegativities, such as most metals, easily donate electrons and oxidize – they are reducing agents. On the contrary, many oxides or ions with high oxidation numbers of their non-oxygen atoms, such as , , , , or , can gain one or two extra electrons and are strong oxidizing agents.
For some main-group element
In chemistry and atomic physics, the main group is the group of elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, and fluorine as arrange ...
s the number of electrons donated or accepted in a redox reaction can be predicted from the electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
of the reactant element. Elements try to reach the low-energy noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemi ...
configuration, and therefore alkali metals and halogens will donate and accept one electron, respectively. Noble gases themselves are chemically inactive.
The overall redox reaction can be balanced by combining the oxidation and reduction half-reactions multiplied by coefficients such that the number of electrons lost in the oxidation equals the number of electrons gained in the reduction.
An important class of redox reactions are the electrolytic electrochemical
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outc ...
reactions, where electrons from the power supply at the negative electrode are used as the reducing agent and electron withdrawal at the positive electrode as the oxidizing agent. These reactions are particularly important for the production of chemical elements, such as chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
or aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
. The reverse process, in which electrons are released in redox reactions and chemical energy is converted to electrical energy, is possible and used in batteries.
Complexation
 In complexation reactions, several
In complexation reactions, several ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
s react with a metal atom to form a coordination complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
. This is achieved by providing lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
s of the ligand into empty orbitals of the metal atom and forming dipolar bond
In coordination chemistry, a coordinate covalent bond, also known as a dative bond, dipolar bond, or coordinate bond is a kind of two-center, two-electron covalent bond in which the two electrons derive from the same atom. The bonding of metal ...
s. The ligands are Lewis bases, they can be both ions and neutral molecules, such as carbon monoxide, ammonia or water. The number of ligands that react with a central metal atom can be found using the 18-electron rule, saying that the valence shell
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
s of a transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
will collectively accommodate 18 electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s, whereas the symmetry of the resulting complex can be predicted with the crystal field theory Crystal field theory (CFT) describes the breaking of degeneracies of electron orbital states, usually ''d'' or ''f'' orbitals, due to a static electric field produced by a surrounding charge distribution (anion neighbors). This theory has been used ...
and ligand field theory
Ligand field theory (LFT) describes the bonding, orbital arrangement, and other characteristics of coordination complexes. It represents an application of molecular orbital theory to transition metal complexes. A transition metal ion has nine valen ...
. Complexation reactions also include ligand exchange, in which one or more ligands are replaced by another, and redox processes which change the oxidation state of the central metal atom.
Acid–base reactions
In theBrønsted–Lowry acid–base theory
The Brønsted–Lowry theory (also called proton theory of acids and bases) is an acid–base reaction theory which was proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923. The fundamental concept of this theory ...
, an acid–base reaction
An acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their applica ...
involves a transfer of proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s (H+) from one species (the acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
) to another (the base). When a proton is removed from an acid, the resulting species is termed that acid's conjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a ...
. When the proton is accepted by a base, the resulting species is termed that base's conjugate acid
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a ...
. In other words, acids act as proton donors and bases act as proton acceptors according to the following equation:
salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
.
Acid-base reactions can have different definitions depending on the acid-base concept employed. Some of the most common are:
* Arrhenius definition: Acids dissociate in water releasing H3O+ ions; bases dissociate in water releasing OH− ions.
* Brønsted–Lowry definition: Acids are proton (H+) donors, bases are proton acceptors; this includes the Arrhenius definition.
* Lewis
Lewis may refer to:
Names
* Lewis (given name), including a list of people with the given name
* Lewis (surname), including a list of people with the surname
Music
* Lewis (musician), Canadian singer
* "Lewis (Mistreated)", a song by Radiohead ...
definition: Acids are electron-pair acceptors, and bases are electron-pair donors; this includes the Brønsted-Lowry definition.
Precipitation
Precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls under gravitational pull from clouds. The main forms of precipitation include drizzle, rain, sleet, snow, ice pellets, graupel and hail. ...
is the formation of a solid in a solution or inside another solid during a chemical reaction. It usually takes place when the concentration of dissolved ions exceeds the solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
limit and forms an insoluble salt. This process can be assisted by adding a precipitating agent or by the removal of the solvent. Rapid precipitation results in an amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal.
Etymology
The term comes from the Greek ''a'' ("wi ...
or microcrystalline residue and a slow process can yield single crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
s. The latter can also be obtained by recrystallization from microcrystalline salts.
Solid-state reactions
Reactions can take place between two solids. However, because of the relatively smalldiffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
rates in solids, the corresponding chemical reactions are very slow in comparison to liquid and gas phase reactions. They are accelerated by increasing the reaction temperature and finely dividing the reactant to increase the contacting surface area.
Reactions at the solid/gas interface
The reaction can take place at the solid, gas interface, surfaces at very low pressure such asultra-high vacuum
Ultra-high vacuum (UHV) is the vacuum regime characterised by pressures lower than about . UHV conditions are created by pumping the gas out of a UHV chamber. At these low pressures the mean free path of a gas molecule is greater than approximately ...
. Via scanning tunneling microscopy
A scanning tunneling microscope (STM) is a type of microscope used for imaging surfaces at the atomic level. Its development in 1981 earned its inventors, Gerd Binnig and Heinrich Rohrer, then at IBM Zürich, the Nobel Prize in Physics in 1986. ...
, it is possible to observe reactions at the solid, gas interface in real space, if the time scale of the reaction is in the correct range. Reactions at the solid, gas interface are in some cases related to catalysis.
Photochemical reactions
photochemical reactions Organic photochemistry encompasses organic reactions that are induced by the action of light. The absorption of ultraviolet light by organic molecules often leads to reactions. In the earliest days, sunlight was employed, while in more modern times ...
, atoms and molecules absorb energy (photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless, so they always ...
s) of the illumination light and convert it into an excited state
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to a ...
. They can then release this energy by breaking chemical bonds, thereby producing radicals. Photochemical reactions include hydrogen–oxygen reactions, radical polymerization
In polymer chemistry, free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks ( repeat units). Free radicals can be formed by a number of different mechani ...
, chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
Chain reactions are one way that syst ...
s and rearrangement reactions.
Many important processes involve photochemistry. The premier example is photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
, in which most plants use solar energy to convert carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
and water into glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using ...
, disposing of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
as a side-product. Humans rely on photochemistry for the formation of vitamin D, and vision
Vision, Visions, or The Vision may refer to:
Perception Optical perception
* Visual perception, the sense of sight
* Visual system, the physical mechanism of eyesight
* Computer vision, a field dealing with how computers can be made to gain un ...
is initiated by a photochemical reaction of rhodopsin
Rhodopsin, also known as visual purple, is a protein encoded by the RHO gene and a G-protein-coupled receptor (GPCR). It is the opsin of the rod cells in the retina and a light-sensitive receptor protein that triggers visual phototransduction ...
. In fireflies
The Lampyridae are a family of elateroid beetles with more than 2,000 described species, many of which are light-emitting. They are soft-bodied beetles commonly called fireflies, lightning bugs, or glowworms for their conspicuous production ...
, an enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
in the abdomen catalyzes a reaction that results in bioluminescence
Bioluminescence is the production and emission of light by living organisms. It is a form of chemiluminescence. Bioluminescence occurs widely in marine vertebrates and invertebrates, as well as in some fungi, microorganisms including some b ...
. Many significant photochemical reactions, such as ozone formation, occur in the Earth atmosphere and constitute atmospheric chemistry
Atmospheric chemistry is a branch of atmospheric science in which the chemistry of the Earth's atmosphere and that of other planets is studied. It is a multidisciplinary approach of research and draws on environmental chemistry, physics, meteoro ...
.
Catalysis
catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
, the reaction does not proceed directly, but through a reaction with a third substance known as catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
. Although the catalyst takes part in the reaction, forming weak bonds with reactants or intermediates, it is returned to its original state by the end of the reaction and so is not consumed. However, it can be inhibited, deactivated or destroyed by secondary processes. Catalysts can be used in a different phase (heterogeneous
Homogeneity and heterogeneity are concepts often used in the sciences and statistics relating to the uniformity of a substance or organism. A material or image that is homogeneous is uniform in composition or character (i.e. color, shape, siz ...
) or in the same phase (homogeneous
Homogeneity and heterogeneity are concepts often used in the sciences and statistics relating to the uniformity of a substance or organism. A material or image that is homogeneous is uniform in composition or character (i.e. color, shape, siz ...
) as the reactants. In heterogeneous catalysis, typical secondary processes include coking
Coking is the heating of coal in the absence of oxygen to a temperature above 600 °C to drive off the volatile components of the raw coal, leaving a hard, strong, porous material of high carbon content called coke. Coke consists almost ent ...
where the catalyst becomes covered by polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
ic side products. Additionally, heterogeneous catalysts can dissolve into the solution in a solid-liquid system or evaporate in a solid–gas system. Catalysts can only speed up the reaction – chemicals that slow down the reaction are called inhibitors. Substances that increase the activity of catalysts are called promoters, and substances that deactivate catalysts are called catalytic poisons. With a catalyst, a reaction that is kinetically inhibited by high activation energy can take place in the circumvention of this activation energy.
Heterogeneous catalysts are usually solids, powdered in order to maximize their surface area. Of particular importance in heterogeneous catalysis are the platinum group
The platinum-group metals (abbreviated as the PGMs; alternatively, the platinoids, platinides, platidises, platinum group, platinum metals, platinum family or platinum-group elements (PGEs)) are six noble, precious metallic elements clustered t ...
metals and other transition metals, which are used in hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or S ...
s, catalytic reforming
Catalytic reforming is a chemical process used to convert petroleum refinery naphthas distilled from crude oil (typically having low octane ratings) into high-octane liquid products called reformates, which are premium blending stocks for high-oc ...
and in the synthesis of commodity chemicals such as nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
and ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
. Acids are an example of a homogeneous catalyst, they increase the nucleophilicity of carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
s, allowing a reaction that would not otherwise proceed with electrophiles. The advantage of homogeneous catalysts is the ease of mixing them with the reactants, but they may also be difficult to separate from the products. Therefore, heterogeneous catalysts are preferred in many industrial processes.
Reactions in organic chemistry
In organic chemistry, in addition to oxidation, reduction or acid-base reactions, a number of other reactions can take place which involvescovalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
s between carbon atoms or carbon and heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is usually used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecula ...
s (such as oxygen, nitrogen, halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
s, etc.). Many specific reactions in organic chemistry are name reaction A name reaction is a chemical reaction named after its discoverers or developers. Among the tens of thousands of organic reactions that are known, hundreds of such reactions are well-known enough to be named after people. Well-known examples include ...
s designated after their discoverers.
Substitution
In asubstitution reaction
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions ar ...
, a functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
in a particular chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
is replaced by another group. These reactions can be distinguished by the type of substituting species into a nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
, electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carri ...
or radical substitution
In organic chemistry, a radical-substitution reaction is a substitution reaction involving free radicals as a reactive intermediate.March Jerry; (1985). Advanced organic chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wile ...
.
In the first type, a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
, an atom or molecule with an excess of electrons and thus a negative charge or partial charge A partial charge is a non-integer charge value when measured in elementary charge units. Partial charge is more commonly called net atomic charge. It is represented by the Greek lowercase letter 𝛿, namely 𝛿− or 𝛿+.
Partial charges are c ...
, replaces another atom or part of the "substrate" molecule. The electron pair from the nucleophile attacks the substrate forming a new bond, while the leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited t ...
departs with an electron pair. The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. Examples of nucleophiles are hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. I ...
ion, alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, whe ...
s, amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
s and halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
s. This type of reaction is found mainly in aliphatic hydrocarbons, and rarely in aromatic hydrocarbon
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past grouping ...
. The latter have high electron density and enter nucleophilic aromatic substitution
A nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring. Aromatic rings are usually nucleophilic, but some aromatic compou ...
only with very strong electron withdrawing groups. Nucleophilic substitution can take place by two different mechanisms, SN1 and SN2. In their names, S stands for substitution, N for nucleophilic, and the number represents the kinetic order of the reaction, unimolecular or bimolecular.
The SN1 reaction proceeds in two steps. First, the leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited t ...
is eliminated creating a carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountere ...
. This is followed by a rapid reaction with the nucleophile.
In the SN2 mechanisms, the nucleophile forms a transition state with the attacked molecule, and only then the leaving group is cleaved. These two mechanisms differ in the stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereois ...
of the products. SN1 leads to the non-stereospecific addition and does not result in a chiral center, but rather in a set of geometric isomers
Geometry (; ) is, with arithmetic, one of the oldest branches of mathematics. It is concerned with properties of space such as the distance, shape, size, and relative position of figures. A mathematician who works in the field of geometry is ...
(''cis/trans''). In contrast, a reversal (Walden inversion
Walden inversion is the inversion of a stereogenic center in a chiral molecule in a chemical reaction. Since a molecule can form two enantiomers around a stereogenic center, the Walden inversion converts the configuration of the molecule fro ...
) of the previously existing stereochemistry is observed in the SN2 mechanism.
Electrophilic substitution is the counterpart of the nucleophilic substitution in that the attacking atom or molecule, an electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries ...
, has low electron density and thus a positive charge. Typical electrophiles are the carbon atom of carbonyl group
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
s, carbocations or sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
or nitronium cations. This reaction takes place almost exclusively in aromatic hydrocarbons, where it is called electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic ni ...
. The electrophile attack results in the so-called σ-complex, a transition state in which the aromatic system is abolished. Then, the leaving group, usually a proton, is split off and the aromaticity is restored. An alternative to aromatic substitution is electrophilic aliphatic substitution. It is similar to the nucleophilic aliphatic substitution and also has two major types, SE1 and SE2
radical
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
. This process usually takes the form of a chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
Chain reactions are one way that syst ...
, for example in the reaction of alkanes with halogens. In the first step, light or heat disintegrates the halogen-containing molecules producing radicals. Then the reaction proceeds as an avalanche until two radicals meet and recombine.
:;Addition and elimination
Theaddition
Addition (usually signified by the Plus and minus signs#Plus sign, plus symbol ) is one of the four basic Operation (mathematics), operations of arithmetic, the other three being subtraction, multiplication and Division (mathematics), division. ...
and its counterpart, the elimination, are reactions that change the number of substituents on the carbon atom, and form or cleave multiple bonds. Double
A double is a look-alike or doppelgänger; one person or being that resembles another.
Double, The Double or Dubble may also refer to:
Film and television
* Double (filmmaking), someone who substitutes for the credited actor of a character
* Th ...
and triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
s can be produced by eliminating a suitable leaving group. Similar to the nucleophilic substitution, there are several possible reaction mechanisms that are named after the respective reaction order. In the E1 mechanism, the leaving group is ejected first, forming a carbocation. The next step, the formation of the double bond, takes place with the elimination of a proton (deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
). The leaving order is reversed in the E1cb mechanism, that is the proton is split off first. This mechanism requires the participation of a base. Because of the similar conditions, both reactions in the E1 or E1cb elimination always compete with the SN1 substitution.
electrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where a chemical compound containing a double or triple bond has a π bond broken, with the formation of two new σ bonds.March, Jerry; (1985). Advanced Organic Che ...
of hydrogen bromide, an electrophile (proton) attacks the double bond forming a carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountere ...
, which then reacts with the nucleophile (bromine). The carbocation can be formed on either side of the double bond depending on the groups attached to its ends, and the preferred configuration can be predicted with the Markovnikov's rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule describes the outcome of some addition reactions. The rule was formulated by Russian chemist Vladimir Markovnikov in 1870.
Explanation
The rule states that with the addition of a p ...
. This rule states that "In the heterolytic addition of a polar molecule to an alkene or alkyne, the more electronegative (nucleophilic) atom (or part) of the polar molecule becomes attached to the carbon atom bearing the smaller number of hydrogen atoms."
If the addition of a functional group takes place at the less substituted carbon atom of the double bond, then the electrophilic substitution with acids is not possible. In this case, one has to use the hydroboration–oxidation reaction Hydroboration–oxidation reaction is a two-step hydration reaction that converts an alkene into an alcohol. The process results in the syn addition of a hydrogen and a hydroxyl group where the double bond had been. Hydroboration–oxidation is a ...
, wherein the first step, the boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
atom acts as electrophile and adds to the less substituted carbon atom. In the second step, the nucleophilic hydroperoxide
Hydroperoxides or peroxols are compounds containing the hydroperoxide functional group (ROOH). If the R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. ...
or halogen anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
attacks the boron atom.
While the addition to the electron-rich alkenes and alkynes is mainly electrophilic, the nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions di ...
plays an important role in the carbon-heteroatom multiple bonds, and especially its most important representative, the carbonyl group. This process is often associated with elimination so that after the reaction the carbonyl group is present again. It is, therefore, called an addition-elimination reaction and may occur in carboxylic acid derivatives such as chlorides, esters or anhydrides. This reaction is often catalyzed by acids or bases, where the acids increase the electrophilicity of the carbonyl group by binding to the oxygen atom, whereas the bases enhance the nucleophilicity of the attacking nucleophile.
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions di ...
of a carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3C ...
or another nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
to the double bond of an alpha, beta-unsaturated carbonyl compound can proceed via the Michael reaction
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon ...
, which belongs to the larger class of conjugate addition
Nucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polarit ...
s. This is one of the most useful methods for the mild formation of C–C bonds.
Some additions which can not be executed with nucleophiles and electrophiles can be succeeded with free radicals. As with the free-radical substitution, the radical addition
In organic chemistry, free-radical addition is an addition reaction which involves free radicals. The addition may occur between a radical and a non-radical, or between two radicals.
The basic steps with examples of the free-radical addition (al ...
proceeds as a chain reaction, and such reactions are the basis of the free-radical polymerization.
Other organic reaction mechanisms
 In a rearrangement reaction, the carbon skeleton of a
In a rearrangement reaction, the carbon skeleton of a molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
is rearranged to give a structural isomer
In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a chemical compound, compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct chemical bond, b ...
of the original molecule. These include hydride shift
A sigmatropic reaction in organic chemistry is a pericyclic reaction wherein the net result is one σ-bond is changed to another σ-bond in an uncatalyzed intramolecular reaction. The name ''sigmatropic'' is the result of a compounding of the lon ...
reactions such as the Wagner-Meerwein rearrangement, where a hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
, alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ...
or aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
group migrates from one carbon to a neighboring carbon. Most rearrangements are associated with the breaking and formation of new carbon-carbon bonds. Other examples are sigmatropic reaction
A sigmatropic reaction in organic chemistry is a pericyclic reaction wherein the net result is one σ-bond is changed to another σ-bond in an uncatalyzed intramolecular reaction. The name ''sigmatropic'' is the result of a compounding of the lo ...
such as the Cope rearrangement
The Cope rearrangement is an extensively studied organic reaction involving the ,3sigmatropic rearrangement of 1,5-dienes. It was developed by Arthur C. Cope and Elizabeth Hardy. For example, 3-methyl-hexa-1,5-diene heated to 300 °C yield ...
.
Cyclic rearrangements include cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
s and, more generally, pericyclic reaction
In organic chemistry, a pericyclic reaction is the type of organic reaction wherein the transition state of the molecule has a cyclic geometry, the reaction progresses in a concerted fashion, and the bond orbitals involved in the reaction overla ...
s, wherein two or more double bond-containing molecules form a cyclic molecule. An important example of cycloaddition reaction is the Diels–Alder reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a peric ...
(the so-called +2cycloaddition) between a conjugated diene
In organic chemistry a diene ( ) (diolefin ( ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature. ...
and a substituted alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
to form a substituted cyclohexene
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes. Cyclohexene is not very stable upon long term storage with exposure to light a ...
system.
Whether a certain cycloaddition would proceed depends on the electronic orbitals of the participating species, as only orbitals with the same sign of wave function
A wave function in quantum physics is a mathematical description of the quantum state of an isolated quantum system. The wave function is a complex-valued probability amplitude, and the probabilities for the possible results of measurements mad ...
will overlap and interact constructively to form new bonds. Cycloaddition is usually assisted by light or heat. These perturbations result in a different arrangement of electrons in the excited state of the involved molecules and therefore in different effects. For example, the +2Diels-Alder reactions can be assisted by heat whereas the +2cycloaddition is selectively induced by light. Because of the orbital character, the potential for developing stereoisomeric
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms i ...
products upon cycloaddition is limited, as described by the Woodward–Hoffmann rules The Woodward–Hoffmann rules (or the pericyclic selection rules), devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules used to rationalize or predict certain aspects of the stereochemistry and activation energy of pericyclic reac ...
.
Biochemical reactions
enzymes
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecule ...
. These protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
s can specifically catalyze
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
a single reaction so that reactions can be controlled very precisely. The reaction takes place in the active site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate (binding site) a ...
, a small part of the enzyme which is usually found in a cleft or pocket lined by amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
residues, and the rest of the enzyme is used mainly for stabilization. The catalytic action of enzymes relies on several mechanisms including the molecular shape ("induced fit"), bond strain, proximity and orientation of molecules relative to the enzyme, proton donation or withdrawal (acid/base catalysis), electrostatic interactions and many others.
The biochemical reactions that occur in living organisms are collectively known as metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cell ...
. Among the most important of its mechanisms is the anabolism, in which different DNA and enzyme-controlled processes result in the production of large molecules such as protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
s and carbohydrates
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or may ...
from smaller units. Bioenergetics
Bioenergetics is a field in biochemistry and cell biology that concerns energy flow through living systems. This is an active area of biological research that includes the study of the transformation of energy in living organisms and the study of ...
studies the sources of energy for such reactions. Important energy sources are glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using ...
and oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
, which can be produced by plants via photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
or assimilated from food and air, respectively. All organisms use this energy to produce adenosine triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of ...
(ATP), which can then be used to energize other reactions.
Applications
 Chemical reactions are central to
Chemical reactions are central to chemical engineering
Chemical engineering is an engineering field which deals with the study of operation and design of chemical plants as well as methods of improving production. Chemical engineers develop economical commercial processes to convert raw materials int ...
, where they are used for the synthesis of new compounds from natural raw materials such as petroleum
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crud ...
, mineral ore
Ore is natural rock or sediment that contains one or more valuable minerals, typically containing metals, that can be mined, treated and sold at a profit.Encyclopædia Britannica. "Ore". Encyclopædia Britannica Online. Retrieved 7 Apr ...
s, and oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
in air. It is essential to make the reaction as efficient as possible, maximizing the yield and minimizing the number of reagents, energy inputs and waste. Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s are especially helpful for reducing the energy required for the reaction and increasing its reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit ...
.
Some specific reactions have their niche applications. For example, the thermite
Thermite () is a pyrotechnic composition of metal powder and metal oxide. When ignited by heat or chemical reaction, thermite undergoes an exothermic reduction-oxidation (redox) reaction. Most varieties are not explosive, but can create brie ...
reaction is used to generate light and heat in pyrotechnics
Pyrotechnics is the science and craft of creating such things as fireworks, safety matches, oxygen candles, explosive bolts and other fasteners, parts of automotive airbags, as well as gas-pressure blasting in mining, quarrying, and demolition. ...
and welding
Welding is a fabrication (metal), fabrication process that joins materials, usually metals or thermoplastics, by using high heat to melt the parts together and allowing them to cool, causing Fusion welding, fusion. Welding is distinct from lower ...
. Although it is less controllable than the more conventional oxy-fuel welding
Principle of burn cutting
Oxy-fuel welding (commonly called oxyacetylene welding, oxy welding, or gas welding in the United States) and oxy-fuel cutting are processes that use fuel gases (or liquid fuels such as gasoline or petrol, diesel, ...
, arc welding
Arc welding is a welding process that is used to join metal to metal by using electricity to create enough heat to melt metal, and the melted metals, when cool, result in a binding of the metals. It is a type of welding that uses a welding powe ...
and flash welding
Flash welding is a type of resistance welding that does not use any filler metals. The pieces of metal to be welded are set apart at a predetermined distance based on material thickness, material composition, and desired properties of the finished ...
, it requires much less equipment and is still used to mend rails, especially in remote areas.
Monitoring
Mechanisms of monitoring chemical reactions depend strongly on the reaction rate. Relatively slow processes can be analyzed in situ for the concentrations and identities of the individual ingredients. Important tools of real-time analysis are the measurement of pH and analysis of optical absorption (color) and emission spectra. A less accessible but rather efficient method is the introduction of a radioactive isotope into the reaction and monitoring how it changes over time and where it moves to; this method is often used to analyze the redistribution of substances in the human body. Faster reactions are usually studied withultrafast laser spectroscopy
Ultrafast laser spectroscopy is a spectroscopic technique that uses ultrashort pulse lasers for the study of dynamics on extremely short time scales ( attoseconds to nanoseconds). Different methods are used to examine the dynamics of charge car ...
where utilization of femtosecond laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word "laser" is an acronym for "light amplification by stimulated emission of radiation". The fir ...
s allows short-lived transition states to be monitored at a time scaled down to a few femtoseconds.Atkins Atkins may refer to:
Places in the United States
* Atkins, Arkansas, a city
* Atkins, Iowa, a city
* Atkins, Louisiana, an unincorporated community
* Atkins, Nebraska, an unincorporated community
* Atkins, Virginia, a census-designated place
* ...
, p. 987
See also
*Chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between ...
* Chemical reaction
** Substrate
** Reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
** Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
** Product
Product may refer to:
Business
* Product (business), an item that serves as a solution to a specific consumer problem.
* Product (project management), a deliverable or set of deliverables that contribute to a business solution
Mathematics
* Produ ...
* Chemical reaction model
* Chemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties. Chemists carefully describe th ...
* Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
* Combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
* Limiting reagent
The limiting reagent (or limiting reactant or limiting agent) in a chemical reaction is a reactant that is totally consumed when the chemical reaction is completed. The amount of product formed is limited by this reagent, since the reaction canno ...
* List of organic reactions
Well-known reactions and reagents in organic chemistry include
0-9
* 1,2-Wittig rearrangement
* 1,3-Dipolar cycloaddition
* 2,3-Wittig rearrangement
A
* Abramovitch–Shapiro tryptamine synthesis
* Acetalisation
* Acetoacetic ester condensat ...
* Mass balance
In physics, a mass balance, also called a material balance, is an application of conservation of mass to the analysis of physical systems. By accounting for material entering and leaving a system, mass flows can be identified which might have b ...
* Microscopic reversibility The principle of microscopic reversibility in physics and chemistry is twofold:
* First, it states that the microscopic detailed dynamics of particles and fields is time-reversible because the microscopic equations of motion are symmetric with resp ...
* Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, Mechanistic Organ ...
* Reaction progress kinetic analysis
In chemistry, reaction progress kinetic analysis (RPKA) is a subset of a broad range of kinetic techniques utilized to determine the rate laws of chemical reactions and to aid in elucidation of reaction mechanisms. While the concepts guiding reacti ...
* Reversible reaction
A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur simultaneously.
: \mathit aA + \mathit bB \mathit cC + \mathit dD
A and B can react to form C and D or, in the ...
References
Bibliography
* * * * * {{DEFAULTSORT:Chemical Reaction Chemistry Change