|
Glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, the most abundant carbohydrate in the world. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as starch and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form of glucose is -glucose, while -glucose is produced synthetically in comparatively small amounts and is less biologically active. Glucose is a monosaccharide containing six carbon atoms and an aldehyde group, and is therefore an aldohexose. The glucose molecule can exist in an open-chain (acyclic) as well as ring (cyclic) form. Gluco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood Sugar
Glycaemia, also known as blood sugar level, blood sugar concentration, or blood glucose level is the measure of glucose concentrated in the blood of humans or other animals. Approximately 4 grams of glucose, a simple sugar, is present in the blood of a 70 kg (154 lb) human at all times. The body tightly regulates blood glucose levels as a part of metabolic homeostasis. Glucose is stored in skeletal muscle and liver cells in the form of glycogen; in fasting individuals, blood glucose is maintained at a constant level at the expense of glycogen stores in the liver and skeletal muscle. In humans, a blood glucose level of 4 grams, or about a teaspoon, is critical for normal function in a number of tissues, and the human brain consumes approximately 60% of blood glucose in fasting, sedentary individuals. A persistent elevation in blood glucose leads to glucose toxicity, which contributes to cell dysfunction and the pathology grouped together as complications of diabetes. Gl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or may not be different from ''n''), which does not mean the H has covalent bonds with O (for example with , H has a covalent bond with C but not with O). However, not all carbohydrates conform to this precise stoichiometric definition (e.g., uronic acids, deoxy-sugars such as fucose), nor are all chemicals that do conform to this definition automatically classified as carbohydrates (e.g. formaldehyde and acetic acid). The term is most common in biochemistry, where it is a synonym of saccharide (), a group that includes sugars, starch, and cellulose. The saccharides are divided into four chemical groups: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. Monosaccharides and disaccharides, the smallest (lower molecular wei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycogen
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. The polysaccharide structure represents the main storage form of glucose in the body. Glycogen functions as one of two forms of energy reserves, glycogen being for short-term and the other form being triglyceride stores in adipose tissue (i.e., body fat) for long-term storage. In humans, glycogen is made and stored primarily in the cells of the liver and skeletal muscle. In the liver, glycogen can make up 5–6% of the organ's fresh weight, and the liver of an adult, weighing 1.5 kg, can store roughly 100–120 grams of glycogen. In skeletal muscle, glycogen is found in a low concentration (1–2% of the muscle mass) and the skeletal muscle of an adult weighing 70 kg stores roughly 400 grams of glycogen. The amount of glycogen stored in the body—particularly within the muscles and liver—mostly depends on physical training, bas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double sugars, are molecules made of two bonded monosaccharides; common examples are sucrose (glucose + fructose), lactose (glucose + galactose), and maltose (two molecules of glucose). White sugar is a refined form of sucrose. In the body, compound sugars are hydrolysed into simple sugars. Longer chains of monosaccharides (>2) are not regarded as sugars, and are called oligosaccharides or polysaccharides. Starch is a glucose polymer found in plants, the most abundant source of energy in human food. Some other chemical substances, such as glycerol and sugar alcohols, may have a sweet taste, but are not classified as sugar. Sugars are found in the tissues of most plants. Honey and fruits are abundant natural sources of simple sugars. Suc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Starch
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diets, and is contained in large amounts in staple foods such as wheat, potatoes, maize (corn), rice, and cassava (manioc). Pure starch is a white, tasteless and odorless powder that is insoluble in cold water or alcohol. It consists of two types of molecules: the linear and helical amylose and the branched amylopectin. Depending on the plant, starch generally contains 20 to 25% amylose and 75 to 80% amylopectin by weight. Glycogen, the energy reserve of animals, is a more highly branched version of amylopectin. In industry, starch is often converted into sugars, for example by malting. These sugars may be fermented to produce ethanol in the manufacture of beer, whisky and biofuel. In addition, sugars produced from processed starch are used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycogenolysis
Glycogenolysis is the breakdown of glycogen (n) to glucose-1-phosphate and glycogen (n-1). Glycogen branches are catabolized by the sequential removal of glucose monomers via phosphorolysis, by the enzyme glycogen phosphorylase. Mechanism The overall reaction for the breakdown of glycogen to glucose-1-phosphate is: : glycogen(n residues) + Pi glycogen(n-1 residues) + glucose-1-phosphate Here, glycogen phosphorylase cleaves the bond linking a terminal glucose residue to a glycogen branch by substitution of a phosphoryl group for the α →4linkage. Glucose-1-phosphate is converted to glucose-6-phosphate (which often ends up in glycolysis) by the enzyme phosphoglucomutase. Glucose residues are phosphorolysed from branches of glycogen until four residues before a glucose that is branched with a α →6linkage. Glycogen debranching enzyme then transfers three of the remaining four glucose units to the end of another glycogen branch. This exposes the α →6branching point, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldohexose
In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol. Hexoses exist in two forms, open-chain or cyclic, that easily convert into each other in aqueous solutions. The open-chain form of a hexose, which usually is favored in solutions, has the general structure H–(CHOH)''n''−1–C(=O)–(CHOH)4−''n''–H, where ''n'' is 1, 2, or 3. Namely, five of the carbons have one hydroxyl functional group (–OH) each, connected by a single bond, and one has an oxo group (=O), forming a carbonyl group (C=O). The remaining bonds of the carbon atoms are satisfied by seven hydrogen atoms. The carbons are commonly numbered 1 to 6 starting at the end closest to the carbonyl. Hexoses are extremely important in biochemistry, both as isolated molecules (such as glucose and fructose) and as building blocks of other compounds such as starch, cellulose, and glycosides. Hexose ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
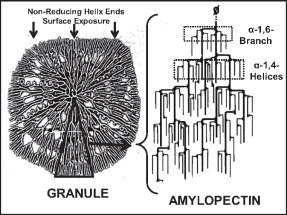
Amylopectin
Amylopectin is a water-insoluble polysaccharide and highly branched polymer of α-glucose units found in plants. It is one of the two components of starch, the other being amylose. Plants store starch within specialized organelles called amyloplasts. To generate energy, the plant hydrolyzes the starch, releasing the glucose subunits. Humans and other animals that eat plant foods also use amylase, an enzyme that assists in breaking down amylopectin, to initiate the hydrolyzation of starch. Starch is made of about 70–80% amylopectin by weight, though it varies depending on the source. For example, it ranges from lower percent content in long-grain rice, amylomaize, and russet potatoes to 100% in glutinous rice, waxy potato starch, and waxy corn. Amylopectin is highly branched, being formed of 2,000 to 200,000 glucose units. Its inner chains are formed of 20–24 glucose subunits. Dissolved amylopectin starch has a lower tendency of retrogradation (a partial recrystallization a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
WHO Model List Of Essential Medicines
The WHO Model List of Essential Medicines (aka Essential Medicines List or EML), published by the World Health Organization (WHO), contains the medications considered to be most effective and safe to meet the most important needs in a health system. The list is frequently used by countries to help develop their own local lists of essential medicines. , more than 155 countries have created national lists of essential medicines based on the World Health Organization's model list. This includes both developed and developing countries. The list is divided into core items and complementary items. The core items are deemed to be the most cost-effective options for key health problems and are usable with little additional health care resources. The complementary items either require additional infrastructure such as specially trained health care providers or diagnostic equipment or have a lower cost–benefit ratio. About 25% of items are in the complementary list. Some medicatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fischer Projection
In chemistry, the Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates and used by chemists, particularly in organic chemistry and biochemistry. The use of Fischer projections in non-carbohydrates is discouraged, as such drawings are ambiguous and easily confused with other types of drawing. The main purpose of Fischer projections is to show the chirality of a molecule and to distinguish between a pair of enantiomers. Some notable uses include drawing sugars and depicting isomers. Conventions All bonds are depicted as horizontal or vertical lines. The carbon chain is depicted vertically, with carbon atoms sometimes not shown and represented by the center of crossing lines (see figure below). The orientation of the carbon chain is so that the first carbon (C1) is at the top. In an aldose, C1 is the carbon o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haworth Projection
In chemistry, a Haworth projection is a common way of writing a structural formula to represent the cyclic structure of monosaccharides with a simple three-dimensional perspective. Haworth projection approximate the shapes of the actual molecules better for furanoses -which are in reality nearly planar- than for pyranoses which exist in solution in the chair conformation. Organic chemistry and especially biochemistry are the areas of chemistry that use the Haworth projection the most. The Haworth projection was named after the British chemist Sir Norman Haworth. A Haworth projection has the following characteristics: * Carbon is the implicit type of atom. In the example on the right, the atoms numbered from 1 to 6 are all carbon atoms. Carbon 1 is known as the anomeric carbon. * Hydrogen atoms on carbon are implicit. In the example, atoms 1 to 6 have extra hydrogen atoms not depicted. * A thicker line indicates atoms that are closer to the observer. In the example on the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monosaccharide
Monosaccharides (from Greek ''monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built. They are usually colorless, water-soluble, and crystalline solids. Contrary to their name (sugars), only some monosaccharides have a sweet taste. Most monosaccharides have the formula (though not all molecules with this formula are monosaccharides). Examples of monosaccharides include glucose (dextrose), fructose (levulose), and galactose. Monosaccharides are the building blocks of disaccharides (such as sucrose and lactose) and polysaccharides (such as cellulose and starch). The table sugar used in everyday vernacular is itself a disaccharide sucrose comprising one molecule of each of the two monosaccharides D-glucose and D-fructose. Each carbon atom that supports a hydroxyl group is chiral, except those at the end of the chain. This gives rise to a number of isomeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |