|
Femtochemistry
Femtochemistry is the area of physical chemistry that studies chemical reactions on extremely short timescales (approximately 10−15 seconds or one femtosecond, hence the name) in order to study the very act of atoms within molecules (reactants) rearranging themselves to form new molecules (products). In a 1988 issue of the journal ''Science'', Ahmed Hassan Zewail published an article using this term for the first time, stating "Real-time femtochemistry, that is, chemistry on the femtosecond timescale...". Later in 1999, Zewail received the Nobel Prize in Chemistry for his pioneering work in this field showing that it is possible to see how atoms in a molecule move during a chemical reaction with flashes of laser light. Application of femtochemistry in biological studies has also helped to elucidate the conformational dynamics of stem-loop RNA structures. Many publications have discussed the possibility of controlling chemical reactions by this method, but this remains cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ahmed Zewail
Ahmed Hassan Zewail ( ar, أحمد حسن زويل, ; February 26, 1946 – August 2, 2016) was an Egyptian-American chemist, known as the "father of femtochemistry". He was awarded the 1999 Nobel Prize in Chemistry for his work on femtochemistry and became the first Egyptian to win a Nobel Prize in a scientific field, and the second African to win a Nobel Prize in Chemistry. He was the Linus Pauling Chair Professor of Chemistry, Professor of Physics, and the director of the Physical Biology Center for Ultrafast Science and Technology at the California Institute of Technology. Early life and education Ahmed Hasan Zewail was born on February 26, 1946, in Damanhur, Kingdom of Egypt, Egypt, and was raised in Desouk. He received a Bachelor of Science and Master of Science degrees in Chemistry from Alexandria University before moving to the United States to complete his Doctor of Philosophy, PhD at the University of Pennsylvania supervised by Robin M. Hochstrasser. Career After comple ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Attophysics
Attosecond physics, also known as attophysics, or more generally attosecond science, is a branch of physics that deals with light-matter interaction phenomena wherein attosecond (10−18 s) photon pulses are used to unravel dynamical processes in matter with unprecedented time resolution. Attosecond science mainly employs pump–probe spectroscopic methods to investigate the physical process of interest. Due to the complexity of this field of study, it generally requires a synergistic interplay between state-of-the-art experimental setup and advanced theoretical tools to interpret the data collected from attosecond experiments. The main interests of attosecond physics are: # Atomic physics: investigation of electron correlation effects, photo-emission delay and ionization tunneling. # Molecular physics and molecular chemistry: role of electronic motion in molecular excited states (e.g. charge-transfer processes), light-induced photo-fragmentation, and light-induced electron t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (chemistry), products, which usually have properties different from the reactants. Reactions often consist of a sequence o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Femtosecond
A femtosecond is a unit of time in the International System of Units (SI) equal to 10 or of a second; that is, one quadrillionth, or one millionth of one billionth, of a second. For context, a femtosecond is to a second as a second is to about 31.71 million years; a ray of light travels approximately 0.3 μm (micrometers) in 1 femtosecond, a distance comparable to the diameter of a virus.Compared with overview in: Page 3 The word ''femtosecond'' is formed by the SI prefix ''femto'' and the SI unit ''second''. Its symbol is fs. A femtosecond is equal to 1000 attoseconds, or 1/1000 picosecond. Because the next higher SI unit is 1000 times larger, times of 10−14 and 10−13 seconds are typically expressed as tens or hundreds of femtoseconds. * Typical time steps for molecular dynamics simulations are on the order of 1 fs. * The periods of the waves of visible light have a duration of about 2 femtoseconds. = = 2.0 \times 10^~ The precise duration depends on the ener ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultrafast Spectroscopy
In optics, an ultrashort pulse, also known as an ultrafast event, is an electromagnetic pulse whose time duration is of the order of a picosecond (10−12 second) or less. Such pulses have a broadband optical spectrum, and can be created by mode-locked oscillators. Amplification of ultrashort pulses almost always requires the technique of chirped pulse amplification, in order to avoid damage to the gain medium of the amplifier. They are characterized by a high peak intensity (or more correctly, irradiance) that usually leads to nonlinear interactions in various materials, including air. These processes are studied in the field of nonlinear optics. In the specialized literature, "ultrashort" refers to the femtosecond (fs) and picosecond (ps) range, although such pulses no longer hold the record for the shortest pulses artificially generated. Indeed, x-ray pulses with durations on the attosecond time scale have been reported. The 1999 Nobel Prize in Chemistry was awarded ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flash Photolysis
Flash photolysis is a pump-probe laboratory technique, in which a sample is first excited by a strong pulse of light from a pulsed laser of nanosecond, picosecond, or femtosecond pulse width or by another short-pulse light source such as a flash lamp. This first strong pulse is called the pump pulse and starts a chemical reaction or leads to an increased population for energy levels other than the ground state within a sample of atoms or molecules. Typically the absorption of light by the sample is recorded within short time intervals (by a so-called test or probe pulses) to monitor relaxation or reaction processes initiated by the pump pulse. Flash photolysis was developed shortly after World War II as an outgrowth of attempts by military scientists to build cameras fast enough to photograph missiles in flight. The technique was developed in 1949 by Manfred Eigen, Ronald George Wreyford Norrish and George Porter, who won the 1967 Nobel Prize in Chemistry for this invention. O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultrashort Pulse
In optics, an ultrashort pulse, also known as an ultrafast event, is an electromagnetic pulse whose time duration is of the order of a picosecond (10−12 second) or less. Such pulses have a broadband optical spectrum, and can be created by mode-locked oscillators. Amplification of ultrashort pulses almost always requires the technique of chirped pulse amplification, in order to avoid damage to the gain medium of the amplifier. They are characterized by a high peak intensity (or more correctly, irradiance) that usually leads to nonlinear interactions in various materials, including air. These processes are studied in the field of nonlinear optics. In the specialized literature, "ultrashort" refers to the femtosecond (fs) and picosecond (ps) range, although such pulses no longer hold the record for the shortest pulses artificially generated. Indeed, x-ray pulses with durations on the attosecond time scale have been reported. The 1999 Nobel Prize in Chemistry was awarded ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Femtotechnology
Femtotechnology is a hypothetical term used in reference to structuring of matter on the scale of a femtometer, which is 10−15 m. This is a smaller scale in comparison with nanotechnology and picotechnology which refer to 10−9 m and 10−12 m respectively. Theory Work in the femtometer range involves manipulation of excited energy states within atomic nuclei, specifically nuclear isomers, to produce metastable (or otherwise stabilized) states with unusual properties. In the extreme case, excited states of the individual nucleons that make up the atomic nucleus (protons and neutrons) are considered, ostensibly to tailor the behavioral properties of these particles. The most advanced form of molecular nanotechnology is often imagined to involve self-replicating molecular machines, and there have been some speculations suggesting something similar might be possible with analogues of molecules composed of nucleons rather than atoms. For example, the astrophysici ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physical Chemistry
Physical chemistry is the study of macroscopic and microscopic phenomena in chemical systems in terms of the principles, practices, and concepts of physics such as motion, energy, force, time, thermodynamics, quantum chemistry, statistical mechanics, analytical dynamics and chemical equilibria. Physical chemistry, in contrast to chemical physics, is predominantly (but not always) a supra-molecular science, as the majority of the principles on which it was founded relate to the bulk rather than the molecular or atomic structure alone (for example, chemical equilibrium and colloids). Some of the relationships that physical chemistry strives to resolve include the effects of: # Intermolecular forces that act upon the physical properties of materials ( plasticity, tensile strength, surface tension in liquids). # Reaction kinetics on the rate of a reaction. # The identity of ions and the electrical conductivity of materials. # Surface science and electrochemistry of cell membrane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
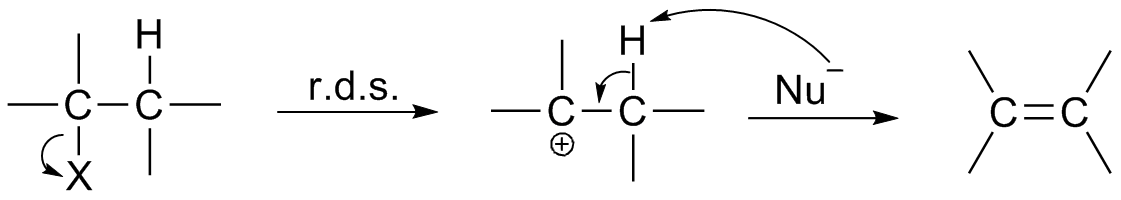
Reaction Intermediate
In chemistry, a reaction intermediate or an intermediate is a molecular entity that is formed from the reactants (or preceding intermediates) but is consumed in further reactions in stepwise chemical reactions that contain multiple elementary steps. Intermediates are the reaction product of one elementary step, but do not appear in the chemical equation for an overall chemical equation. For example, consider this hypothetical stepwise reaction: :A + B -> C + D The reaction includes two elementary steps: :A + B -> X :X -> C + D In this example, X is a reaction intermediate. IUPAC definition The IUPAC Gold Book defines an ''intermediate'' as a compound that has a lifetime greater than a molecular vibration that is formed (directly or indirectly) from the reactants and reacts further to give (either directly or indirectly) the products of a chemical reaction. The lifetime condition distinguishes true, chemically distinct intermediates from vibrational states or such transition st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig (in 1825) and Antoine Jérôme Balard (in 1826), its name was derived from the Ancient Greek (bromos) meaning "stench", referring to its sharp and pungent smell. Elemental bromine is very reactive and thus does not occur as a native element in nature but it occurs in colourless soluble crystalline mineral halide salts, analogous to table salt. In fact, bromine and all the halogens are so reactive that they form bonds in pairs—never in single atoms. While it is rather rare in the Earth's crust, the high solubility of the bromide ion (Br) has caused its accumulation in the oceans. Commercial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |