Cocaine (from , from , ultimately from
Quechua
Quechua may refer to:
*Quechua people, several indigenous ethnic groups in South America, especially in Peru
*Quechuan languages, a Native South American language family spoken primarily in the Andes, derived from a common ancestral language
**So ...
: ''kúka'') is a
central nervous system
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain and spinal cord. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all par ...
(CNS)
stimulant
Stimulants (also often referred to as psychostimulants or colloquially as uppers) is an overarching term that covers many drugs including those that increase activity of the central nervous system and the body, drugs that are pleasurable and inv ...
mainly
used recreationally for its
euphoric
Euphoria ( ) is the experience (or affect) of pleasure or excitement and intense feelings of well-being and happiness. Certain natural rewards and social activities, such as aerobic exercise, laughter, listening to or making music and da ...
effects. It is primarily obtained from the leaves of two
Coca
Coca is any of the four cultivated plants in the family Erythroxylaceae, native to western South America. Coca is known worldwide for its psychoactive alkaloid, cocaine.
The plant is grown as a cash crop in the Argentine Northwest, Bolivia, Al ...
species native to South America, ''
Erythroxylum coca
''Erythroxylum coca'' is one of two species of cultivated coca.
Description
The coca plant resembles a blackthorn bush, and grows to a height of . The branches are straight, and the leaves, which have a green tint, are thin, opaque, oval, and tap ...
'' and ''
Erythroxylum novogranatense
''Erythroxylum novogranatense'' is a neotropical species of ''Erythroxylum'' (Erythroxylaceae). Cocaine is produced from the leaves.
Name
"Novogranatense" is derived from Latin: ''novo'' (new) and ''granatense'' (Granada). It was named by Willi ...
''.
After extraction from coca leaves and further processing into cocaine hydrochloride (powdered cocaine), the drug is often
snorted, applied
topically
A topical medication is a medication that is applied to a particular place on or in the body. Most often topical medication means application to body surfaces such as the skin or mucous membranes to treat ailments via a large range of classes ...
to the
mouth
In animal anatomy, the mouth, also known as the oral cavity, or in Latin cavum oris, is the opening through which many animals take in food and issue vocal sounds. It is also the cavity lying at the upper end of the alimentary canal, bounded on ...
, or dissolved and
injected into a
vein
Veins are blood vessels in humans and most other animals that carry blood towards the heart. Most veins carry deoxygenated blood from the tissues back to the heart; exceptions are the pulmonary and umbilical veins, both of which carry oxygenated b ...
. It can also then be turned into
free base
Free base (freebase, free-base) is the conjugate base (deprotonated) form of an amine, as opposed to its conjugate acid (protonated) form. The amine is often an alkaloid, such as nicotine, cocaine, morphine, and ephedrine, or derivatives thereo ...
form (
crack cocaine
Crack cocaine, commonly known simply as crack, and also known as rock, is a free base form of the stimulant cocaine that can be smoked. Crack offers a short, intense high to smokers. The ''Manual of Adolescent Substance Abuse Treatment'' calls ...
), in which it can be heated until sublimated and then the vapours can be
inhaled
Inhalation (or Inspiration) happens when air or other gases enter the lungs.
Inhalation of air
Inhalation of air, as part of the cycle of breathing, is a vital process for all human life. The process is autonomic (though there are exceptions ...
.
Cocaine stimulates the
reward pathway
The mesolimbic pathway, sometimes referred to as the reward pathway, is a dopaminergic pathway in the brain. The pathway connects the ventral tegmental area in the midbrain to the ventral striatum of the basal ganglia in the forebrain. The ventra ...
in the brain.
[ Mental effects may include an intense feeling of happiness, ]sexual arousal
Sexual arousal (also known as sexual excitement) describes the physiological and psychological responses in preparation for sexual intercourse or when exposed to sexual stimuli. A number of physiological responses occur in the body and mind as ...
, loss of contact with reality, or agitation.fast heart rate
Tachycardia, also called tachyarrhythmia, is a heart rate that exceeds the normal resting rate. In general, a resting heart rate over 100 beats per minute is accepted as tachycardia in adults. Heart rates above the resting rate may be normal (su ...
, sweating, and dilated pupils
Mydriasis is the dilation of the pupil, usually having a non-physiological cause, or sometimes a physiological pupillary response. Non-physiological causes of mydriasis include disease, trauma, or the use of certain types of drugs.
Normally, as ...
.high blood pressure
Hypertension (HTN or HT), also known as high blood pressure (HBP), is a long-term medical condition in which the blood pressure in the arteries is persistently elevated. High blood pressure usually does not cause symptoms. Long-term high bl ...
or high body temperature. Effects begin within seconds to minutes of use and last between five and ninety minutes.throat
In vertebrate anatomy, the throat is the front part of the neck, internally positioned in front of the vertebrae. It contains the pharynx and larynx. An important section of it is the epiglottis, separating the esophagus from the trachea (windpipe ...
or inside of the nose to control pain, bleeding, and vocal cord spasm.
Cocaine crosses the blood–brain barrier
The blood–brain barrier (BBB) is a highly selective semipermeable membrane, semipermeable border of endothelium, endothelial cells that prevents solutes in the circulating blood from ''non-selectively'' crossing into the extracellular fluid of ...
via a proton-coupled organic cation antiporterdopamine transporter
The dopamine transporter (also dopamine active transporter, DAT, SLC6A3) is a membrane-spanning protein that pumps the neurotransmitter dopamine out of the synaptic cleft back into cytosol. In the cytosol, other transporters sequester the dopam ...
, inhibiting reuptake
Reuptake is the reabsorption of a neurotransmitter by a neurotransmitter transporter located along the plasma membrane of an axon terminal (i.e., the pre-synaptic neuron at a synapse) or glial cell after it has performed its function of transm ...
of dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic compound, organic chemical of the catecholamine and phenethylamine families. Dopamine const ...
from the synaptic cleft
Chemical synapses are biological junctions through which neurons' signals can be sent to each other and to non-neuronal cells such as those in muscles or glands. Chemical synapses allow neurons to form circuits within the central nervous syste ...
into the pre-synaptic axon terminal
Axon terminals (also called synaptic boutons, terminal boutons, or end-feet) are distal terminations of the telodendria (branches) of an axon. An axon, also called a nerve fiber, is a long, slender projection of a nerve cell, or neuron, that condu ...
; the higher dopamine levels in the synaptic cleft increase dopamine receptor
Dopamine receptors are a class of G protein-coupled receptors that are prominent in the vertebrate central nervous system (CNS). Dopamine receptors activate different effectors through not only G-protein coupling, but also signaling through diffe ...
activation in the post-synaptic neuron, causing euphoria and arousal. Cocaine also blocks the serotonin transporter
The serotonin transporter (SERT or 5-HTT) also known as the sodium-dependent serotonin transporter and solute carrier family 6 member 4 is a protein that in humans is encoded by the SLC6A4 gene. SERT is a type of monoamine transporter protein tha ...
and norepinephrine transporter
The norepinephrine transporter (NET), also known as noradrenaline transporter (NAT), is a protein that in humans is encoded by the solute carrier family 6 member 2 (SLC6A2) gene.
NET is a monoamine transporter and is responsible for the sodium- ...
, inhibiting reuptake of serotonin
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vas ...
and norepinephrine
Norepinephrine (NE), also called noradrenaline (NA) or noradrenalin, is an organic chemical in the catecholamine family that functions in the brain and body as both a hormone and neurotransmitter. The name "noradrenaline" (from Latin '' ad'', ...
from the synaptic cleft into the pre-synaptic axon terminal
Axon terminals (also called synaptic boutons, terminal boutons, or end-feet) are distal terminations of the telodendria (branches) of an axon. An axon, also called a nerve fiber, is a long, slender projection of a nerve cell, or neuron, that condu ...
and increasing activation of serotonin receptor
5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neur ...
s and norepinephrine receptors in the post-synaptic neuron, contributing to the mental and physical effects of cocaine exposure.addiction
Addiction is a neuropsychological disorder characterized by a persistent and intense urge to engage in certain behaviors, one of which is the usage of a drug, despite substantial harm and other negative consequences. Repetitive drug use o ...
. Addicts who abstain from cocaine experience cocaine craving and drug withdrawal
Drug withdrawal, drug withdrawal syndrome, or substance withdrawal syndrome, is the group of symptoms that occur upon the abrupt discontinuation or decrease in the intake of pharmaceutical or recreational drugs.
In order for the symptoms of with ...
, with depression, decreased libido, decreased ability to feel pleasure and fatigue.[ Use of cocaine increases the overall risk of death and intravenous use particularly increases the risk of trauma and infectious diseases such as blood infections and ]HIV
The human immunodeficiency viruses (HIV) are two species of ''Lentivirus'' (a subgroup of retrovirus) that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the immune ...
. It also increases risk of stroke
A stroke is a medical condition in which poor blood flow to the brain causes cell death. There are two main types of stroke: ischemic, due to lack of blood flow, and hemorrhagic, due to bleeding. Both cause parts of the brain to stop functionin ...
, heart attack
A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow decreases or stops to the coronary artery of the heart, causing damage to the heart muscle. The most common symptom is chest pain or discomfort which may tr ...
, cardiac arrhythmia, lung injury (when smoked), and sudden cardiac death
Cardiac arrest is when the heart suddenly and unexpectedly stops beating. It is a medical emergency that, without immediate medical intervention, will result in sudden cardiac death within minutes. Cardiopulmonary resuscitation (CPR) and possib ...
.fentanyl
Fentanyl, also spelled fentanil, is a very potent synthetic opioid used as a pain medication. Together with other drugs, fentanyl is used for anesthesia. It is also used illicitly as a recreational drug, sometimes mixed with heroin, cocaine ...
, local anesthetics
A local anesthetic (LA) is a medication that causes absence of pain sensation. In the context of surgery, a local anesthetic creates an absence of pain in a specific location of the body without a loss of consciousness, as opposed to a general an ...
, levamisole
Levamisole, sold under the brand name Ergamisol among others, is a medication used to treat parasitic worm infections, specifically ascariasis and hookworm infections. It is taken by mouth.
Side effects may include abdominal pain, vomiting, ...
, cornstarch, quinine
Quinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to ''Plasmodium falciparum'' that is resistant to chloroquine when artesunate is not available. While sometimes used for nocturnal leg cr ...
, or sugar, which can result in additional toxicity.
Uses
Coca leaves have been used by Andean civilizations
The Andean civilizations were civilization, complex societies of many Indigenous peoples of South America, cultures and peoples mainly developed in the river valleys of the coastal deserts of Peru. They stretched from the Andes of southern Colomb ...
since ancient times
Ancient history is a time period from the beginning of writing and recorded human history to as far as late antiquity. The span of recorded history is roughly 5,000 years, beginning with the Sumerian cuneiform script. Ancient history cov ...
.[ In ancient ]Wari culture
The Wari ( es, Huari) were a Middle Horizon civilization that flourished in the south-central Andes and coastal area of modern-day Peru, from about 500 to 1000 AD.
Wari, as the former capital city was called, is located north-east of the mo ...
,Incan
The Inca Empire (also known as the Incan Empire and the Inka Empire), called ''Tawantinsuyu'' by its subjects, (Quechua for the "Realm of the Four Parts", "four parts together" ) was the largest empire in pre-Columbian America. The admin ...
culture, and through modern successor indigenous
Indigenous may refer to:
*Indigenous peoples
*Indigenous (ecology), presence in a region as the result of only natural processes, with no human intervention
*Indigenous (band), an American blues-rock band
*Indigenous (horse), a Hong Kong racehorse ...
cultures of the Andes mountains
The Andes, Andes Mountains or Andean Mountains (; ) are the List of mountain ranges#Mountain ranges by length, longest continental mountain range in the world, forming a continuous highland along the western edge of South America. The range i ...
, coca leaves are chewed, taken orally in the form of a tea
Tea is an aromatic beverage prepared by pouring hot or boiling water over cured or fresh leaves of '' Camellia sinensis'', an evergreen shrub native to East Asia which probably originated in the borderlands of southwestern China and northe ...
, or alternatively, prepared in a sachet wrapped around alkaline burnt ashes, and held in the mouth against the inner cheek; it has traditionally been used to combat the effects of cold, hunger, and altitude sickness
Altitude sickness, the mildest form being acute mountain sickness (AMS), is the harmful effect of high altitude, caused by rapid exposure to low amounts of oxygen at high elevation. People can respond to high altitude in different ways. Sympt ...
.[
Globally, in 2019, cocaine was used by an estimated 20 million people (0.4% of adults aged 15 to 64 years). The highest prevalence of cocaine use was in Australia and New Zealand (2.1%), followed by North America (2.1%), Western and Central Europe (1.4%), and South and Central America (1.0%).]Single Convention on Narcotic Drugs
The Single Convention on Narcotic Drugs, 1961 (Single Convention, 1961 Convention, or C61) is an international treaty that controls activities (cultivation, production, supply, trade, transport) of specific narcotic drugs and lays down a syst ...
has required countries to make recreational use of cocaine a crime
In ordinary language, a crime is an unlawful act punishable by a State (polity), state or other authority. The term ''crime'' does not, in modern criminal law, have any simple and universally accepted definition,Farmer, Lindsay: "Crime, definit ...
. In the United States, cocaine is regulated as a Schedule II drug under the Controlled Substances Act
The Controlled Substances Act (CSA) is the statute establishing federal government of the United States, federal drug policy of the United States, U.S. drug policy under which the manufacture, importation, possession, use, and distribution of ...
, meaning that it has a high potential for abuse and has an accepted medical use for treatment.
Medical
 Topical cocaine is sometimes used as a local numbing agent and
Topical cocaine is sometimes used as a local numbing agent and vasoconstrictor
Vasoconstriction is the narrowing of the blood vessels resulting from contraction of the muscular wall of the vessels, in particular the large arteries and small arterioles. The process is the opposite of vasodilation, the widening of blood vessel ...
to help control pain and bleeding with surgery of the nose, mouth, throat or lacrimal duct. Although some absorption and systemic effects may occur, the use of cocaine as a topical anesthetic and vasoconstrictor is generally safe, rarely causing cardiovascular
The blood circulatory system is a system of organs that includes the heart, blood vessels, and blood which is circulated throughout the entire body of a human or other vertebrate. It includes the cardiovascular system, or vascular system, tha ...
toxicity, glaucoma
Glaucoma is a group of eye diseases that result in damage to the optic nerve (or retina) and cause vision loss. The most common type is open-angle (wide angle, chronic simple) glaucoma, in which the drainage angle for fluid within the eye rem ...
, and pupil dilation
Pupillary response is a physiological response that varies the size of the pupil, via the optic and oculomotor cranial nerve.
A constriction response (miosis), is the narrowing of the pupil, which may be caused by scleral buckles or drugs such a ...
.adrenaline
Adrenaline, also known as epinephrine, is a hormone and medication which is involved in regulating visceral functions (e.g., respiration). It appears as a white microcrystalline granule. Adrenaline is normally produced by the adrenal glands and ...
and sodium bicarbonate
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−) ...
and used topically for surgery, a formulation called Moffett's solution.
Cocaine hydrochloride
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base (e.g. an amine). An alternative name is chlorhydrate, which comes from French. An archaic alternative na ...
(Goprelto), an ester local anesthetic, was approved for medical use in the United States in December 2017, and is indicated for the introduction of local anesthesia of the mucous membranes for diagnostic procedures and surgeries on or through the nasal cavities of adults.epistaxis
A nosebleed, also known as epistaxis, is bleeding from the nose. Blood can flow down into the stomach, and cause nausea and vomiting. In more severe cases, blood may come out of both nostrils. Rarely, bleeding may be so significant that low bloo ...
.
Recreational
Cocaine is a central nervous system
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain and spinal cord. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all par ...
stimulant.Crack cocaine
Crack cocaine, commonly known simply as crack, and also known as rock, is a free base form of the stimulant cocaine that can be smoked. Crack offers a short, intense high to smokers. The ''Manual of Adolescent Substance Abuse Treatment'' calls ...
is a smokeable form of cocaine made into small "rocks" by processing cocaine with sodium bicarbonate (baking soda) and water.euphoria
Euphoria ( ) is the experience (or affect) of pleasure or excitement and intense feelings of well-being and happiness. Certain natural rewards and social activities, such as aerobic exercise, laughter, listening to or making music and da ...
, increased energy and motor activity, and increased feelings of competence and sexuality.correlation
In statistics, correlation or dependence is any statistical relationship, whether causal or not, between two random variables or bivariate data. Although in the broadest sense, "correlation" may indicate any type of association, in statistics ...
between the use of 18 various psychoactive substance
A psychoactive drug, psychopharmaceutical, psychoactive agent or psychotropic drug is a chemical substance, that changes functions of the nervous system, and results in alterations in perception, mood, consciousness, cognition or behavior.
Th ...
s shows that cocaine use correlates with other " party drugs" (such as ecstasy or amphetamines
Substituted amphetamines are a class of compounds based upon the amphetamine structure; it includes all derivative compounds which are formed by replacing, or substituting, one or more hydrogen atoms in the amphetamine core structure with sub ...
), as well as with heroin
Heroin, also known as diacetylmorphine and diamorphine among other names, is a potent opioid mainly used as a recreational drug for its euphoric effects. Medical grade diamorphine is used as a pure hydrochloride salt. Various white and brow ...
and benzodiazepine
Benzodiazepines (BZD, BDZ, BZs), sometimes called "benzos", are a class of depressant drugs whose core chemical structure is the fusion of a benzene ring and a diazepine ring. They are prescribed to treat conditions such as anxiety disorders, ...
s use, and can be considered as a bridge between the use of different groups of drugs.
Coca leaves
It is legal for people to use Coca
Coca is any of the four cultivated plants in the family Erythroxylaceae, native to western South America. Coca is known worldwide for its psychoactive alkaloid, cocaine.
The plant is grown as a cash crop in the Argentine Northwest, Bolivia, Al ...
leaves in some Andean nations, such as Peru and Bolivia, where they are chewed, consumed in the form of tea, or are sometimes incorporated into food products. Coca leaves are typically mixed with an alkaline substance (such as lime
Lime commonly refers to:
* Lime (fruit), a green citrus fruit
* Lime (material), inorganic materials containing calcium, usually calcium oxide or calcium hydroxide
* Lime (color), a color between yellow and green
Lime may also refer to:
Botany ...
) and chewed into a wad that is retained in the buccal pouch (mouth between gum and cheek, much the same as chewing tobacco
Chewing tobacco is a type of smokeless tobacco product that is placed between the cheek and lower gum to draw out its flavor. Some users chew it, others do not. It consists of coarsely chopped aged tobacco that is flavored and often sweetened; ...
is chewed) and sucked of its juices. The juices are absorbed slowly by the mucous membrane of the inner cheek and by the gastrointestinal tract when swallowed. Alternatively, coca leaves can be infused in liquid and consumed like tea. Coca tea
Coca tea, also called mate de coca, is an herbal tea (infusion) made using the raw or dried leaves of the coca plant, which is native to South America. It is made either by submerging the coca leaf or dipping a tea bag in hot water. The tea is mo ...
, an infusion of coca leaves, is also a traditional method of consumption. The tea has often been recommended for travelers in the Andes to prevent altitude sickness
Altitude sickness, the mildest form being acute mountain sickness (AMS), is the harmful effect of high altitude, caused by rapid exposure to low amounts of oxygen at high elevation. People can respond to high altitude in different ways. Sympt ...
.[ ] Its actual effectiveness has never been systematically studied.Journal of the American Medical Association
''The Journal of the American Medical Association'' (''JAMA'') is a peer-reviewed medical journal published 48 times a year by the American Medical Association. It publishes original research, reviews, and editorials covering all aspects of bio ...
'' revealed that U.S. health food store
A health food store (or health food shop) is a type of grocery store that primarily sells health foods, organic foods, local produce, and often nutritional supplements. Health food stores typically offer a wider or more specialized selection of fo ...
s were selling dried coca leaves to be prepared as an infusion as "Health Inca Tea". While the packaging claimed it had been "decocainized", no such process had actually taken place. The article stated that drinking two cups of the tea per day gave a mild stimulation
Stimulation is the encouragement of development or the cause of activity generally. For example, "The press provides stimulation of political discourse." An interesting or fun activity can be described as "stimulating", regardless of its physica ...
, increased heart rate
Heart rate (or pulse rate) is the frequency of the heartbeat measured by the number of contractions (beats) of the heart per minute (bpm). The heart rate can vary according to the body's physical needs, including the need to absorb oxygen and excr ...
, and mood elevation, and the tea was essentially harmless.
Insufflation
 Nasal
Nasal insufflation
In religious and magical practice, insufflation and exsufflation are ritual acts of blowing, breathing, hissing, or puffing that signify variously expulsion or renunciation of evil or of the devil (the Evil One), or infilling or blessing with goo ...
(known colloquially as "snorting", "sniffing", or "blowing") is a common method of ingestion of recreational powdered cocaine. The drug coats and is absorbed through the mucous membrane
A mucous membrane or mucosa is a membrane that lines various cavities in the body of an organism and covers the surface of internal organs. It consists of one or more layers of epithelial cells overlying a layer of loose connective tissue. It is ...
s lining the nasal passages
The human nose is the most protruding part of the face. It bears the nostrils and is the first organ of the respiratory system. It is also the principal organ in the olfactory system. The shape of the nose is determined by the nasal bones ...
. Cocaine's desired euphoric effects are delayed when snorted through the nose by about five minutes. This occurs because cocaine's absorption is slowed by its constricting effect on the blood vessels of the nose.banknotes
A banknote—also called a bill (North American English), paper money, or simply a note—is a type of negotiable instrument, negotiable promissory note, made by a bank or other licensed authority, payable to the bearer on demand.
Banknotes w ...
, hollowed-out pen
A pen is a common writing instrument that applies ink to a surface, usually paper, for writing or drawing. Early pens such as reed pens, quill pens, dip pens and ruling pens held a small amount of ink on a nib or in a small void or cavity wh ...
s, cut straws, pointed ends of keys, specialized spoons, long fingernails
A nail is a claw-like plate found at the tip of the fingers and toes on most primates. Nails correspond to the claws found in other animals. Fingernails and toenails are made of a tough protective protein called alpha-keratin, which is a polymer. ...
, and (clean) tampon applicators are often used to insufflate cocaine. The cocaine typically is poured onto a flat, hard surface (such as a mobile phone screen, mirror, CD case or book) and divided into "bumps", "lines" or "rails", and then insufflated. A 2001 study reported that the sharing of straws used to "snort" cocaine can spread blood diseases such as hepatitis C
Hepatitis C is an infectious disease caused by the hepatitis C virus (HCV) that primarily affects the liver; it is a type of viral hepatitis. During the initial infection people often have mild or no symptoms. Occasionally a fever, dark urine, a ...
.
Injection
Subjective effects not commonly shared with other methods of administration include a ringing in the ears moments after injection (usually when over 120 milligrams) lasting two to 5 minutes including tinnitus
Tinnitus is the perception of sound when no corresponding external sound is present. Nearly everyone experiences a faint "normal tinnitus" in a completely quiet room; but it is of concern only if it is bothersome, interferes with normal hearin ...
and audio distortion. This is colloquially referred to as a "bell ringer". In a study of cocaine users, the average time taken to reach peak subjective effects was 3.1 minutes.emboli
An embolism is the lodging of an embolus, a blockage-causing piece of material, inside a blood vessel. The embolus may be a blood clot (thrombus), a fat globule ( fat embolism), a bubble of air or other gas (gas embolism), amniotic fluid (amniot ...
from the insoluble substances that may be used to cut the drug. As with all injected illicit substances, there is a risk of the user contracting blood-borne infections if sterile injecting equipment is not available or used.
An injected mixture of cocaine and heroin
Heroin, also known as diacetylmorphine and diamorphine among other names, is a potent opioid mainly used as a recreational drug for its euphoric effects. Medical grade diamorphine is used as a pure hydrochloride salt. Various white and brow ...
, known as " speedball", is a particularly dangerous combination, as the converse effects of the drugs actually complement each other, but may also mask the symptoms of an overdose. It has been responsible for numerous deaths, including celebrities such as comedians/actors John Belushi
John Adam Belushi (January 24, 1949 – March 5, 1982) was an American comedian, actor, and musician, best known for being one of the seven original cast members of the NBC sketch comedy show ''Saturday Night Live'' (''SNL''). Throughout his ca ...
and Chris Farley
Christopher Crosby Farley (February 15, 1964 – December 18, 1997) was an American actor and comedian. Farley was known for his loud, energetic comedic style, and was a member of Chicago's Second City Theatre and later a cast member of the ...
, Mitch Hedberg
Mitchell Lee Hedberg (February 24, 1968 – March 30, 2005) was an American stand-up comedian known for his surreal humor and deadpan delivery. His comedy typically featured short, sometimes one-line jokes mixed with absurd elements and non seq ...
, River Phoenix
River Jude Phoenix (; August 23, 1970 – October 31, 1993) was an American actor, musician and activist.
Phoenix grew up in an itinerant family, as the older brother of Rain Phoenix, Joaquin Phoenix, Liberty Phoenix, and Summer Phoenix. He ha ...
, grunge singer Layne Staley
Layne Thomas Staley (born Layne Rutherford Staley; August 22, 1967 – April 5, 2002) was an American musician, songwriter and the original lead singer of the rock band Alice in Chains, which rose to international fame in the early 1990s as part ...
and actor Philip Seymour Hoffman
Philip Seymour Hoffman (July 23, 1967 – February 2, 2014) was an American actor. Known for his distinctive supporting and character roles—typically lowlifes, eccentrics, underdogs, and misfits—he acted in many films and theatrical produ ...
. Experimentally, cocaine injections can be delivered to animals such as fruit flies
Fruit fly may refer to:
Organisms
* Drosophilidae, a family of small flies, including:
** ''Drosophila'', the genus of small fruit flies and vinegar flies
** ''Drosophila melanogaster'' or common fruit fly
** ''Drosophila suzukii'' or Asian fruit ...
to study the mechanisms of cocaine addiction.
Inhalation
The onset of cocaine's euphoric effects is fastest with inhalation, beginning after 3–5 seconds.Pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''py ...
products of cocaine that occur only when heated/smoked have been shown to change the effect profile, ''i.e.'' anhydroecgonine methyl ester, when co-administered with cocaine, increases the dopamine in CPu and NAc brain regions, and has M1− and M3− receptor affinity.love rose
A love rose is a glass tube with a paper or plastic rose inside of it, and a bit of cork or foil on the ends to keep the rose from falling out. While ostensibly intended as romantic gifts, their primary known use is as a pipe to smoke drugs such a ...
s", small glass tubes with a paper rose that are promoted as romantic gifts. These are sometimes called "stems", "horns", "blasters" and "straight shooters". A small piece of clean heavy copper or occasionally stainless steel scouring padoften called a "brillo" (actual Brillo Pad
Brillo is a trade name for a scouring pad, used for cleaning dishes, and made from steel wool impregnated with soap. The concept was patented in 1913, at a time when aluminium pots and pans were replacing cast iron in the kitchen; the new coo ...
s contain soap, and are not used) or "chore" (named for Chore Boy Chore Boy is a brand name for a coarse scouring pad made of steel wool, copper wool or non-metallic terry cloth and all-purpose sponges. It is ideal for cleaning BBQ grills, cookware, glassware, oven racks and stove burners. During the first half o ...
brand copper scouring pads)serves as a reduction base and flow modulator in which the "rock" can be melted and boiled to vapor. Crack is smoked by placing it at the end of the pipe; a flame held close to it produces vapor, which is then inhaled by the smoker. The effects felt almost immediately after smoking, are very intense and do not last long usually 2 to 10 minutes. When smoked, cocaine is sometimes combined with other drugs, such as cannabis
''Cannabis'' () is a genus of flowering plants in the family Cannabaceae. The number of species within the genus is disputed. Three species may be recognized: ''Cannabis sativa'', '' C. indica'', and '' C. ruderalis''. Alternatively ...
, often rolled into a joint or blunt
Blunt may refer to:
* Blunt (surname), a surname (and list of people with the name)
* Blunt (cigar), a term used in the cigar industry to designate blunt-tipped, usually factory-rolled cigars
* Blunt (cannabis), a slang term used in cannabis cult ...
.
Effects
 File:US timeline. Opioid involvement in cocaine overdose.jpg, Opioid involvement in cocaine overdose deaths. The green line is cocaine and any opioid (top line in 2017). The gray line is cocaine without any opioids (bottom line in 2017). The yellow line is cocaine and other
File:US timeline. Opioid involvement in cocaine overdose.jpg, Opioid involvement in cocaine overdose deaths. The green line is cocaine and any opioid (top line in 2017). The gray line is cocaine without any opioids (bottom line in 2017). The yellow line is cocaine and other synthetic opioids
Opioids are substances that act on opioid receptors to produce morphine-like effects. Medically they are primarily used for pain relief, including anesthesia. Other medical uses include suppression of diarrhea, replacement therapy for opioid us ...
(middle line in 2017).
Acute
Acute exposure to cocaine has many effects on humans, including euphoria, increases in heart rate and blood pressure, and increases in cortisol secretion from the adrenal gland. In humans with acute exposure followed by continuous exposure to cocaine at a constant blood concentration, the acute tolerance to the chronotropic cardiac effects of cocaine begins after about 10 minutes, while acute tolerance to the euphoric effects of cocaine begins after about one hour.itch
Itch (also known as pruritus) is a sensation that causes the desire or reflex to scratch. Itch has resisted many attempts to be classified as any one type of sensory experience. Itch has many similarities to pain, and while both are unpleasant ...
ing, fast heart rate
Tachycardia, also called tachyarrhythmia, is a heart rate that exceeds the normal resting rate. In general, a resting heart rate over 100 beats per minute is accepted as tachycardia in adults. Heart rates above the resting rate may be normal (su ...
, and paranoid delusions or sensations of insects crawling on the skin.psychosis
Psychosis is a condition of the mind that results in difficulties determining what is real and what is not real. Symptoms may include delusions and hallucinations, among other features. Additional symptoms are incoherent speech and behavior ...
characterized by paranoia, impaired reality testing Reality testing is the psychotherapeutic function by which the objective or real world and one's relationship to it are reflected on and evaluated by the observer. This process of distinguishing the internal world of thoughts and feelings from the e ...
, hallucinations
A hallucination is a perception in the absence of an external stimulus that has the qualities of a real perception. Hallucinations are vivid, substantial, and are perceived to be located in external objective space. Hallucination is a combinatio ...
, irritability, and physical aggression. Cocaine intoxication
Cocaine intoxication refers to the subjective, desired and adverse effects of cocaine on the mind and behavior of users. Both self-induced and involuntary cocaine intoxication have medical and legal implications (even in absence of relevant advers ...
can cause hyperawareness, hypervigilance
Hypervigilance (more accurately understood as Hyper-awareness) is a condition in which the nervous system is filtering sensory information and the individual is in an enhanced state of sensory sensitivity or sensory domination. The name itself is ...
, and psychomotor agitation and delirium
Delirium (also known as acute confusional state) is an organically caused decline from a previous baseline of mental function that develops over a short period of time, typically hours to days. Delirium is a syndrome encompassing disturbances in ...
. Consumption of large doses of cocaine can cause violent outbursts, especially by those with preexisting psychosis. Crack-related violence is also systemic, relating to disputes between crack dealers and users. Acute exposure may induce cardiac arrhythmias, including atrial fibrillation, supraventricular tachycardia
Supraventricular tachycardia (SVT) is an umbrella term for fast heart rhythms arising from the upper part of the heart. This is in contrast to the other group of fast heart rhythms – ventricular tachycardia, which start within the lower cham ...
, ventricular tachycardia
Ventricular tachycardia (V-tach or VT) is a fast heart rate arising from the lower chambers of the heart. Although a few seconds of VT may not result in permanent problems, longer periods are dangerous; and multiple episodes over a short period ...
, and ventricular fibrillation
Ventricular fibrillation (V-fib or VF) is an abnormal heart rhythm in which the ventricles of the heart quiver. It is due to disorganized electrical activity. Ventricular fibrillation results in cardiac arrest with loss of consciousness and no p ...
. Acute exposure may also lead to angina
Angina, also known as angina pectoris, is chest pain or pressure, usually caused by ischemia, insufficient blood flow to the Cardiac muscle, heart muscle (myocardium). It is most commonly a symptom of coronary artery disease.
Angina is typical ...
, heart attack
A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow decreases or stops to the coronary artery of the heart, causing damage to the heart muscle. The most common symptom is chest pain or discomfort which may tr ...
, and congestive heart failure
Heart failure (HF), also known as congestive heart failure (CHF), is a syndrome, a group of signs and symptoms caused by an impairment of the heart's blood pumping function. Symptoms typically include shortness of breath, excessive fatigue, a ...
. Cocaine overdose may cause seizures
An epileptic seizure, informally known as a seizure, is a period of symptoms due to abnormally excessive or neural oscillation, synchronous neuronal activity in the brain. Outward effects vary from uncontrolled shaking movements involving much o ...
, abnormally high body temperature and a marked elevation of blood pressure, which can be life-threatening,paranoia
Paranoia is an instinct or thought process that is believed to be heavily influenced by anxiety or fear, often to the point of delusion and irrationality. Paranoid thinking typically includes persecutory beliefs, or beliefs of conspiracy concer ...
, and restlessness can also occur, especially during the comedown. With excessive dosage, tremors, convulsions and increased body temperature are observed.sudden cardiac death
Cardiac arrest is when the heart suddenly and unexpectedly stops beating. It is a medical emergency that, without immediate medical intervention, will result in sudden cardiac death within minutes. Cardiopulmonary resuscitation (CPR) and possib ...
, become a serious risk at high doses due to cocaine's blocking effect on cardiac sodium channels.cornea
The cornea is the transparent front part of the eye that covers the iris, pupil, and anterior chamber. Along with the anterior chamber and lens, the cornea refracts light, accounting for approximately two-thirds of the eye's total optical power ...
and long-term loss of visual acuity.
Chronic
 Although it has been commonly asserted, the available evidence does not show that chronic use of cocaine is associated with broad cognitive deficits. Research is inconclusive on age-related loss of striatal
Although it has been commonly asserted, the available evidence does not show that chronic use of cocaine is associated with broad cognitive deficits. Research is inconclusive on age-related loss of striatal dopamine transporter
The dopamine transporter (also dopamine active transporter, DAT, SLC6A3) is a membrane-spanning protein that pumps the neurotransmitter dopamine out of the synaptic cleft back into cytosol. In the cytosol, other transporters sequester the dopam ...
(DAT) sites, suggesting cocaine has neuroprotective or neurodegenerative properties for dopamine neurons. Exposure to cocaine may lead to the breakdown of the blood–brain barrier.
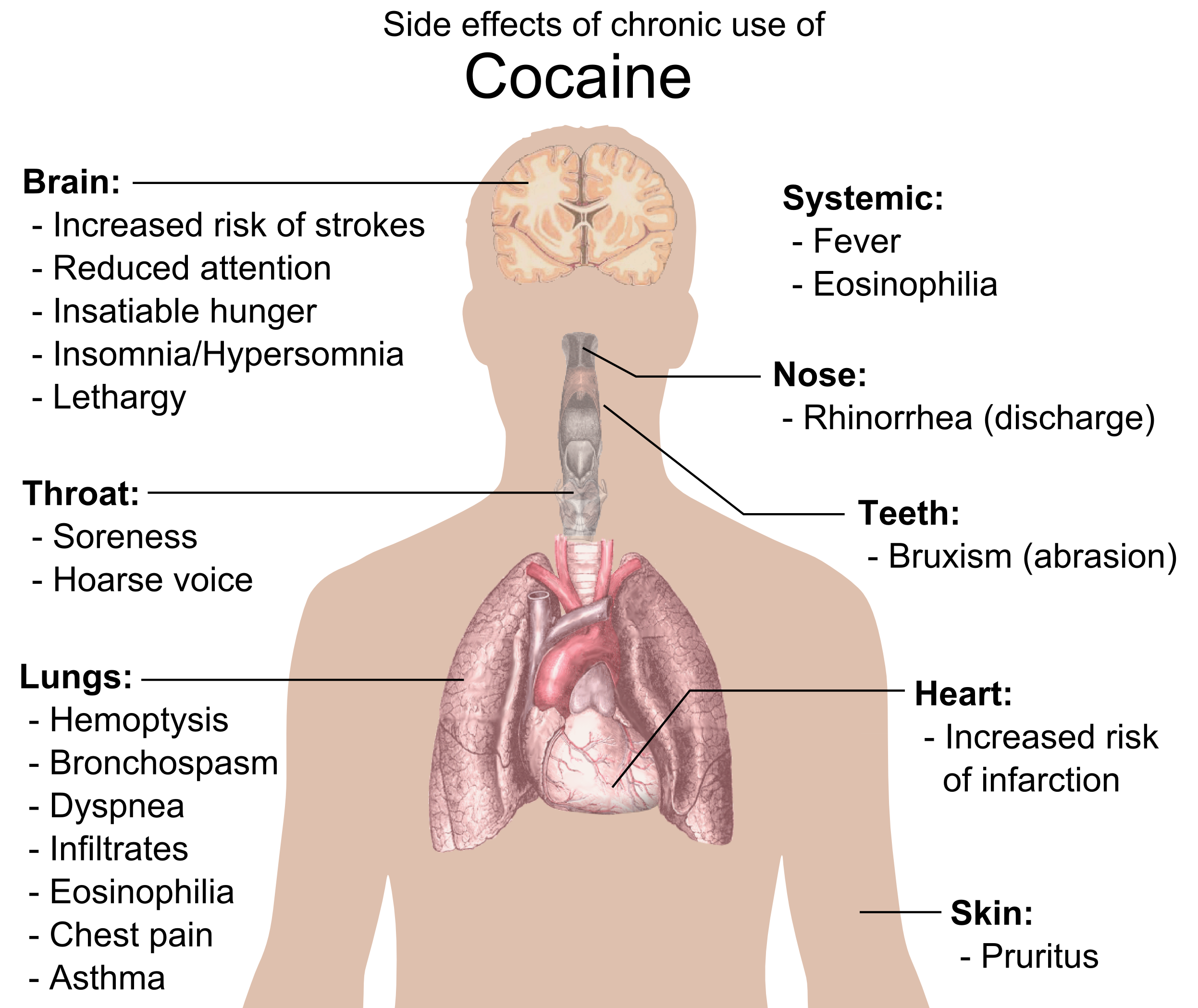
Physical side effects from chronic smoking of cocaine include coughing up blood, bronchospasm
Bronchospasm or a bronchial spasm is a sudden constriction of the muscles in the walls of the bronchioles. It is caused by the release (degranulation) of substances from mast cells or basophils under the influence of anaphylatoxins. It causes di ...
, itching
Itch (also known as pruritus) is a sensation that causes the desire or reflex to scratch. Itch has resisted many attempts to be classified as any one type of sensory experience. Itch has many similarities to pain, and while both are unpleasan ...
, fever
Fever, also referred to as pyrexia, is defined as having a body temperature, temperature above the human body temperature, normal range due to an increase in the body's temperature Human body temperature#Fever, set point. There is not a single ...
, diffuse alveolar infiltrates without effusions, pulmonary and systemic eosinophilia, chest pain, lung trauma, sore throat, asthma
Asthma is a long-term inflammatory disease of the airways of the lungs. It is characterized by variable and recurring symptoms, reversible airflow obstruction, and easily triggered bronchospasms. Symptoms include episodes of wheezing, cou ...
, hoarse voice, dyspnea
Shortness of breath (SOB), also medically known as dyspnea (in AmE) or dyspnoea (in BrE), is an uncomfortable feeling of not being able to breathing, breathe well enough. The American Thoracic Society defines it as "a subjective experience of brea ...
(shortness of breath), and an aching, flu
Influenza, commonly known as "the flu", is an infectious disease caused by influenza viruses. Symptoms range from mild to severe and often include fever, runny nose, sore throat, muscle pain, headache, coughing, and fatigue. These symptom ...
-like syndrome. Cocaine constricts blood vessels, dilates pupils, and increases body temperature, heart rate, and blood pressure. It can also cause headaches and gastrointestinal complications such as abdominal pain and nausea. A common but untrue belief is that the smoking of cocaine chemically breaks down tooth enamel
Tooth enamel is one of the four major Tissue (biology), tissues that make up the tooth in humans and many other animals, including some species of fish. It makes up the normally visible part of the tooth, covering the Crown (tooth), crown. The ...
and causes tooth decay
Tooth decay, also known as cavities or caries, is the breakdown of teeth due to acids produced by bacteria. The cavities may be a number of different colors from yellow to black. Symptoms may include pain and difficulty with eating. Complicatio ...
. Cocaine can cause involuntary tooth grinding, known as bruxism
Bruxism is excessive teeth grinding or jaw clenching. It is an oral parafunctional activity; i.e., it is unrelated to normal function such as eating or talking. Bruxism is a common behavior; reports of prevalence range from 8% to 31% in the gene ...
, which can deteriorate tooth enamel and lead to gingivitis
Gingivitis is a non-destructive disease that causes inflammation of the gums. The most common form of gingivitis, and the most common form of periodontal disease overall, is in response to bacterial biofilms (also called plaque) that is attached ...
. Additionally, stimulants like cocaine, methamphetamine, and even caffeine cause dehydration and dry mouth
Xerostomia, also known as dry mouth, is dryness in the mouth, which may be associated with a change in the composition of saliva, or reduced salivary flow, or have no identifiable cause.
This symptom is very common and is often seen as a side eff ...
. Since saliva is an important mechanism in maintaining one's oral pH level, people who use cocaine over a long period of time who do not hydrate sufficiently may experience demineralization of their teeth due to the pH of the tooth surface dropping too low (below 5.5). Cocaine use also promotes the formation of blood clots.platelet
Platelets, also called thrombocytes (from Greek θρόμβος, "clot" and κύτος, "cell"), are a component of blood whose function (along with the coagulation factors) is to react to bleeding from blood vessel injury by clumping, thereby ini ...
s.cartilage
Cartilage is a resilient and smooth type of connective tissue. In tetrapods, it covers and protects the ends of long bones at the joints as articular cartilage, and is a structural component of many body parts including the rib cage, the neck an ...
separating the nostrils
A nostril (or naris , plural ''nares'' ) is either of the two orifices of the nose. They enable the entry and exit of air and other gasses through the nasal cavities. In birds and mammals, they contain branched bones or cartilages called turbi ...
(the septum nasi), leading eventually to its complete disappearance. Due to the absorption of the cocaine from cocaine hydrochloride, the remaining hydrochloride forms a dilute hydrochloric acid.stroke
A stroke is a medical condition in which poor blood flow to the brain causes cell death. There are two main types of stroke: ischemic, due to lack of blood flow, and hemorrhagic, due to bleeding. Both cause parts of the brain to stop functionin ...
s.heart attack
A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow decreases or stops to the coronary artery of the heart, causing damage to the heart muscle. The most common symptom is chest pain or discomfort which may tr ...
.
Addiction
Relatives of persons with cocaine addiction have an increased risk of cocaine addiction. Cocaine addiction
Cocaine dependence is a neurological disorder that is characterized by withdrawal symptoms upon cessation from cocaine use. It also often coincides with cocaine addiction which is a biopsychosocial disorder characterized by persistent use of coc ...
occurs through ΔFosB
Protein fosB, also known as FosB and G0/G1 switch regulatory protein 3 (G0S3), is a protein that in humans is encoded by the FBJ murine osteosarcoma viral oncogene homolog B (''FOSB'') gene.
The FOS gene family consists of four members: FOS, F ...
overexpression in the nucleus accumbens
The nucleus accumbens (NAc or NAcc; also known as the accumbens nucleus, or formerly as the ''nucleus accumbens septi'', Latin for "nucleus adjacent to the septum") is a region in the basal forebrain rostral to the preoptic area of the hypotha ...
, which results in altered transcriptional regulation
In molecular biology and genetics, transcriptional regulation is the means by which a cell regulates the conversion of DNA to RNA (transcription), thereby orchestrating gene activity. A single gene can be regulated in a range of ways, from alt ...
in neurons within the nucleus accumbens
The nucleus accumbens (NAc or NAcc; also known as the accumbens nucleus, or formerly as the ''nucleus accumbens septi'', Latin for "nucleus adjacent to the septum") is a region in the basal forebrain rostral to the preoptic area of the hypotha ...
. ΔFosB levels have been found to increase upon the use of cocaine.BDNF
Brain-derived neurotrophic factor (BDNF), or abrineurin, is a protein found in the and the periphery. that, in humans, is encoded by the ''BDNF'' gene. BDNF is a member of the neurotrophin family of growth factors, which are related to the cano ...
) levels, which in turn increases the number of dendritic
Dendrite derives from the Greek word "dendron" meaning ( "tree-like"), and may refer to:
Biology
*Dendrite, a branched projection of a neuron
*Dendrite (non-neuronal), branching projections of certain skin cells and immune cells
Physical
* Dendr ...
branches and spines present on neurons involved with the nucleus accumbens and prefrontal cortex
In mammalian brain anatomy, the prefrontal cortex (PFC) covers the front part of the frontal lobe of the cerebral cortex. The PFC contains the Brodmann areas BA8, BA9, BA10, BA11, BA12, BA13, BA14, BA24, BA25, BA32, BA44, BA45, BA46, ...
areas of the brain. This change can be identified rather quickly, and may be sustained weeks after the last dose of the drug.
Transgenic mice exhibiting inducible expression of ΔFosB primarily in the nucleus accumbens and dorsal striatum
The striatum, or corpus striatum (also called the striate nucleus), is a nucleus (a cluster of neurons) in the subcortical basal ganglia of the forebrain. The striatum is a critical component of the motor and reward systems; receives glutama ...
exhibit sensitized behavioural responses to cocaine.relapse
In internal medicine, relapse or recidivism is a recurrence of a past (typically medical) condition. For example, multiple sclerosis and malaria often exhibit peaks of activity and sometimes very long periods of dormancy, followed by relapse or r ...
when the drug is withheld.AMPA receptor
The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (also known as AMPA receptor, AMPAR, or quisqualate receptor) is an ionotropic receptor, ionotropic transmembrane receptor for glutamate (iGluR) that mediates fast synapse, synap ...
subunit GluR2dynorphin
Dynorphins (Dyn) are a class of opioid peptides that arise from the precursor protein prodynorphin. When prodynorphin is cleaved during processing by proprotein convertase 2 (PC2), multiple active peptides are released: dynorphin A, dynorphin B, a ...
, thereby enhancing sensitivity to reward.DNA damage
DNA repair is a collection of processes by which a cell identifies and corrects damage to the DNA molecules that encode its genome. In human cells, both normal metabolic activities and environmental factors such as radiation can cause DNA da ...
is increased in the brain of rodents by administration of cocaine.DNA repair
DNA repair is a collection of processes by which a cell identifies and corrects damage to the DNA molecules that encode its genome. In human cells, both normal metabolic activities and environmental factors such as radiation can cause DNA dam ...
of such damages, persistent chromatin alterations may occur such as methylation of DNA or the acetylation or methylation of histones at the sites of repair.chromatin
Chromatin is a complex of DNA and protein found in eukaryotic cells. The primary function is to package long DNA molecules into more compact, denser structures. This prevents the strands from becoming tangled and also plays important roles in r ...
that contribute to the persistent epigenetic changes found in cocaine addiction
Cocaine dependence is a neurological disorder that is characterized by withdrawal symptoms upon cessation from cocaine use. It also often coincides with cocaine addiction which is a biopsychosocial disorder characterized by persistent use of coc ...
.
Dependence and withdrawal
Cocaine dependence
Cocaine dependence is a neurological disorder that is characterized by withdrawal symptoms upon cessation from cocaine use. It also often coincides with cocaine addiction which is a biopsychosocial disorder characterized by persistent use of coc ...
develops after even brief periods of regular cocaine use and produces a withdrawal
Withdrawal means "an act of taking out" and may refer to:
* Anchoresis (withdrawal from the world for religious or ethical reasons)
* ''Coitus interruptus'' (the withdrawal method)
* Drug withdrawal
* Social withdrawal
* Taking of money from a ban ...
state with emotional-motivational deficits upon cessation of cocaine use.
During pregnancy
''Crack baby'' is a term for a child born to a mother who used crack cocaine during her pregnancy. The threat that cocaine use during pregnancy
Pregnancy is the time during which one or more offspring develops ( gestates) inside a woman's uterus (womb). A multiple pregnancy involves more than one offspring, such as with twins.
Pregnancy usually occurs by sexual intercourse, but ca ...
poses to the fetus
A fetus or foetus (; plural fetuses, feti, foetuses, or foeti) is the unborn offspring that develops from an animal embryo. Following embryonic development the fetal stage of development takes place. In human prenatal development, fetal deve ...
is now considered exaggerated. Studies show that prenatal cocaine exposure (independent of other effects such as, for example, alcohol, tobacco, or physical environment) has no appreciable effect on childhood growth and development.
However, the official opinion of the National Institute on Drug Abuse
The National Institute on Drug Abuse (NIDA) is a United States federal government research institute whose mission is to "advance science on the causes and consequences of drug use and addiction and to apply that knowledge to improve individual a ...
of the United States warns about health risks while cautioning against stereotyping:
There are also warnings about the threat of breastfeeding
Breastfeeding, or nursing, is the process by which human breast milk is fed to a child. Breast milk may be from the breast, or may be expressed by hand or pumped and fed to the infant. The World Health Organization (WHO) recommends that br ...
: The March of Dimes
March of Dimes is a United States nonprofit organization that works to improve the health of mothers and babies. The organization was founded by President Franklin D. Roosevelt in 1938, as the National Foundation for Infantile Paralysis, to comba ...
said "it is likely that cocaine will reach the baby through breast milk," and advises the following regarding cocaine use during pregnancy:
Mortality
Persons with regular or problematic use of cocaine have a significantly higher rate of death, and are specifically at higher risk of traumatic deaths and deaths attributable to infectious disease.
Pharmacology
Pharmacokinetics
The extent of absorption of cocaine into the systemic circulation after nasal insufflation is similar to that after oral ingestion. The rate of absorption after nasal insufflation is limited by cocaine-induced vasoconstriction of capillaries in the nasal mucosa. Onset of absorption after oral ingestion is delayed because cocaine is a weak base with a pKa of 8.6, and is thus in an ionized form that is poorly absorbed from the acidic stomach and easily absorbed from the alkaline duodenum.blood–brain barrier
The blood–brain barrier (BBB) is a highly selective semipermeable membrane, semipermeable border of endothelium, endothelial cells that prevents solutes in the circulating blood from ''non-selectively'' crossing into the extracellular fluid of ...
via both a proton-coupled organic cation antiportermetabolized
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cell ...
by plasma
Plasma or plasm may refer to:
Science
* Plasma (physics), one of the four fundamental states of matter
* Plasma (mineral), a green translucent silica mineral
* Quark–gluon plasma, a state of matter in quantum chromodynamics
Biology
* Blood pla ...
esterases and also by liver cholinesterase
The enzyme cholinesterase (EC 3.1.1.8, choline esterase; systematic name acylcholine acylhydrolase) catalyses the hydrolysis of choline-based esters:
: an acylcholine + H2O = choline + a carboxylate
Several of these serve as neurotransmitters ...
s, with only about 1% excreted unchanged in the urine.hydrolytic
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysi ...
ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
cleavage, so the eliminated metabolites consist mostly of benzoylecgonine
Benzoylecgonine is the main metabolite of cocaine, formed by the liver and excreted in the urine. It is the compound tested for in most cocaine urine drug screens.
Pharmacokinetics
Chemically, benzoylecgonine is the benzoate ester of ecgonine. I ...
(BE), the major metabolite
In biochemistry, a metabolite is an intermediate or end product of metabolism.
The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, c ...
, and other metabolites in lesser amounts such as ecgonine methyl ester (EME) and ecgonine
Ecgonine (tropane derivative) is a tropane alkaloid found naturally in coca leaves. It has a close structural relation to cocaine: it is both a metabolite and a precursor, and as such, it is a controlled substance in many jurisdictions, as are so ...
.norcocaine
Norcocaine is a minor metabolite of cocaine. It is the only confirmed pharmacologically active metabolite of cocaine, although salicylmethylecgonine
Salicylmethylecgonine, (2′-Hydroxycocaine) is a tropane derivative drug which is both a syn ...
, p-hydroxycocaine, m-hydroxycocaine, p-hydroxybenzoylecgonine (pOHBE), and m-hydroxybenzoylecgonine. If consumed with alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
, cocaine combines with alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
in the liver
The liver is a major Organ (anatomy), organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of proteins and biochemicals necessary for ...
to form cocaethylene
Cocaethylene (ethylbenzoylecgonine) is the ethyl ester of benzoylecgonine. It is structurally similar to cocaine, which is the methyl ester of benzoylecgonine. Cocaethylene is formed by the liver when cocaine and ethanol coexist in the blood. In ...
.euphoric
Euphoria ( ) is the experience (or affect) of pleasure or excitement and intense feelings of well-being and happiness. Certain natural rewards and social activities, such as aerobic exercise, laughter, listening to or making music and da ...
, and has a higher cardiovascular
The blood circulatory system is a system of organs that includes the heart, blood vessels, and blood which is circulated throughout the entire body of a human or other vertebrate. It includes the cardiovascular system, or vascular system, tha ...
toxicity than cocaine by itself.liver
The liver is a major Organ (anatomy), organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of proteins and biochemicals necessary for ...
and kidney
The kidneys are two reddish-brown bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and in adult humans are about in length. They receive blood from the paired renal arteries; blood ...
function, cocaine metabolites are detectable in urine. Benzoylecgonine
Benzoylecgonine is the main metabolite of cocaine, formed by the liver and excreted in the urine. It is the compound tested for in most cocaine urine drug screens.
Pharmacokinetics
Chemically, benzoylecgonine is the benzoate ester of ecgonine. I ...
can be detected in urine within four hours after cocaine intake and remains detectable in concentrations greater than 150 ng/mL typically for up to eight days after cocaine is used. Detection of cocaine metabolite
In biochemistry, a metabolite is an intermediate or end product of metabolism.
The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, c ...
s in hair is possible in regular users until after the sections of hair grown during the period of cocaine use are cut or fall out.
Pharmacodynamics
The pharmacodynamics
Pharmacodynamics (PD) is the study of the biochemical and physiologic effects of drugs (especially pharmaceutical drugs). The effects can include those manifested within animals (including humans), microorganisms, or combinations of organisms (fo ...
of cocaine involve the complex relationships of neurotransmitters (inhibiting monoamine
Monoamine neurotransmitters are neurotransmitters and neuromodulators that contain one amino group connected to an aromatic ring by a two-carbon chain (such as -CH2-CH2-). Examples are dopamine, norepinephrine and serotonin.
All monoamines are ...
uptake in rats with ratios of about: serotonin
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vas ...
:dopamine = 2:3, serotonin:norepinephrine
Norepinephrine (NE), also called noradrenaline (NA) or noradrenalin, is an organic chemical in the catecholamine family that functions in the brain and body as both a hormone and neurotransmitter. The name "noradrenaline" (from Latin '' ad'', ...
= 2:5).[ The most extensively studied effect of cocaine on the ]central nervous system
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain and spinal cord. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all par ...
is the blockade of the dopamine transporter
The dopamine transporter (also dopamine active transporter, DAT, SLC6A3) is a membrane-spanning protein that pumps the neurotransmitter dopamine out of the synaptic cleft back into cytosol. In the cytosol, other transporters sequester the dopam ...
protein. Dopamine neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, any main body part or target cell, may be another neuron, but could also be a gland or muscle cell.
Neuro ...
released during neural signaling is normally recycled via the transporter; i.e., the transporter binds the transmitter and pumps it out of the synaptic cleft back into the presynaptic
In the nervous system, a synapse is a structure that permits a neuron (or nerve cell) to pass an electrical or chemical signal to another neuron or to the target effector cell.
Synapses are essential to the transmission of nervous impulses from ...
neuron
A neuron, neurone, or nerve cell is an electrically excitable cell that communicates with other cells via specialized connections called synapses. The neuron is the main component of nervous tissue in all animals except sponges and placozoa. N ...
, where it is taken up into storage vesicles
Vesicle may refer to:
; In cellular biology or chemistry
* Vesicle (biology and chemistry), a supramolecular assembly of lipid molecules, like a cell membrane
* Synaptic vesicle
; In human embryology
* Vesicle (embryology), bulge-like features o ...
. Cocaine binds tightly at the dopamine transporter forming a complex that blocks the transporter's function. The dopamine transporter can no longer perform its reuptake function, and thus dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic compound, organic chemical of the catecholamine and phenethylamine families. Dopamine const ...
accumulates in the synaptic cleft
Chemical synapses are biological junctions through which neurons' signals can be sent to each other and to non-neuronal cells such as those in muscles or glands. Chemical synapses allow neurons to form circuits within the central nervous syste ...
. The increased concentration of dopamine in the synapse activates post-synaptic dopamine receptors, which makes the drug rewarding and promotes the compulsive use of cocaine.
Cocaine affects certain serotonin (5-HT) receptors; in particular, it has been shown to antagonize the 5-HT3 receptor
The 5-HT3 receptor belongs to the Cys-loop superfamily of ligand-gated ion channels (LGICs) and therefore differs structurally and functionally from all other 5-HT receptors (5-hydroxytryptamine, or serotonin receptors) which are G protein-coupled ...
, which is a ligand-gated ion channel
Ligand-gated ion channels (LICs, LGIC), also commonly referred to as ionotropic receptors, are a group of transmembrane ion-channel proteins which open to allow ions such as Na+, K+, Ca2+, and/or Cl− to pass through the membrane in res ...
. An overabundance of 5-HT3 receptors is reported in cocaine-conditioned rats, though 5-HT3's role is unclear. The 5-HT2 receptor
The 5-HT2 receptors are a subfamily of 5-HT receptors that bind the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). The 5-HT2 subfamily consists of three G protein-coupled receptors (GPCRs) which are coupled to Gq/G11 and m ...
(particularly the subtypes 5-HT2A
The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations. 5-HT is s ...
, 5-HT2B
5-Hydroxytryptamine receptor 2B (5-HT2B) also known as serotonin receptor 2B is a protein that in humans is encoded by the ''HTR2B'' gene. 5-HT2B is a member of the 5-HT2 receptor family that binds the neurotransmitter serotonin (5-hydroxytrypta ...
and 5-HT2C
The 5-HT2C receptor is a subtype of 5-HT receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). It is a G protein-coupled receptor (GPCR) that is coupled to Gq/G11 and mediates excitatory neurotransmission. ...
) are involved in the locomotor-activating effects of cocaine.
Cocaine has been demonstrated to bind as to directly stabilize the DAT transporter on the open outward-facing conformation. Further, cocaine binds in such a way as to inhibit a hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
bond innate to DAT. Cocaine's binding properties are such that it attaches so this hydrogen bond will not form and is blocked from formation due to the tightly locked orientation of the cocaine molecule. Research studies have suggested that the affinity for the transporter is not what is involved in the habituation of the substance so much as the conformation and binding properties to where and how on the transporter the molecule binds.
Sigma receptor
Sigma receptors (σ-receptors) are protein cell surface receptors that bind ligands such as 4-PPBP (4-phenyl-1-(4-phenylbutyl) piperidine), SA 4503 (cutamesine), ditolylguanidine, dimethyltryptamine, and siramesine. There are two subtypes, ...
s are affected by cocaine, as cocaine functions as a sigma ligand agonist. Further specific receptors it has been demonstrated to function on are NMDA
''N''-methyl--aspartic acid or ''N''-methyl--aspartate (NMDA) is an amino acid derivative that acts as a specific agonist at the NMDA receptor mimicking the action of glutamate, the neurotransmitter which normally acts at that receptor. Unlike ...
and the D1 dopamine receptor.
Cocaine also blocks sodium channels
Sodium channels are integral membrane proteins that form ion channels, conducting sodium ions (Na+) through a cell's membrane. They belong to the superfamily of cation channels and can be classified according to the trigger that opens the chann ...
, thereby interfering with the propagation of action potential
An action potential occurs when the membrane potential of a specific cell location rapidly rises and falls. This depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of animal cells, ...
s;lignocaine
Lidocaine, also known as lignocaine and sold under the brand name Xylocaine among others, is a local anesthetic of the amino amide type. It is also used to treat ventricular tachycardia. When used for local anaesthesia or in nerve blocks, lidoca ...
and novocaine
Procaine is a local anesthetic drug of the amino ester group. It is most commonly used in dental procedures to numb the area around a tooth and is also used to reduce the pain of intramuscular injection of penicillin. Owing to the ubiquity of ...
, it acts as a local anesthetic. It also functions on the binding sites to the dopamine and serotonin sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
dependent transport area as targets as separate mechanisms from its reuptake of those transporters; unique to its local anesthetic value which makes it in a class of functionality different from both its own derived phenyltropane
Phenyltropanes (PTs) were originally developed to reduce cocaine addiction and dependency. In general these compounds act as inhibitors of the plasmalemmal monoamine reuptake transporters. Although RTI holds a strong position in this field, the ...
s analogues which have that removed. In addition to this, cocaine has some target binding to the site of the Kappa-opioid receptor. Cocaine also causes vasoconstriction
Vasoconstriction is the narrowing of the blood vessels resulting from contraction of the muscular wall of the vessels, in particular the large arteries and small arterioles. The process is the opposite of vasodilation, the widening of blood vessel ...
, thus reducing bleeding during minor surgical procedures. Recent research points to an important role of circadian mechanisms and clock genes
A clock or a timepiece is a device used to measure and indicate time. The clock is one of the oldest human inventions, meeting the need to measure intervals of time shorter than the natural units such as the day, the lunar month and th ...
in behavioral actions of cocaine.
Cocaine is known to suppress hunger and appetite by increasing co-localization of sigma σ1R receptors and ghrelin GHS-R1a receptors at the neuronal cell surface, thereby increasing ghrelin-mediated signaling of satiety and possibly via other effects on appetitive hormones. Chronic users may lose their appetite
Appetite is the desire to eat food items, usually due to hunger. Appealing foods can stimulate appetite even when hunger is absent, although appetite can be greatly reduced by satiety. Appetite exists in all higher life-forms, and serves to regu ...
and can experience severe malnutrition
Malnutrition occurs when an organism gets too few or too many nutrients, resulting in health problems. Specifically, it is "a deficiency, excess, or imbalance of energy, protein and other nutrients" which adversely affects the body's tissues a ...
and significant weight loss.
Cocaine effects, further, are shown to be potentiated for the user when used in conjunction with new surroundings and stimuli, and otherwise novel environs.
Chemistry
Appearance

 Cocaine in its purest form is a white, pearly product. Cocaine appearing in powder form is a
Cocaine in its purest form is a white, pearly product. Cocaine appearing in powder form is a salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
, typically cocaine hydrochloride
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base (e.g. an amine). An alternative name is chlorhydrate, which comes from French. An archaic alternative na ...
. Street cocaine is often adulterated or "cut" with talc
Talc, or talcum, is a Clay minerals, clay mineral, composed of hydrated magnesium silicate with the chemical formula Mg3Si4O10(OH)2. Talc in powdered form, often combined with corn starch, is used as baby powder. This mineral is used as a thi ...
, lactose
Lactose is a disaccharide sugar synthesized by galactose and glucose subunits and has the molecular formula C12H22O11. Lactose makes up around 2–8% of milk (by mass). The name comes from ' (gen. '), the Latin word for milk, plus the suffix '' - ...
, sucrose
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula .
For human consumption, sucrose is extracted and refined ...
, glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using ...
, mannitol
Mannitol is a type of sugar alcohol used as a sweetener and medication. It is used as a low calorie sweetener as it is poorly absorbed by the intestines. As a medication, it is used to decrease pressure in the eyes, as in glaucoma, and to lower ...
, inositol
Inositol, or more precisely ''myo''-inositol, is a carbocyclic sugar that is abundant in the brain and other mammalian tissues; it mediates cell signal transduction in response to a variety of hormones, neurotransmitters, and growth factors and ...
, caffeine
Caffeine is a central nervous system (CNS) stimulant of the methylxanthine class. It is mainly used recreationally as a cognitive enhancer, increasing alertness and attentional performance. Caffeine acts by blocking binding of adenosine t ...
, procaine
Procaine is a local anesthetic drug of the amino ester group. It is most commonly used in dental procedures to numb the area around a tooth and is also used to reduce the pain of intramuscular injection of penicillin. Owing to the ubiquity ...
, phencyclidine
Phencyclidine or phenylcyclohexyl piperidine (PCP), also known as angel dust among other names, is a dissociative anesthetic mainly used recreationally for its significant mind-altering effects. PCP may cause hallucinations, distorted perceptio ...
, phenytoin
Phenytoin (PHT), sold under the brand name Dilantin among others, is an anti-seizure medication. It is useful for the prevention of tonic-clonic seizures (also known as grand mal seizures) and focal seizures, but not absence seizures. The intr ...
, lignocaine
Lidocaine, also known as lignocaine and sold under the brand name Xylocaine among others, is a local anesthetic of the amino amide type. It is also used to treat ventricular tachycardia. When used for local anaesthesia or in nerve blocks, lidoca ...
, strychnine
Strychnine (, , US chiefly ) is a highly toxic, colorless, bitter, crystalline alkaloid used as a pesticide, particularly for killing small vertebrates such as birds and rodents. Strychnine, when inhaled, swallowed, or absorbed through the eye ...
, levamisole
Levamisole, sold under the brand name Ergamisol among others, is a medication used to treat parasitic worm infections, specifically ascariasis and hookworm infections. It is taken by mouth.
Side effects may include abdominal pain, vomiting, ...
, amphetamine
Amphetamine (contracted from alpha- methylphenethylamine) is a strong central nervous system (CNS) stimulant that is used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, and obesity. It is also commonly used ...
, or heroin
Heroin, also known as diacetylmorphine and diamorphine among other names, is a potent opioid mainly used as a recreational drug for its euphoric effects. Medical grade diamorphine is used as a pure hydrochloride salt. Various white and brow ...
.
The color of "crack" cocaine depends upon several factors including the origin of the cocaine used, the method of preparation – with ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
or baking soda
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−) ...
– and the presence of impurities. It will generally range from white to a yellowish cream to a light brown. Its texture will also depend on the adulterants, origin, and processing of the powdered cocaine, and the method of converting the base. It ranges from a crumbly texture, sometimes extremely oily, to a hard, almost crystalline nature.
Forms
Salts
Cocaine – a tropane alkaloid
Tropane alkaloids are a class of bicyclic .2.1alkaloids and secondary metabolites that contain a tropane ring in their chemical structure. Tropane alkaloids occur naturally in many members of the plant family Solanaceae. Certain tropane alkal ...
– is a weakly alkaline compound, and can therefore combine with acidic compounds to form salts. The hydrochloride
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base (e.g. an amine). An alternative name is chlorhydrate, which comes from French. An archaic alternative na ...
(HCl) salt of cocaine is by far the most commonly encountered, although the sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ar ...
(SO42-) and the nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
(NO3−) salts are occasionally seen. Different salts dissolve to a greater or lesser extent in various solvents – the hydrochloride salt is polar in character and is quite soluble in water.
Base
As the name implies, "freebase" is the base form of cocaine, as opposed to the salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
form. It is practically insoluble in water whereas hydrochloride salt is water-soluble.
Smoking freebase cocaine has the additional effect of releasing methylecgonidine
Methylecgonidine (anhydromethylecgonine; anhydroecgonine methyl ester; AEME) is a chemical intermediate derived from ecgonine or cocaine.
Methylecgonidine is a pyrolysis product formed when crack cocaine is smoked, making this substance a useful ...
into the user's system due to the pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''py ...
of the substance (a side effect which insufflating or injecting powder cocaine does not create). Some research suggests that smoking freebase cocaine can be even more cardiotoxic than other routes of administration
A route of administration in pharmacology and toxicology is the way by which a medication, drug, fluid, poison, or other substance is taken into the body.
Routes of administration are generally classified by the location at which the substance i ...
because of methylecgonidine's effects on lung tissue and liver tissue.
Pure cocaine is prepared by neutralizing its compounding salt with an alkaline solution, which will precipitate non-polar basic cocaine. It is further refined through aqueous-solvent liquid–liquid extraction
Liquid–liquid extraction (LLE), also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubilities in two different immiscible liquids, usually water (polar) and an org ...
.
Crack cocaine

 Crack is usually smoked in a glass pipe, and once inhaled, it passes from the lungs directly to the
Crack is usually smoked in a glass pipe, and once inhaled, it passes from the lungs directly to the central nervous system
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain and spinal cord. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all par ...
, producing an almost immediate "high" that can be very powerful – this initial crescendo of stimulation is known as a "rush". This is followed by an equally intense low, leaving the user craving more drug. Addiction to crack usually occurs with four to six weeks; much more rapidly than with regular cocaine.onomatopoeic
Onomatopoeia is the process of creating a word that phonetically imitates, resembles, or suggests the sound that it describes. Such a word itself is also called an onomatopoeia. Common onomatopoeias include animal noises such as ''oink'', ''m ...
moniker "crack") that is produced when the cocaine and its impurities (i.e. water, sodium bicarbonate) are heated past the point of vaporization.
Coca leaf infusions
Coca herbal infusion
Infusion is the process of extracting chemical compounds or flavors from plant material in a solvent such as water, oil or alcohol, by allowing the material to remain suspended in the solvent over time (a process often called steeping). An inf ...
(also referred to as coca tea
Coca tea, also called mate de coca, is an herbal tea (infusion) made using the raw or dried leaves of the coca plant, which is native to South America. It is made either by submerging the coca leaf or dipping a tea bag in hot water. The tea is mo ...
) is used in coca-leaf producing countries much as any herbal medicinal infusion would elsewhere in the world. The free and legal commercialization of dried coca leaves under the form of filtration bags to be used as "coca tea" has been actively promoted by the governments of Peru
, image_flag = Flag of Peru.svg
, image_coat = Escudo nacional del Perú.svg
, other_symbol = Great Seal of the State
, other_symbol_type = Seal (emblem), National seal
, national_motto = "Fi ...
and Bolivia
, image_flag = Bandera de Bolivia (Estado).svg
, flag_alt = Horizontal tricolor (red, yellow, and green from top to bottom) with the coat of arms of Bolivia in the center
, flag_alt2 = 7 × 7 square p ...
for many years as a drink having medicinal powers. In Peru, the National Coca Company
The National Company of the Coca (Spanish: ''Empresa Nacional de la Coca'', ENACO) is a Peruvian state company dedicated to the commercialization of the coca leaf and derivatives. It is the only state company that has a monopoly on the commercializ ...
, a state-run corporation, sells cocaine-infused teas and other medicinal products and also exports leaves to the U.S. for medicinal use.
Visitors to the city of Cuzco
Cusco, often spelled Cuzco (; qu, Qusqu ()), is a city in Southeastern Peru near the Urubamba Valley of the Andes mountain range. It is the capital of the Cusco Region and of the Cusco Province. The city is the seventh most populous in Peru; ...
in Peru, and La Paz
La Paz (), officially known as Nuestra Señora de La Paz (Spanish pronunciation: ), is the seat of government of the Bolivia, Plurinational State of Bolivia. With an estimated 816,044 residents as of 2020, La Paz is the List of Bolivian cities ...
in Bolivia are greeted with the offering of coca leaf infusions (prepared in teapots with whole coca leaves) purportedly to help the newly arrived traveler overcome the malaise of high altitude sickness.Lima
Lima ( ; ), originally founded as Ciudad de Los Reyes (City of The Kings) is the capital and the largest city of Peru. It is located in the valleys of the Chillón River, Chillón, Rímac River, Rímac and Lurín Rivers, in the desert zone of t ...
, Peru found that the relapses rate fell from 4.35 times per month on average before coca tea treatment to one during treatment. The duration of abstinence increased from an average of 32 days before treatment to 217.2 days during treatment. This suggests that coca leaf infusion plus counseling may be effective at preventing relapse during cocaine addiction treatment.solid-phase extraction
Solid-phase extraction (SPE) is an extractive technique by which compounds that are dissolved or suspended in a liquid mixture are separated from other compounds in the mixture according to their physical and chemical properties. Analytical labor ...
and gas chromatography–mass spectrometry
Gas chromatography–mass spectrometry (GC-MS) is an analytical method that combines the features of gas-chromatography and mass spectrometry to identify different substances within a test sample. Applications of GC-MS include drug detection, fir ...
(SPE-GC/MS) of Peruvian and Bolivian tea bags indicated the presence of significant amounts of cocaine, the metabolite benzoylecgonine
Benzoylecgonine is the main metabolite of cocaine, formed by the liver and excreted in the urine. It is the compound tested for in most cocaine urine drug screens.
Pharmacokinetics
Chemically, benzoylecgonine is the benzoate ester of ecgonine. I ...
, ecgonine methyl ester and trans-cinnamoylcocaine in coca tea bags and coca tea. Urine
Urine is a liquid by-product of metabolism in humans and in many other animals. Urine flows from the kidneys through the ureters to the urinary bladder. Urination results in urine being excretion, excreted from the body through the urethra.
Cel ...
specimens were also analyzed from an individual who consumed one cup of coca tea and it was determined that enough cocaine and cocaine-related metabolites were present to produce a positive drug test.
Synthesis
Biosynthesis
 The first synthesis and elucidation of the cocaine molecule was by
The first synthesis and elucidation of the cocaine molecule was by Richard Willstätter
Richard Martin Willstätter FRS(For) HFRSE (, 13 August 1872 – 3 August 1942) was a German organic chemist whose study of the structure of plant pigments, chlorophyll included, won him the 1915 Nobel Prize for Chemistry. Willstätter invented ...
in 1898.tropinone
Tropinone is an alkaloid, famously synthesised in 1917 by Robert Robinson as a synthetic precursor to atropine, a scarce commodity during World War I. Tropinone and the alkaloids cocaine and atropine all share the same tropane core structure. It ...
. Since then, Robert Robinson and Edward Leete have made significant contributions to the mechanism of the synthesis. (-NO3)
The additional carbon atoms required for the synthesis of cocaine are derived from acetyl-CoA, by addition of two acetyl-CoA units to the ''N''-methyl-Δ1-pyrrolinium cation. The first addition is a Mannich-like reaction with the enolate anion from acetyl-CoA acting as a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
towards the pyrrolinium cation. The second addition occurs through a Claisen condensation. This produces a racemic mixture of the 2-substituted pyrrolidine, with the retention of the thioester from the Claisen condensation. In formation of tropinone
Tropinone is an alkaloid, famously synthesised in 1917 by Robert Robinson as a synthetic precursor to atropine, a scarce commodity during World War I. Tropinone and the alkaloids cocaine and atropine all share the same tropane core structure. It ...
from racemic
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
ethyl ,3-13C2sub>4(Nmethyl-2-pyrrolidinyl)-3-oxobutanoate there is no preference for either stereoisomer. In cocaine biosynthesis, only the (S)-enantiomer can cyclize to form the tropane ring system of cocaine. The stereoselectivity of this reaction was further investigated through study of prochiral methylene hydrogen discrimination. This is due to the extra chiral center at C-2. This process occurs through an oxidation, which regenerates the pyrrolinium cation and formation of an enolate anion, and an intramolecular Mannich reaction. The tropane ring system undergoes hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
, SAM-dependent methylation, and reduction via NADPH
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NAD ...
for the formation of methylecgonine
Methylecgonine is a prominent tropane alkaloid found in coca leaves. It is metabolite of cocaine, and maybe is used as precursor for it. It also occurs as minor alkaloid in roots of many '' Datura'' species such as ''Datura stramonium'' and ''Datu ...
. The benzoyl
In organic chemistry, benzoyl (, ) is the functional group with the formula C6H5CO-. It can be viewed as benzaldehyde missing one hydrogen.
The term "benzoyl" should not be confused with benzyl, which has the formula C6H5CH2. The benzoyl grou ...
moiety required for the formation of the cocaine diester is synthesized from phenylalanine via cinnamic acid. Benzoyl-CoA then combines the two units to form cocaine.
=''N''-methyl-pyrrolinium cation
=
The biosynthesis
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. ...
begins with L-Glutamine
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral, ...
, which is derived to L-ornithine
Ornithine is a non-proteinogenic amino acid that plays a role in the urea cycle. Ornithine is abnormally accumulated in the body in ornithine transcarbamylase deficiency. The radical is ornithyl.
Role in urea cycle
L-Ornithine is one of the produ ...
in plants. The major contribution of L-ornithine and L-arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the am ...
as a precursor to the tropane
Tropane is a nitrogenous Bicyclic molecule, bicyclic organic compound. It is mainly known for the other alkaloids derived from it, which include atropine and cocaine, among others. Tropane alkaloids occur in plants of the families Erythroxylaceae ...
ring was confirmed by Edward Leete. Ornithine then undergoes a pyridoxal phosphate
Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent ac ...
-dependent decarboxylation to form putrescine. In some animals, the urea cycle derives putrescine from ornithine. L-ornithine is converted to L-arginine, which is then decarboxylated via PLP to form agmatine. Hydrolysis of the imine derives ''N''-carbamoylputrescine followed with hydrolysis of the urea to form putrescine. The separate pathways of converting ornithine to putrescine in plants and animals have converged. A SAM-dependent ''N''-methylation of putrescine gives the ''N''-methylputrescine product, which then undergoes oxidative deamination by the action of diamine oxidase to yield the aminoaldehyde. Schiff base formation confirms the biosynthesis of the ''N''-methyl-Δ1-pyrrolinium cation.

=Robert Robinson's acetonedicarboxylate
=
The biosynthesis of the tropane alkaloid
Tropane alkaloids are a class of bicyclic .2.1alkaloids and secondary metabolites that contain a tropane ring in their chemical structure. Tropane alkaloids occur naturally in many members of the plant family Solanaceae. Certain tropane alkal ...
is still not understood. Hemscheidt proposes that Robinson's acetonedicarboxylate emerges as a potential intermediate for this reaction. Condensation of ''N''-methylpyrrolinium and acetonedicarboxylate would generate the oxobutyrate. Decarboxylation leads to tropane alkaloid formation.
=Reduction of tropinone
=
The reduction of tropinone is mediated by NADPH
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NAD ...
-dependent reductase enzymes, which have been characterized in multiple plant species. These plant species all contain two types of the reductase enzymes, tropinone reductase I and tropinone reductase II. TRI produces tropine and TRII produces pseudotropine. Due to differing kinetic and pH/activity characteristics of the enzymes and by the 25-fold higher activity of TRI over TRII, the majority of the tropinone reduction is from TRI to form tropine.

GMO synthesis
=Research
=
In 2022, a GMO produced '' N. benthamiana'' were discovered that were able to produce 25% of the amount of cocaine
Cocaine (from , from , ultimately from Quechuan languages, Quechua: ''kúka'') is a central nervous system (CNS) stimulant mainly recreational drug use, used recreationally for its euphoria, euphoric effects. It is primarily obtained from t ...
found in a coca plant.
Detection in body fluids
Cocaine and its major metabolites may be quantified in blood, plasma, or urine to monitor for use, confirm a diagnosis of poisoning, or assist in the forensic investigation of a traffic or other criminal violation or sudden death. Most commercial cocaine immunoassay screening tests cross-react appreciably with the major cocaine metabolites, but chromatographic techniques can easily distinguish and separately measure each of these substances. When interpreting the results of a test, it is important to consider the cocaine usage history of the individual, since a chronic user can develop tolerance to doses that would incapacitate a cocaine-naive individual, and the chronic user often has high baseline values of the metabolites in his system. Cautious interpretation of testing results may allow a distinction between passive or active usage, and between smoking versus other routes of administration.
Field analysis
Cocaine may be detected by law enforcement using the Scott reagent. The test can easily generate false positives for common substances and must be confirmed with a laboratory test.
Approximate cocaine purity can be determined using 1 mL 2% cupric sulfate pentahydrate in dilute HCl, 1 mL 2% potassium thiocyanate and 2 mL of chloroform
Chloroform, or trichloromethane, is an organic compound with chemical formula, formula Carbon, CHydrogen, HChlorine, Cl3 and a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to ...
. The shade of brown shown by the chloroform is proportional to the cocaine content. This test is not cross sensitive to heroin, methamphetamine, benzocaine, procaine and a number of other drugs but other chemicals could cause false positives.
Usage
According to a 2016 United Nations report, England and Wales
England and Wales () is one of the three legal jurisdictions of the United Kingdom. It covers the constituent countries England and Wales and was formed by the Laws in Wales Acts 1535 and 1542. The substantive law of the jurisdiction is Eng ...
are the countries with the highest rate of cocaine usage (2.4% of adults in the previous year).
Europe
Cocaine is the second most popular illegal recreational drug in Europe (behind cannabis
''Cannabis'' () is a genus of flowering plants in the family Cannabaceae. The number of species within the genus is disputed. Three species may be recognized: ''Cannabis sativa'', '' C. indica'', and '' C. ruderalis''. Alternatively ...
). Since the mid-1990s, overall cocaine usage in Europe has been on the rise, but usage rates and attitudes tend to vary between countries. European countries with the highest usage rates are the United Kingdom, Spain, Italy, and the Republic of Ireland.
Approximately 17 million Europeans (5.1%) have used cocaine at least once and 3.5 million (1.1%) in the last year. About 1.9% (2.3 million) of young adults (15–34 years old) have used cocaine in the last year (latest data available as of 2018).
Usage is particularly prevalent among this demographic: 4% to 7% of males have used cocaine in the last year in Spain, Denmark, the Republic of Ireland, Italy, and the United Kingdom. The ratio of male to female users is approximately 3.8:1, but this statistic varies from 1:1 to 13:1 depending on country.
United States
Cocaine is the second most popular illegal recreational drug in the United States (behind cannabis
''Cannabis'' () is a genus of flowering plants in the family Cannabaceae. The number of species within the genus is disputed. Three species may be recognized: ''Cannabis sativa'', '' C. indica'', and '' C. ruderalis''. Alternatively ...
) and the U.S. is the world's largest consumer of cocaine.disco
Disco is a genre of dance music and a subculture that emerged in the 1970s from the United States' urban nightlife scene. Its sound is typified by four-on-the-floor beats, syncopated basslines, string sections, brass and horns, electric pia ...
culture as cocaine usage was very common and popular in many discos such as Studio 54
Studio 54 is a Broadway theater and a former disco nightclub at 254 West 54th Street in the Midtown Manhattan neighborhood of New York City. Operated by the Roundabout Theatre Company, Studio 54 has 1,006 seats on two levels. The theater was ...
.
Dependence treatment
Treatment with contingency management
Contingency management (CM) is the application of the three-term contingency (or operant conditioning), which uses stimulus control and consequences to change behavior. CM originally derived from the science of applied behavior analysis (ABA), bu ...
, such as providing vouchers for retail items contingent upon objectively-verified abstinence from recent drug use, has been shown in randomized clinical trials to significantly reduce the likelihood of having a positive test for the presence of cocaine.
Vaccine
TA-CD TA-CD is an active vaccine developed by the Xenova Group which is used to negate the effects of cocaine, making it suitable for use in treatment of addiction. It is created by combining norcocaine with inactivated cholera toxin.
It works in much t ...
is an active vaccine
A vaccine is a biological Dosage form, preparation that provides active acquired immunity to a particular infectious disease, infectious or cancer, malignant disease. The safety and effectiveness of vaccines has been widely studied and verifie ...
addiction
Addiction is a neuropsychological disorder characterized by a persistent and intense urge to engage in certain behaviors, one of which is the usage of a drug, despite substantial harm and other negative consequences. Repetitive drug use o ...
. It is created by combining norcocaine
Norcocaine is a minor metabolite of cocaine. It is the only confirmed pharmacologically active metabolite of cocaine, although salicylmethylecgonine
Salicylmethylecgonine, (2′-Hydroxycocaine) is a tropane derivative drug which is both a syn ...
with inactivated cholera
Cholera is an infection of the small intestine by some strains of the bacterium ''Vibrio cholerae''. Symptoms may range from none, to mild, to severe. The classic symptom is large amounts of watery diarrhea that lasts a few days. Vomiting and ...
toxin.
History
Discovery

Indigenous peoples of South America
The Indigenous peoples of South America or South American Indigenous peoples, are the pre-Colombian peoples of South America and their descendants. These peoples contrast with South Americans of European ancestry and those of African descent.
...
have chewed the leaves of ''Erythroxylon coca
Coca is any of the four cultivated plants in the family Erythroxylaceae, native to western South America. Coca is known worldwide for its psychoactive alkaloid, cocaine.
The plant is grown as a cash crop in the Argentine Northwest, Bolivia, ...
'' – a plant that contains vital nutrients as well as numerous alkaloids
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar st ...
, including cocaine – for over a thousand years. The coca leaf was, and still is, chewed almost universally by some indigenous communities. The remains of coca leaves have been found with ancient Peruvian mummies, and pottery from the time period depicts humans with bulged cheeks, indicating the presence of something on which they are chewing.trepanation
Trepanning, also known as trepanation, trephination, trephining or making a burr hole (the verb ''trepan'' derives from Old French from Medieval Latin from Greek , literally "borer, auger"), is a surgical intervention in which a hole is drill ...
.Nicolás Monardes
Nicolás Bautista Monardes (1493 – 10 October 1588) was a Spanish physician and botanist.
Monardes published several books of varying importance. In ''Diálogo llamado pharmacodilosis'' (1536), he examines humanism and suggests studying s ...
described the indigenous peoples' practice of chewing a mixture of tobacco and coca leaves to induce "great contentment":
In 1609, Padre
__NOTOC__
Padre means father in many Romance languages, and it may also refer to:
Music
* "Padre" (song)
People
* A military chaplain
* A Latin Catholic priest
* A member of the San Diego Padres baseball team
Places
* Padre Island, a barrier i ...
Blas Valera
Blas Valera (1544-1597) was a Roman Catholic priest of the Jesuit Order in Peru, a historian, and a linguist. The son of a Spaniard and an indigenous woman, he was one of the first mestizo priests in Peru. He wrote a history of Peru titled ''Hi ...
wrote:
Isolation and naming
Although the stimulant and hunger-suppressant properties of coca had been known for many centuries, the isolation of the cocaine alkaloid
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar ...
was not achieved until 1855. Various European scientists had attempted to isolate cocaine, but none had been successful for two reasons: the knowledge of chemistry required was insufficient at the time, and contemporary conditions of sea-shipping from South America could degrade the cocaine in the plant samples available to European chemists.
The cocaine alkaloid was first isolated by the German chemist Friedrich Gaedcke
Friedrich Georg Carl (Friedrich) Gaedcke (5 June 1828 – 19 September 1890) was a German chemist. He was the first person to isolate the cocaine alkaloid in 1855.
Gaedcke named the alkaloid “erythroxyline,” and published a description in the ...
in 1855. Gaedcke named the alkaloid "erythroxyline", and published a description in the journal ''Archiv der Pharmazie
The ''Archiv der Pharmazie'' (German pronunciation: � arˈçiːf ˈdeːɐ̯ farmaˈtsiː English: ''Archive of Pharmacy'') is a monthly peer-reviewed scientific journal covering all aspects of chemistry in the life sciences. The journal was estab ...
.''
In 1856, Friedrich Wöhler
Friedrich Wöhler () FRS(For) HonFRSE (31 July 180023 September 1882) was a German chemist known for his work in inorganic chemistry, being the first to isolate the chemical elements beryllium and yttrium in pure metallic form. He was the firs ...
asked Dr. Carl Scherzer
Karl Ritter von Scherzer (sometimes written Carl; 1 May 1821 in Vienna – 19 February 1903 in Görz) was an Austrian explorer, diplomat and natural scientist.
Biography
He began his working life as a printer. After inheriting a fortune, Scherze ...
, a scientist aboard the ''Novara
Novara (, Novarese: ) is the capital city of the province of Novara in the Piedmont region in northwest Italy, to the west of Milan. With 101,916 inhabitants (on 1 January 2021), it is the second most populous city in Piedmont after Turin. It is ...
'' (an Austrian frigate
A frigate () is a type of warship. In different eras, the roles and capabilities of ships classified as frigates have varied somewhat.
The name frigate in the 17th to early 18th centuries was given to any full-rigged ship built for speed and ...
sent by Emperor Franz Joseph
Franz Joseph I or Francis Joseph I (german: Franz Joseph Karl, hu, Ferenc József Károly, 18 August 1830 – 21 November 1916) was Emperor of Austria, King of Hungary, and the other states of the Habsburg monarchy from 2 December 1848 until his ...
to circle the globe), to bring him a large amount of coca leaves from South America. In 1859, the ship finished its travels and Wöhler received a trunk full of coca. Wöhler passed on the leaves to Albert Niemann, a PhD student at the University of Göttingen
The University of Göttingen, officially the Georg August University of Göttingen, (german: Georg-August-Universität Göttingen, known informally as Georgia Augusta) is a public research university in the city of Göttingen, Germany. Founded ...
in Germany, who then developed an improved purification process.Über eine neue organische Base in den Cocablättern
''On a New Organic Base in the Coca Leaves'' is an 1860 dissertation written by Albert Niemann. Its title in German is ''Über eine neue organische Base in den Cocablättern''. The piece describes, in detail, how Niemann isolated cocaine, a cryst ...
'' (''On a New Organic Base in the Coca Leaves''), which was published in 1860 and earned him his Ph.D. He wrote of the alkaloid's "colourless transparent prisms" and said that "Its solutions have an alkaline reaction, a bitter taste, promote the flow of saliva and leave a peculiar numbness, followed by a sense of cold when applied to the tongue." Niemann named the alkaloid "cocaine" from "coca" (from Quechua
Quechua may refer to:
*Quechua people, several indigenous ethnic groups in South America, especially in Peru
*Quechuan languages, a Native South American language family spoken primarily in the Andes, derived from a common ancestral language
**So ...
"kúka") + suffix
In linguistics, a suffix is an affix which is placed after the stem of a word. Common examples are case endings, which indicate the grammatical case of nouns, adjectives, and verb endings, which form the conjugation of verbs. Suffixes can carry ...
"ine".Richard Willstätter
Richard Martin Willstätter FRS(For) HFRSE (, 13 August 1872 – 3 August 1942) was a German organic chemist whose study of the structure of plant pigments, chlorophyll included, won him the 1915 Nobel Prize for Chemistry. Willstätter invented ...
in 1898.biomimetic
Biomimetics or biomimicry is the emulation of the models, systems, and elements of nature for the purpose of solving complex human problems. The terms "biomimetics" and "biomimicry" are derived from grc, βίος (''bios''), life, and μίμησ ...
synthesis of an organic structure recorded in academic chemical literature.tropinone
Tropinone is an alkaloid, famously synthesised in 1917 by Robert Robinson as a synthetic precursor to atropine, a scarce commodity during World War I. Tropinone and the alkaloids cocaine and atropine all share the same tropane core structure. It ...
, a related natural product and took five steps.
Because of the former use of cocaine as a local anesthetic
A local anesthetic (LA) is a medication that causes absence of pain sensation. In the context of surgery, a local anesthetic creates an absence of pain in a specific location of the body without a loss of consciousness, as opposed to a general an ...
, a suffix "-caine" was later extracted and used to form names of synthetic local anesthetic
A local anesthetic (LA) is a medication that causes absence of pain sensation. In the context of surgery, a local anesthetic creates an absence of pain in a specific location of the body without a loss of consciousness, as opposed to a general an ...
s.
Medicalization
 With the discovery of this new alkaloid, Western medicine was quick to exploit the possible uses of this plant.
In 1879, Vassili von Anrep, of the
With the discovery of this new alkaloid, Western medicine was quick to exploit the possible uses of this plant.
In 1879, Vassili von Anrep, of the University of Würzburg
The Julius Maximilian University of Würzburg (also referred to as the University of Würzburg, in German ''Julius-Maximilians-Universität Würzburg'') is a public research university in Würzburg, Germany. The University of Würzburg is one of ...
, devised an experiment to demonstrate the analgesic properties of the newly discovered alkaloid. He prepared two separate jars, one containing a cocaine-salt solution, with the other containing merely saltwater. He then submerged a frog's legs into the two jars, one leg in the treatment and one in the control solution, and proceeded to stimulate the legs in several different ways. The leg that had been immersed in the cocaine solution reacted very differently from the leg that had been immersed in saltwater.Sigmund Freud
Sigmund Freud ( , ; born Sigismund Schlomo Freud; 6 May 1856 – 23 September 1939) was an Austrian neurologist and the founder of psychoanalysis, a clinical method for evaluating and treating psychopathology, pathologies explained as originatin ...
, who would write about cocaine later) experimented with cocaine for ophthalmic usage. In an infamous experiment in 1884, he experimented upon himself by applying a cocaine solution to his own eye and then pricking it with pins. His findings were presented to the Heidelberg Ophthalmological Society. Also in 1884, Jellinek demonstrated the effects of cocaine as a respiratory system
The respiratory system (also respiratory apparatus, ventilatory system) is a biological system consisting of specific organs and structures used for gas exchange in animals and plants. The anatomy and physiology that make this happen varies grea ...
anesthetic. In 1885, William Halsted
William Stewart Halsted, M.D. (September 23, 1852 – September 7, 1922) was an American surgeon who emphasized strict aseptic technique during surgical procedures, was an early champion of newly discovered anesthetics, and introduced several ...
demonstrated nerve-block anesthesia, and James Leonard Corning demonstrated peridural
Epidural administration (from Ancient Greek ἐπί, , upon" + ''dura mater'') is a method of medication administration in which a medicine is injected into the epidural space around the spinal cord. The epidural route is used by physicians and ...
anesthesia. 1898 saw Heinrich Quincke
Heinrich Irenaeus Quincke (26 August 1842 – 19 May 1922) was a German internist and surgeon. His main contribution to internal medicine was the introduction of the lumbar puncture for diagnostic and therapeutic purposes. After 1874, his main ...
use cocaine for spinal anesthesia
Spinal anaesthesia (or spinal anesthesia), also called spinal block, subarachnoid block, intradural block and intrathecal block, is a form of neuraxial regional anaesthesia involving the injection of a local anaesthetic or opioid into the subarac ...
.
Popularization
 In 1859, an Italian
In 1859, an Italian doctor
Doctor or The Doctor may refer to:
Personal titles
* Doctor (title), the holder of an accredited academic degree
* A medical practitioner, including:
** Physician
** Surgeon
** Dentist
** Veterinary physician
** Optometrist
*Other roles
** ...
, Paolo Mantegazza, returned from Peru
, image_flag = Flag of Peru.svg
, image_coat = Escudo nacional del Perú.svg
, other_symbol = Great Seal of the State
, other_symbol_type = Seal (emblem), National seal
, national_motto = "Fi ...
, where he had witnessed first-hand the use of coca by the local indigenous peoples. He proceeded to experiment on himself and upon his return to Milan
Milan ( , , Lombard: ; it, Milano ) is a city in northern Italy, capital of Lombardy, and the second-most populous city proper in Italy after Rome. The city proper has a population of about 1.4 million, while its metropolitan city h ...
, he wrote a paper in which he described the effects. In this paper, he declared coca and cocaine (at the time they were assumed to be the same) as being useful medicinally, in the treatment of "a furred tongue in the morning, flatulence
Flatulence, in humans, is the expulsion of gas from the intestines via the anus, commonly referred to as farting. "Flatus" is the medical word for gas generated in the stomach or bowels. A proportion of intestinal gas may be swallowed environm ...
, and whitening of the teeth."
A chemist named Angelo Mariani who read Mantegazza's paper became immediately intrigued with coca and its economic potential. In 1863, Mariani started marketing a wine
Wine is an alcoholic drink typically made from fermented grapes. Yeast consumes the sugar in the grapes and converts it to ethanol and carbon dioxide, releasing heat in the process. Different varieties of grapes and strains of yeasts are m ...
called Vin Mariani
Vin Mariani ( French: ''Mariani wine'') was a coca wine and patent medicine created in the 1860s by Angelo Mariani, a French chemist from the island of Corsica. Mariani became intrigued with coca and its medical and economic potential after read ...
, which had been treated with coca leaves, to become coca wine
Coca wine is an alcoholic beverage combining wine with cocaine. One popular brand was ''Vin Mariani'', developed in 1863 by French-Corsican chemist and entrepreneur Angelo Mariani.
At the end of the 19th century, the fear of drug abuse made coc ...
. The ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ...
in wine acted as a solvent and extracted the cocaine from the coca leaves, altering the drink's effect. It contained 6 mg cocaine per ounce of wine, but Vin Mariani which was to be exported contained 7.2 mg per ounce, to compete with the higher cocaine content of similar drinks in the United States. A "pinch of coca leaves" was included in John Styth Pemberton's original 1886 recipe for Coca-Cola
Coca-Cola, or Coke, is a carbonated soft drink manufactured by the Coca-Cola Company. Originally marketed as a temperance drink and intended as a patent medicine, it was invented in the late 19th century by John Stith Pemberton in Atlanta ...
, though the company began using decocainized leaves in 1906 when the Pure Food and Drug Act
The Pure Food and Drug Act of 1906, also known as Dr. Wiley's Law, was the first of a series of significant consumer protection laws which was enacted by Congress in the 20th century and led to the creation of the Food and Drug Administration. ...
was passed.
In 1879 cocaine began to be used to treat morphine
Morphine is a strong opiate that is found naturally in opium, a dark brown resin in poppies (''Papaver somniferum''). It is mainly used as a analgesic, pain medication, and is also commonly used recreational drug, recreationally, or to make ...
addiction. Cocaine was introduced into clinical use as a local anesthetic
A local anesthetic (LA) is a medication that causes absence of pain sensation. In the context of surgery, a local anesthetic creates an absence of pain in a specific location of the body without a loss of consciousness, as opposed to a general an ...
in Germany in 1884, about the same time as Sigmund Freud
Sigmund Freud ( , ; born Sigismund Schlomo Freud; 6 May 1856 – 23 September 1939) was an Austrian neurologist and the founder of psychoanalysis, a clinical method for evaluating and treating psychopathology, pathologies explained as originatin ...
published his work ''Über Coca'', in which he wrote that cocaine causes:
By 1885 the U.S. manufacturer Parke-Davis
Parke-Davis is a subsidiary of the pharmaceutical company Pfizer. Although Parke, Davis & Co. is no longer an independent corporation, it was once America's oldest and largest drug maker, and played an important role in medical history. In 1970 ...
sold coca-leaf cigarettes and cheroots, a cocaine inhalant, a Coca Cordial, cocaine crystals, and cocaine solution for intravenous injection. The company promised that its cocaine products would "supply the place of food, make the coward brave, the silent eloquent and render the sufferer insensitive to pain."
 By the late
By the late Victorian era
In the history of the United Kingdom and the British Empire, the Victorian era was the period of Queen Victoria's reign, from 20 June 1837 until her death on 22 January 1901. The era followed the Georgian period and preceded the Edwardia ...
, cocaine use had appeared as a vice in literature
Literature is any collection of written work, but it is also used more narrowly for writings specifically considered to be an art form, especially prose fiction, drama, and poetry. In recent centuries, the definition has expanded to include ...
. For example, it was injected by Arthur Conan Doyle
Sir Arthur Ignatius Conan Doyle (22 May 1859 – 7 July 1930) was a British writer and physician. He created the character Sherlock Holmes in 1887 for ''A Study in Scarlet'', the first of four novels and fifty-six short stories about Ho ...
's fictional Sherlock Holmes
Sherlock Holmes () is a fictional detective created by British author Arthur Conan Doyle. Referring to himself as a " consulting detective" in the stories, Holmes is known for his proficiency with observation, deduction, forensic science and ...
, generally to offset the boredom he felt when he was not working on a case.
In early 20th-century Memphis, Tennessee
Memphis is a city in the U.S. state of Tennessee. It is the seat of Shelby County in the southwest part of the state; it is situated along the Mississippi River. With a population of 633,104 at the 2020 U.S. census, Memphis is the second-mos ...
, cocaine was sold in neighborhood drugstores on Beale Street
Beale Street is a street in Downtown Memphis, Tennessee, which runs from the Mississippi River to East Street, a distance of approximately . It is a significant location in the city's history, as well as in the history of blues music. Today, th ...
, costing five or ten cents for a small boxful. Stevedores along the Mississippi River used the drug as a stimulant, and white employers encouraged its use by black laborers.Ernest Shackleton
Sir Ernest Henry Shackleton (15 February 1874 – 5 January 1922) was an Anglo-Irish Antarctic explorer who led three British expeditions to the Antarctic. He was one of the principal figures of the period known as the Heroic Age of ...
took "Forced March" brand cocaine tablets to Antarctica
Antarctica () is Earth's southernmost and least-populated continent. Situated almost entirely south of the Antarctic Circle and surrounded by the Southern Ocean, it contains the geographic South Pole. Antarctica is the fifth-largest contine ...
, as did Captain Scott
Captain Robert Falcon Scott, , (6 June 1868 – c. 29 March 1912) was a British Royal Navy officer and explorer who led two expeditions to the Antarctic regions: the ''Discovery'' expedition of 1901–1904 and the ill-fated ''Terra Nov ...
a year later on his ill-fated journey to the South Pole
The South Pole, also known as the Geographic South Pole, Terrestrial South Pole or 90th Parallel South, is one of the two points where Earth's axis of rotation intersects its surface. It is the southernmost point on Earth and lies antipod ...
.Minnie the Moocher
"Minnie the Moocher" is a jazz- scat song first recorded in 1931 by Cab Calloway and His Orchestra, selling over a million copies. "Minnie the Moocher" is most famous for its nonsensical ad libbed (" scat") lyrics (for example, "Hi De Hi De Hi ...
", Cab Calloway
Cabell Calloway III (December 25, 1907 – November 18, 1994) was an American singer, songwriter, bandleader, conductor and dancer. He was associated with the Cotton Club in Harlem, where he was a regular performer and became a popular vocalist ...
heavily references cocaine use. He uses the phrase "kicking the gong around", slang for cocaine use; describes titular character Minnie as "tall and skinny;" and describes Smokey Joe as "cokey". In the 1932 comedy musical film ''The Big Broadcast
''The Big Broadcast'' is a 1932 American pre-Code musical comedy film directed by Frank Tuttle and starring Bing Crosby, Stuart Erwin, and Leila Hyams. Based on the play ''Wild Waves'' by William Ford Manley, the film is about a radio-singer wh ...
'', Cab Calloway performs the song with his orchestra and mimes snorting cocaine in between verses.
During the mid-1940s, amidst World War II, cocaine was considered for inclusion as an ingredient of a future generation of 'pep pills' for the German military, code named D-IX
D-IX is a methamphetamine-based experimental Performance-enhancing drugs, performance enhancer developed by Nazi Germany in 1944 for military application. The researcher who rediscovered this project, Wolf Kemper, said that "the aim was to use D-IX ...
.
In modern popular culture, references to cocaine are common. The drug has a glamorous image associated with the wealthy, famous and powerful, and is said to make users "feel rich and beautiful".[
]
Modern usage
 In many countries, cocaine is a popular
In many countries, cocaine is a popular recreational drug
Recreational drug use indicates the use of one or more psychoactive drugs to induce an altered state of consciousness either for pleasure or for some other casual purpose or pastime by modifying the perceptions and emotions of the user. When a ...
. In the United States, the development of "crack" cocaine introduced the substance to a generally poorer inner-city market. The use of the powder form has stayed relatively constant, experiencing a new height of use during the late 1990s and early 2000s in the U.S., and has become much more popular in the last few years in the UK.
Cocaine use is prevalent across all socioeconomic strata, including age, demographics, economic, social, political, religious, and livelihood.Starbucks
Starbucks Corporation is an American multinational chain of coffeehouses and roastery reserves headquartered in Seattle, Washington. It is the world's largest coffeehouse chain.
As of November 2021, the company had 33,833 stores in 80 c ...
. Cocaine's status as a club drug
Club drugs, also called rave drugs or party drugs, are a loosely defined category of recreational drugs which are associated with discothèques in the 1970s and nightclubs, dance clubs, electronic dance music (EDM) parties, and raves in the 198 ...
shows its immense popularity among the "party crowd".World Health Organization
The World Health Organization (WHO) is a specialized agency of the United Nations responsible for international public health. The WHO Constitution states its main objective as "the attainment by all peoples of the highest possible level of h ...
(WHO) and the (UNICRI) announced in a press release the publication of the results of the largest global study on cocaine use ever undertaken. An American representative in the World Health Assembly
The World Health Assembly (WHA) is the forum through which the World Health Organization (WHO) is governed by its 194 member states. It is the world's highest health policy setting body and is composed of health ministers from member states.
T ...
banned the publication of the study, because it seemed to make a case for the positive uses of cocaine. An excerpt of the report strongly conflicted with accepted paradigms, for example, "that occasional cocaine use does not typically lead to severe or even minor physical or social problems." In the sixth meeting of the B committee, the US representative threatened that "If World Health Organization activities relating to drugs failed to reinforce proven drug control approaches, funds for the relevant programs should be curtailed". This led to the decision to discontinue publication. A part of the study was recuperated and published in 2010, including profiles of cocaine use in 20 countries, but are unavailable .Novocain
Procaine is a local anesthetic drug of the amino ester group. It is most commonly used in dental procedures to numb the area around a tooth and is also used to reduce the pain of intramuscular injection of penicillin. Owing to the ubiquity of ...
(procaine) producing temporary anesthesia, as many users believe a strong numbing effect is the result of strong and/or pure cocaine, ephedrine or similar stimulants that are to produce an increased heart rate. The normal adulterants for profit are inactive sugars, usually mannitol, creatine, or glucose, so introducing active adulterants gives the illusion of purity and to 'stretch' or make it so a dealer can sell more product than without the adulterants. The adulterant of sugars allows the dealer to sell the product for a higher price because of the illusion of purity and allows the sale of more of the product at that higher price, enabling dealers to significantly increase revenue with little additional cost for the adulterants. A 2007 study by the European Monitoring Centre for Drugs and Drug Addiction
The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) is an agency of the European Union located in Lisbon, Portugal, and established in 1993. In June 2022, the Council of the European Union approved a reform of the organization w ...
showed that the purity levels for street purchased cocaine was often under 5% and on average under 50% pure.
Society and culture
Legal status
The production, distribution, and sale of cocaine products is restricted (and illegal in most contexts) in most countries as regulated by the Single Convention on Narcotic Drugs
The Single Convention on Narcotic Drugs, 1961 (Single Convention, 1961 Convention, or C61) is an international treaty that controls activities (cultivation, production, supply, trade, transport) of specific narcotic drugs and lays down a syst ...
, and the . In the United States the manufacture, importation, possession, and distribution of cocaine are additionally regulated by the 1970 Controlled Substances Act
The Controlled Substances Act (CSA) is the statute establishing federal government of the United States, federal drug policy of the United States, U.S. drug policy under which the manufacture, importation, possession, use, and distribution of ...
.
Some countries, such as Peru and Bolivia, permit the cultivation of coca leaf for traditional consumption by the local indigenous population
Indigenous peoples are culturally distinct ethnic groups whose members are directly descended from the earliest known inhabitants of a particular geographic region and, to some extent, maintain the language and culture of those original people ...
, but nevertheless, prohibit the production, sale, and consumption of cocaine. The provisions as to how much a coca farmer can yield annually is protected by laws such as the Bolivian Cato accord. In addition, some parts of Europe, the United States, and Australia allow processed cocaine for medicinal uses only.
Australia
Cocaine is a Schedule 8 prohibited substance in Australia under the Poisons Standard (July 2016).Western Australia
Western Australia (commonly abbreviated as WA) is a state of Australia occupying the western percent of the land area of Australia excluding external territories. It is bounded by the Indian Ocean to the north and west, the Southern Ocean to th ...
under the Misuse of Drugs Act 1981 4.0g of cocaine is the amount of prohibited drugs determining a court of trial, 2.0g is the amount of cocaine required for the presumption of intention to sell or supply and 28.0g is the amount of cocaine required for purposes of drug trafficking.
United States
 The US federal government instituted a national labeling requirement for cocaine and cocaine-containing products through the Pure Food and Drug Act of 1906. The next important federal regulation was the
The US federal government instituted a national labeling requirement for cocaine and cocaine-containing products through the Pure Food and Drug Act of 1906. The next important federal regulation was the Harrison Narcotics Tax Act
The Harrison Narcotics Tax Act (Ch. 1, ) was a United States federal law that regulated and taxed the production, importation, and distribution of opiates and coca products. The act was proposed by United States Representative, Representative Fra ...
of 1914. While this act is often seen as the start of prohibition, the act itself was not actually a prohibition on cocaine, but instead set up a regulatory and licensing regime. The Harrison Act did not recognize addiction as a treatable condition and therefore the therapeutic use of cocaine, heroin, or morphine to such individuals was outlawed leading a 1915 editorial in the journal ''American Medicine'' to remark that the addict "is denied the medical care he urgently needs, open, above-board sources from which he formerly obtained his drug supply are closed to him, and he is driven to the underworld where he can get his drug, but of course, surreptitiously and in violation of the law." The Harrison Act left manufacturers of cocaine untouched so long as they met certain purity and labeling standards. Despite that cocaine was typically illegal to sell and legal outlets were rarer, the quantities of legal cocaine produced declined very little. Legal cocaine quantities did not decrease until the Jones–Miller Act of 1922 put serious restrictions on cocaine manufactures.
Before the early 1900s, the primary problem caused by cocaine use was portrayed by newspapers to be addiction, not violence or crime, and the cocaine user was represented as an upper- or middle-class White person. In 1914, ''The New York Times'' published an article titled "Negro Cocaine 'Fiends' Are a New Southern Menace", portraying Black cocaine users as dangerous and able to withstand wounds that would normally be fatal. The Anti-Drug Abuse Act of 1986
The Anti-Drug Abuse Act of 1986 was a law pertaining to the War on Drugs passed by the United States Congress, U.S. Congress and signed into law by U.S. President Ronald Reagan. Among other things, they changed the system of federal supervised re ...
mandated prison sentences for 500 grams of powdered cocaine and 5 grams of crack cocaine. In the National Survey on Drug Use and Health
The National Survey on Drug Use and Health, often abbreviated NSDUH, is an annual nationwide survey on the use of legal and illegal drugs, as well as mental disorders, that has been conducted by the United States federal government since 1971. ...
, Whites reported a higher rate of powdered cocaine use, and Blacks reported a higher rate of crack cocaine use.
Interdiction

 In 2004, according to the
In 2004, according to the United Nations
The United Nations (UN) is an intergovernmental organization whose stated purposes are to maintain international peace and international security, security, develop friendly relations among nations, achieve international cooperation, and be ...
, 589 tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
s of cocaine were seized globally by law enforcement authorities. Colombia
Colombia (, ; ), officially the Republic of Colombia, is a country in South America with insular regions in North America—near Nicaragua's Caribbean coast—as well as in the Pacific Ocean. The Colombian mainland is bordered by the Car ...
seized 188 t, the United States 166 t, Europe 79 t, Peru 14 t, Bolivia 9 t, and the rest of the world 133 t.
Production
Colombia is as of 2019 the world's largest cocaine producer, with production more than tripling since 2013.[Colombia](_blank)
. CIA World Factbookhectare
The hectare (; SI symbol: ha) is a non-SI metric unit of area equal to a square with 100-metre sides (1 hm2), or 10,000 m2, and is primarily used in the measurement of land. There are 100 hectares in one square kilometre. An acre is a ...
, six times per year. The leaves were dried for half a day, then chopped into small pieces with a string trimmer and sprinkled with a small amount of powdered cement (replacing sodium carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions ...
from former times). Several hundred pounds of this mixture were soaked in of gasoline for a day, then the gasoline was removed and the leaves were pressed for the remaining liquid, after which they could be discarded. Then battery acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular f ...
(weak sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
) was used, one bucket per of leaves, to create a phase
Phase or phases may refer to:
Science
*State of matter, or phase, one of the distinct forms in which matter can exist
*Phase (matter), a region of space throughout which all physical properties are essentially uniform
* Phase space, a mathematic ...
separation in which the cocaine free base
Free base (freebase, free-base) is the conjugate base (deprotonated) form of an amine, as opposed to its conjugate acid (protonated) form. The amine is often an alkaloid, such as nicotine, cocaine, morphine, and ephedrine, or derivatives thereo ...
in the gasoline was acidified and extracted into a few buckets of "murky-looking smelly liquid". Once powdered caustic soda
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alka ...
was added to this, the cocaine precipitated and could be removed by filtration through a cloth. The resulting material, when dried, was termed ''pasta
Pasta (, ; ) is a type of food typically made from an unleavened dough of wheat flour mixed with water or eggs, and formed into sheets or other shapes, then cooked by boiling or baking. Rice flour, or legumes such as beans or lentils, are som ...
'' and sold by the farmer. The 3750 pound yearly harvest of leaves from a hectare produced of ''pasta'', approximately 40–60% cocaine. Repeated recrystallization from solvents, producing ''pasta lavada'' and eventually crystalline cocaine were performed at specialized laboratories after the sale.
Attempts to eradicate coca fields through the use of defoliants
A defoliant is any herbicidal chemical sprayed or dusted on plants to cause their leaves to fall off. Defoliants are widely used for the selective removal of weeds in managing croplands and lawns. Worldwide use of defoliants, along with the ...
have devastated part of the farming economy in some coca-growing regions of Colombia, and strains appear to have been developed that are more resistant or immune to their use. Whether these strains are natural mutations or the product of human tampering is unclear. These strains have also shown to be more potent than those previously grown, increasing profits for the drug cartels responsible for the exporting of cocaine. Although production fell temporarily, coca crops rebounded in numerous smaller fields in Colombia, rather than the larger plantations.
The cultivation of coca has become an attractive economic decision for many growers due to the combination of several factors, including the lack of other employment alternatives, the lower profitability of alternative crops in official crop substitution programs, the eradication-related damages to non-drug farms, the spread of new strains of the coca plant due to persistent worldwide demand.
The latest estimate provided by the U.S. authorities on the annual production of cocaine in Colombia refers to 290 metric tons.
As of the end of 2011, the seizure operations of Colombian cocaine
The illegal drug trade in Colombia has, since the 1970s, centered successively on four major drug trafficking cartels: Medellín, Cali, Norte del Valle, and North Coast, as well as several ''bandas criminales'', or BACRIMs. The trade eventuall ...
carried out in different countries have totaled 351.8 metric tons of cocaine, i.e. 121.3% of Colombia's annual production according to the U.S. Department of State's estimates.
Synthesis
Synthesizing cocaine could eliminate the high visibility and low reliability of offshore sources and international smuggling, replacing them with clandestine domestic laboratories, as are common for illicit methamphetamine
Methamphetamine (contracted from ) is a potent central nervous system (CNS) stimulant that is mainly used as a recreational drug and less commonly as a second-line treatment for attention deficit hyperactivity disorder and obesity. Methamph ...
, but is rarely done. Natural cocaine remains the lowest cost and highest quality supply of cocaine. Formation of inactive stereoisomers
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in ...
(cocaine has four chiral centres – 1''R'' 2''R'', 3''S'', and 5''S'', two of them dependent, hence eight possible stereoisomers) plus synthetic by-products limits the yield and purity.
Trafficking and distribution
 Organized criminal gangs operating on a large scale dominate the cocaine trade. Most cocaine is grown and processed in South America, particularly in Colombia,
Organized criminal gangs operating on a large scale dominate the cocaine trade. Most cocaine is grown and processed in South America, particularly in Colombia, Bolivia
, image_flag = Bandera de Bolivia (Estado).svg
, flag_alt = Horizontal tricolor (red, yellow, and green from top to bottom) with the coat of arms of Bolivia in the center
, flag_alt2 = 7 × 7 square p ...
, Peru, and smuggled into the United States and Europe, the United States being the world's largest consumer of cocaine,
=Caribbean and Mexican routes
=
The primary cocaine importation points in the United States have been in Arizona
Arizona ( ; nv, Hoozdo Hahoodzo ; ood, Alĭ ṣonak ) is a state in the Southwestern United States. It is the 6th largest and the 14th most populous of the 50 states. Its capital and largest city is Phoenix. Arizona is part of the Fou ...
, southern California
California is a U.S. state, state in the Western United States, located along the West Coast of the United States, Pacific Coast. With nearly 39.2million residents across a total area of approximately , it is the List of states and territori ...
, southern Florida
Florida is a state located in the Southeastern region of the United States. Florida is bordered to the west by the Gulf of Mexico, to the northwest by Alabama, to the north by Georgia, to the east by the Bahamas and Atlantic Ocean, and to ...
, and Texas
Texas (, ; Spanish language, Spanish: ''Texas'', ''Tejas'') is a state in the South Central United States, South Central region of the United States. At 268,596 square miles (695,662 km2), and with more than 29.1 million residents in 2 ...
. Typically, land vehicles are driven across the U.S.–Mexico border. Sixty-five percent of cocaine enters the United States through Mexico, and the vast majority of the rest enters through Florida. , the Sinaloa Cartel
The Sinaloa Cartel ( es, link=no, Cártel de Sinaloa), also known as the CDS, the Guzmán-Loera Organization, the Pacific Cartel, the Federation and the Blood Alliance, is a large, international organized crime syndicate that specializes in il ...
is the most active drug cartel
A drug cartel is any criminal organization with the intention of supplying drug trafficking operations. They range from loosely managed agreements among various drug traffickers to formalized commercial enterprises. The term was applied when the ...
involved in smuggling illicit drugs like cocaine into the United States and trafficking them throughout the United States.smuggling
Smuggling is the illegal transportation of objects, substances, information or people, such as out of a house or buildings, into a prison, or across an international border, in violation of applicable laws or other regulations.
There are various ...
routes throughout the Caribbean, the Bahama Island chain, and South Florida. They often hire traffickers from Mexico or the Dominican Republic
The Dominican Republic ( ; es, República Dominicana, ) is a country located on the island of Hispaniola in the Greater Antilles archipelago of the Caribbean region. It occupies the eastern five-eighths of the island, which it shares wit ...
to transport the drug using a variety of smuggling techniques to U.S. markets. These include airdrops of in the Bahama Islands
The Bahamas (), officially the Commonwealth of The Bahamas, is an island country within the Lucayan Archipelago of the West Indies in the North Atlantic. It takes up 97% of the Lucayan Archipelago's land area and is home to 88% of the arch ...
or off the coast of Puerto Rico
Puerto Rico (; abbreviated PR; tnq, Boriken, ''Borinquen''), officially the Commonwealth of Puerto Rico ( es, link=yes, Estado Libre Asociado de Puerto Rico, lit=Free Associated State of Puerto Rico), is a Caribbean island and Unincorporated ...
, mid-ocean boat-to-boat transfers of , and the commercial shipment of tonnes of cocaine through the port of Miami
Miami ( ), officially the City of Miami, known as "the 305", "The Magic City", and "Gateway to the Americas", is a East Coast of the United States, coastal metropolis and the County seat, county seat of Miami-Dade County, Florida, Miami-Dade C ...
.
=Chilean route
=
Another route of cocaine traffic goes through Chile, which is primarily used for cocaine produced in Bolivia since the nearest seaports lie in northern Chile. The arid Bolivia–Chile border is easily crossed by 4×4 vehicles that then head to the seaports of Iquique
Iquique () is a port city and commune in northern Chile, capital of both the Iquique Province and Tarapacá Region. It lies on the Pacific coast, west of the Pampa del Tamarugal, which is part of the Atacama Desert. It has a population of 191,468 ...
and Antofagasta
Antofagasta () is a port city in northern Chile, about north of Santiago. It is the capital of Antofagasta Province and Antofagasta Region. According to the 2015 census, the city has a population of 402,669.
After the Spanish American wars ...
. While the price of cocaine is higher in Chile than in Peru and Bolivia, the final destination is usually Europe, especially Spain where drug dealing networks exist among South American immigrants.
=Techniques
=
Cocaine is also carried in small, concealed, kilogram quantities across the border by couriers known as " mules" (or "mulas"), who cross a border either legally, for example, through a port or airport, or illegally elsewhere. The drugs may be strapped to the waist or legs or hidden in bags, or hidden in the body. If the mule gets through without being caught, the gangs will reap most of the profits. If caught, gangs will sever all links and the mule will usually stand trial for trafficking alone.
Bulk cargo ships are also used to smuggle cocaine to staging sites in the western Caribbean–Gulf of Mexico
The Gulf of Mexico ( es, Golfo de México) is an oceanic basin, ocean basin and a marginal sea of the Atlantic Ocean, largely surrounded by the North American continent. It is bounded on the northeast, north and northwest by the Gulf Coast of ...
area. These vessels are typically 150–250-foot (50–80 m) coastal freighters that carry an average cocaine load of approximately 2.5 tonnes. Commercial fishing vessels are also used for smuggling operations. In areas with a high volume of recreational traffic, smugglers use the same types of vessels, such as go-fast boat
A go-fast boat is a small, fast power boat designed with a long narrow platform and a planing hull.
During the United States alcohol prohibition era, these boats were used in " rum-running", transferring illegal liquor from larger vessels wa ...
s, like those used by the local populations.
Sophisticated drug subs are the latest tool drug runners are using to bring cocaine north from Colombia, it was reported on 20 March 2008. Although the vessels were once viewed as a quirky sideshow in the drug war, they are becoming faster, more seaworthy, and capable of carrying bigger loads of drugs than earlier models, according to those charged with catching them.
Sales to consumers
 Cocaine is readily available in all major countries' metropolitan areas. According to the ''Summer 1998 Pulse Check'', published by the U.S.
Cocaine is readily available in all major countries' metropolitan areas. According to the ''Summer 1998 Pulse Check'', published by the U.S. Office of National Drug Control Policy
The Office of National Drug Control Policy (ONDCP) is a component of the Executive Office of the President of the United States.
The Director of the ONDCP, colloquially known as the Drug Czar, heads the office. "Drug Czar" was a term first used ...
, cocaine use had stabilized across the country, with a few increases reported in San Diego
San Diego ( , ; ) is a city on the Pacific Ocean coast of Southern California located immediately adjacent to the Mexico–United States border. With a 2020 population of 1,386,932, it is the List of United States cities by population, eigh ...
, Bridgeport
Bridgeport is the most populous city and a major port in the U.S. state of Connecticut. With a population of 148,654 in 2020, it is also the fifth-most populous in New England. Located in eastern Fairfield County at the mouth of the Pequonnoc ...
, Miami, and Boston
Boston (), officially the City of Boston, is the state capital and most populous city of the Commonwealth of Massachusetts, as well as the cultural and financial center of the New England region of the United States. It is the 24th- mo ...
. In the West, cocaine usage was lower, which was thought to be due to a switch to methamphetamine
Methamphetamine (contracted from ) is a potent central nervous system (CNS) stimulant that is mainly used as a recreational drug and less commonly as a second-line treatment for attention deficit hyperactivity disorder and obesity. Methamph ...
among some users; methamphetamine is cheaper, three and a half times more powerful, and lasts 12–24 times longer with each dose. Nevertheless, the number of cocaine users remain high, with a large concentration among urban youth.
In addition to the amounts previously mentioned, cocaine can be sold in "bill sizes": for example, $10 might purchase a "dime bag", a very small amount (0.1–0.15 g) of cocaine. These amounts and prices are very popular among young people because they are inexpensive and easily concealed on one's body. Quality and price can vary dramatically depending on supply and demand, and on geographic region.
In 2008, the European Monitoring Centre for Drugs and Drug Addiction
The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) is an agency of the European Union located in Lisbon, Portugal, and established in 1993. In June 2022, the Council of the European Union approved a reform of the organization w ...
reports that the typical retail price of cocaine varied between €50 and €75 per gram in most European countries, although Cyprus, Romania, Sweden, and Turkey reported much higher values.
Consumption
World annual cocaine consumption, as of 2000, stood at around 600 tonnes, with the United States consuming around 300 t, 50% of the total, Europe about 150 t, 25% of the total, and the rest of the world the remaining 150 t or 25%.Spain
, image_flag = Bandera de España.svg
, image_coat = Escudo de España (mazonado).svg
, national_motto = ''Plus ultra'' (Latin)(English: "Further Beyond")
, national_anthem = (English: "Royal March")
, i ...
, the United Kingdom
The United Kingdom of Great Britain and Northern Ireland, commonly known as the United Kingdom (UK) or Britain, is a country in Europe, off the north-western coast of the continental mainland. It comprises England, Scotland, Wales and North ...
, Italy
Italy ( it, Italia ), officially the Italian Republic, ) or the Republic of Italy, is a country in Southern Europe. It is located in the middle of the Mediterranean Sea, and its territory largely coincides with the homonymous geographical re ...
, and Ireland
Ireland ( ; ga, Éire ; Ulster Scots dialect, Ulster-Scots: ) is an island in the Atlantic Ocean, North Atlantic Ocean, in Northwestern Europe, north-western Europe. It is separated from Great Britain to its east by the North Channel (Grea ...
.World Drug Report The World Drug Report is a United Nations Office on Drugs and Crime annual publication that analyzes market trends, compiling detailed statistics on drug markets. Using data, it helps draw conclusions about drugs as an issue needing intervention by ...
concluded that "it appears that the North American cocaine market has declined in value from US$47 billion in 1998 to US$38 billion in 2008. Between 2006 and 2008, the value of the market remained basically stable".
See also
* Black cocaine
Black cocaine ( es, italic=no, coca negra) is a mixture of regular cocaine Freebase (chemistry), base or cocaine hydrochloride with various other substances. These other substances are added
* to camouflage the typical appearance (pigments and dy ...
* Coca alkaloid
Coca alkaloids are the alkaloids found in the coca plant, ''Erythroxylum coca''.Variation of Alkaloid Content in Erythroxylum coca Leaves from Leaf Bud to Leaf Drop. Emanuel L. Johnson and Stephen D. Emche, Ann. Bot., 1994, volume 73, issue 6, page ...
s
* Coca eradication
Coca eradication is a strategy promoted by the United States government starting in 1961 as part of its "War on Drugs" to eliminate the cultivation of coca, a plant whose leaves are not only traditionally used by indigenous cultures but also, in m ...
* Cocaine and amphetamine regulated transcript
Cocaine- and amphetamine-regulated transcript, also known as CART, is a neuropeptide protein that in humans is encoded by the ''CARTPT'' gene. CART appears to have roles in reward, feeding, and stress, and it has the functional properties of an e ...
* Cocaine Anonymous Cocaine Anonymous (C.A.) is a twelve-step program formed in 1982 for people who seek recovery from drug addiction. It is patterned very closely after Alcoholics Anonymous, although the two groups are unaffiliated. While many C.A. members have been ...
* '' Cocaine Cowboys''
* Cocaine paste
Coca paste (paco, basuco, oxi) is a crude extract of the coca leaf which contains 40% to 91% cocaine freebase along with companion coca alkaloids and varying quantities of benzoic acid, methanol, and kerosene. In South America, coca paste, also ...
*
* Crack epidemic
The crack epidemic was a surge of crack cocaine use in major cities across the United States throughout the entirety of the 1980s and the early 1990s. This resulted in a number of social consequences, such as increasing crime and violence in Ameri ...
* Illegal drug trade in Latin America
The illegal drug trade in Latin America concerns primarily the production and sale of cocaine and cannabis, including the export of these banned substances to the United States and Europe. The coca cultivation is concentrated in the Andes of Sou ...
* Coca production in Colombia
* Clan del Golfo
The Clan del Golfo (English: The Gulf Clan), also known as Gaitanist Self-Defense Forces of Colombia (Autodefensas Gaitanistas de Colombia – AGC) and formerly called Los Urabeños and Clan Úsuga, is a prominent Colombian Paramilitarism in Colo ...
* Legal status of cocaine
The legal status of cocaine varies worldwide. Even though many countries have banned the sale of cocaine for recreational use, some have legalized it for possession, personal use, transportation, and cultivation, while some have decriminalized it ...
* List of cocaine analogues
This is a list of cocaine analogues. A cocaine analogue is an (usually) artificial construct of a novel chemical compound from (often the starting point of natural) cocaine's molecular structure, with the result product sufficiently similar to co ...
* List of countries by prevalence of cocaine use
* Methylphenidate
* Modafinil
* Pre-Columbian trans-oceanic contact theories#Claims of Egyptian coca and tobacco, Pre-Columbian trans-oceanic evidence for cocaine in ancient Egypt
* Prenatal cocaine exposure
* Route 36 (bar), Route 36, cocaine bar in Bolivia
* Tranquilandia
* Ypadu
References
General bibliography
*
*
*
Further reading
*
External links
*
*
* — A collection of data about cocaine including dose, effects, chemistry, legal status, images and more.
*
* Data on cocaine trafficking worldwide.
{{Authority control
Cocaine,
Alkaloids found in Erythroxylum
Anorectics
Benzoate esters
Carboxylate esters
Cardiac stimulants
CYP2D6 inhibitors
Euphoriants
German inventions
Glycine receptor agonists
Local anesthetics
Methyl esters
Otologicals
Powders
Secondary metabolites
Serotonin–norepinephrine–dopamine reuptake inhibitors
Sigma agonists
Stimulants
Sympathomimetic amines
Teratogens
Tropane alkaloids found in Erythroxylum coca
Vasoconstrictors
Wikipedia medicine articles ready to translate
 Topical cocaine is sometimes used as a local numbing agent and
Topical cocaine is sometimes used as a local numbing agent and  Nasal
Nasal  Although it has been commonly asserted, the available evidence does not show that chronic use of cocaine is associated with broad cognitive deficits. Research is inconclusive on age-related loss of striatal
Although it has been commonly asserted, the available evidence does not show that chronic use of cocaine is associated with broad cognitive deficits. Research is inconclusive on age-related loss of striatal 
 Cocaine in its purest form is a white, pearly product. Cocaine appearing in powder form is a
Cocaine in its purest form is a white, pearly product. Cocaine appearing in powder form is a 
 Crack is usually smoked in a glass pipe, and once inhaled, it passes from the lungs directly to the
Crack is usually smoked in a glass pipe, and once inhaled, it passes from the lungs directly to the  The first synthesis and elucidation of the cocaine molecule was by
The first synthesis and elucidation of the cocaine molecule was by 




 With the discovery of this new alkaloid, Western medicine was quick to exploit the possible uses of this plant.
In 1879, Vassili von Anrep, of the
With the discovery of this new alkaloid, Western medicine was quick to exploit the possible uses of this plant.
In 1879, Vassili von Anrep, of the  In 1859, an Italian
In 1859, an Italian  By the late
By the late 
 In many countries, cocaine is a popular
In many countries, cocaine is a popular  The US federal government instituted a national labeling requirement for cocaine and cocaine-containing products through the Pure Food and Drug Act of 1906. The next important federal regulation was the
The US federal government instituted a national labeling requirement for cocaine and cocaine-containing products through the Pure Food and Drug Act of 1906. The next important federal regulation was the 
 In 2004, according to the
In 2004, according to the  Organized criminal gangs operating on a large scale dominate the cocaine trade. Most cocaine is grown and processed in South America, particularly in Colombia,
Organized criminal gangs operating on a large scale dominate the cocaine trade. Most cocaine is grown and processed in South America, particularly in Colombia,  Cocaine is readily available in all major countries' metropolitan areas. According to the ''Summer 1998 Pulse Check'', published by the U.S.
Cocaine is readily available in all major countries' metropolitan areas. According to the ''Summer 1998 Pulse Check'', published by the U.S.