Trifluoroperoxyacetic Acid on:
[Wikipedia]
[Google]
[Amazon]
Trifluoroperacetic acid (trifluoroperoxyacetic acid, TFPAA) is an organofluorine compound, the
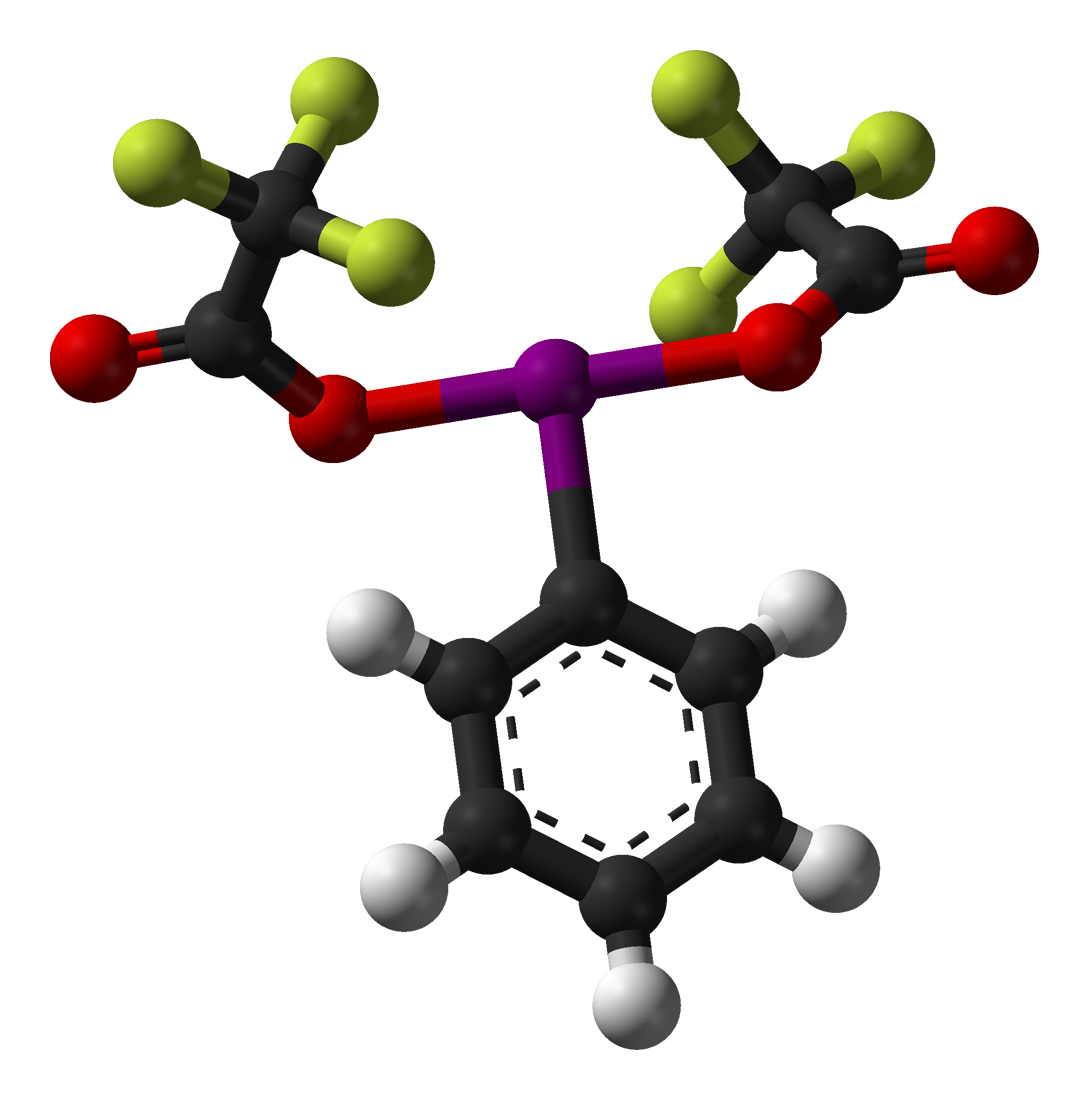 Trifluoroperacetic acid is primarily used as an oxidising agent. In September 1953, the '' Journal of the American Chemical Society'' published work by
Trifluoroperacetic acid is primarily used as an oxidising agent. In September 1953, the '' Journal of the American Chemical Society'' published work by
Emmons is remembered in part as the pioneer and developer of trifluoroperacetic acid as a laboratory reagent, which has since become useful as a
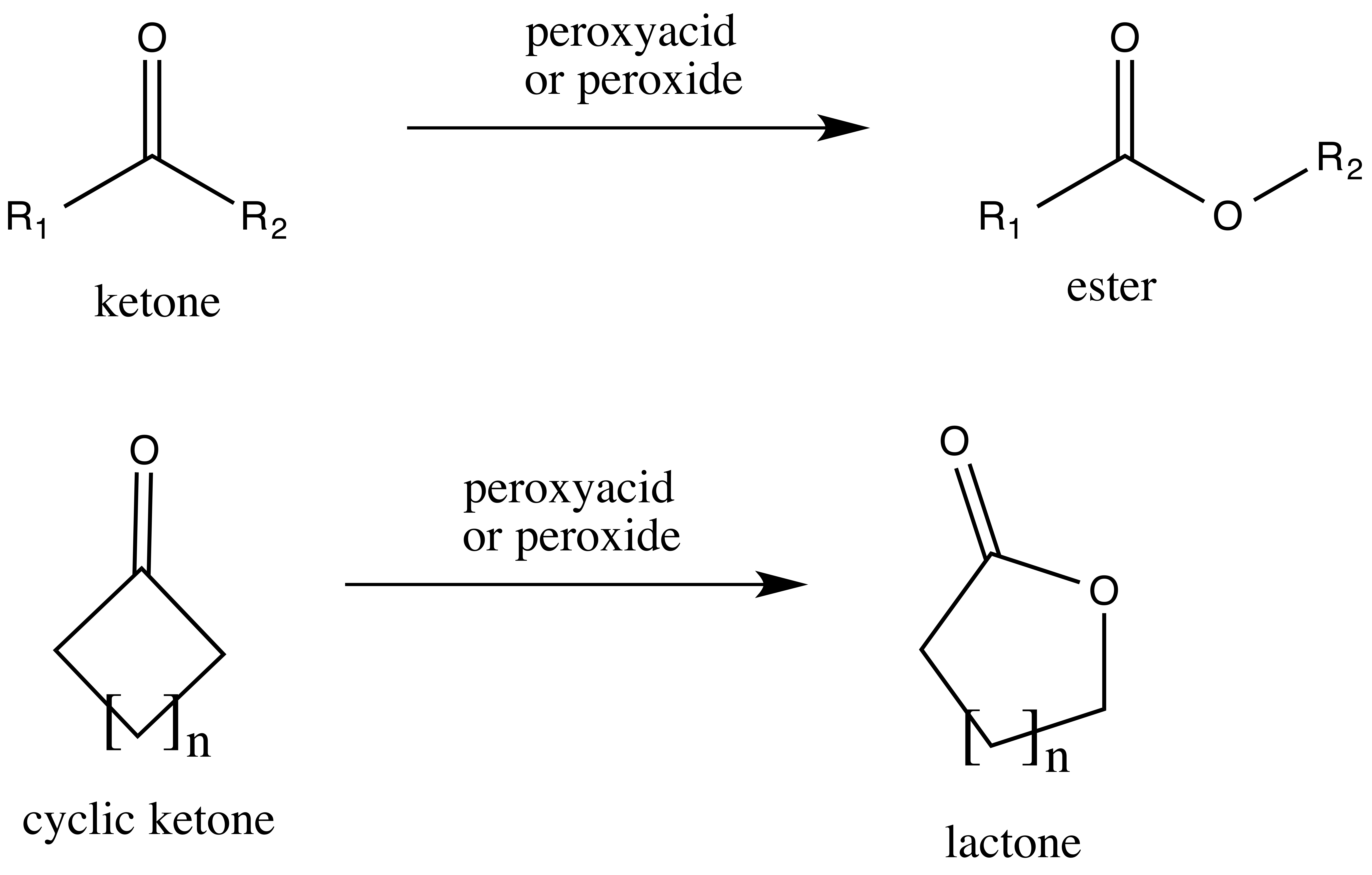 Trifluoroperacetic acid is one of the strongest reagents used for Baeyer–Villiger oxidations, as a consequence of its high acidity relative to similar peracids and peroxides. This reaction converts
Trifluoroperacetic acid is one of the strongest reagents used for Baeyer–Villiger oxidations, as a consequence of its high acidity relative to similar peracids and peroxides. This reaction converts 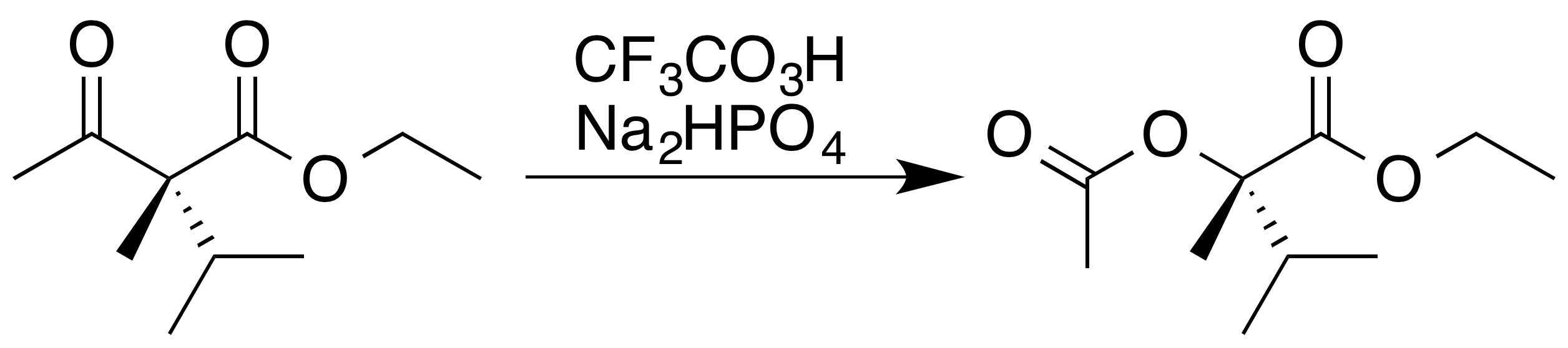
 The high reactivity of trifluoroperacetic acid relative to other peroxy acids allows it to successfully oxidize relatively electron-poor alkenes such as 1-hexene and α,β-unsaturated esters such as
The high reactivity of trifluoroperacetic acid relative to other peroxy acids allows it to successfully oxidize relatively electron-poor alkenes such as 1-hexene and α,β-unsaturated esters such as  In the case of an allyl alcohol compound with a proximate carbonyl functional group, the epoxide can undergo a ring-expansion reaction to form a dioxolane. The process below was used as part of the total synthesis of neosporol, a
In the case of an allyl alcohol compound with a proximate carbonyl functional group, the epoxide can undergo a ring-expansion reaction to form a dioxolane. The process below was used as part of the total synthesis of neosporol, a  The preparation of the isomeric compound sporol involved a similar dioxolane formation. In this case, the use of trifluoroperacetic acid derived from hydrogen peroxide, which therefore presumably contained traces of water, gave mostly a hemiacetal rather than the closed-ring dioxolane. The use of the urea complex, which gave a water-free material, successfully gave the dioxolane as the major product. The dioxolane is expanded to the
The preparation of the isomeric compound sporol involved a similar dioxolane formation. In this case, the use of trifluoroperacetic acid derived from hydrogen peroxide, which therefore presumably contained traces of water, gave mostly a hemiacetal rather than the closed-ring dioxolane. The use of the urea complex, which gave a water-free material, successfully gave the dioxolane as the major product. The dioxolane is expanded to the
 In the case of chalcogen elements, sulfide moieties (R–S–R) can be oxidised by trifluoroperacetic acid to
In the case of chalcogen elements, sulfide moieties (R–S–R) can be oxidised by trifluoroperacetic acid to  The trifluoroperacetic acid oxidation of thiophene illustrates competing pathways for reaction, with both ''S''-oxidation and epoxidation being possible. The major pathway initially forms the sulfoxide, but this chemical promptly undergoes a Diels-Alder-type dimerisation before any further oxidation occurs—neither thiophene-''S''-oxide or thiophene-''S'',''S''-dioxide are found among the products of the reaction. The dimer can then be oxidized further, converting one of the ''S''-oxide moieties to an ''S'',''S''-dioxide. In the minor reaction pathway, a Prilezhaev epoxidation results in the formation of thiophene-2,3-epoxide that rapidly rearranges to the
The trifluoroperacetic acid oxidation of thiophene illustrates competing pathways for reaction, with both ''S''-oxidation and epoxidation being possible. The major pathway initially forms the sulfoxide, but this chemical promptly undergoes a Diels-Alder-type dimerisation before any further oxidation occurs—neither thiophene-''S''-oxide or thiophene-''S'',''S''-dioxide are found among the products of the reaction. The dimer can then be oxidized further, converting one of the ''S''-oxide moieties to an ''S'',''S''-dioxide. In the minor reaction pathway, a Prilezhaev epoxidation results in the formation of thiophene-2,3-epoxide that rapidly rearranges to the 
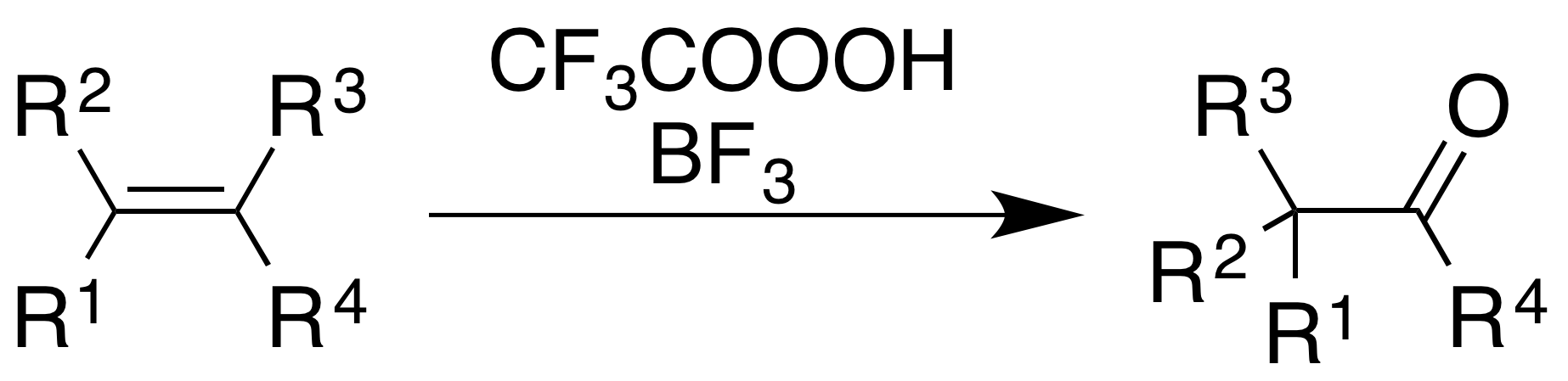 For aromatics, an example demonstrated in an ''Organic Syntheses'' report is the conversion of hexamethylbenzene to 2,3,4,5,6,6-hexamethyl-2,4-cyclohexadienone:
For aromatics, an example demonstrated in an ''Organic Syntheses'' report is the conversion of hexamethylbenzene to 2,3,4,5,6,6-hexamethyl-2,4-cyclohexadienone:

 This selectivity for certain types of bonds allows it to be used to decompose complex mixtures of hydrocarbons, such as coal, in order to determine structural details.
Aromatic systems containing heteroatoms are resistant to this ring-opening as heteroatom oxidation occurs preferentially and deactivates the ring towards electrophilic attack by the peroxy acid. For example, purines, pyridines, and quinolines instead form ''N''-oxides, while sulfur systems like octafluoro dibenzothiophene are converted to sulfones.
Aromatic systems with ring-activating substituents can be oxidised to form phenols instead of undergoing a ring-opening reaction. Mesitylene, for example, reacts with trifluoroperacetic acid to form mesitol (2,4,6-trimethylphenol). Researchers attempting to form a lactone by Baeyer–Villiger oxidation of 7-oxodeacetamido colchicine were unable to prepare the desired product, but did achieve oxidation of the aromatic ring to produce a phenol-derivative in high yield:
This selectivity for certain types of bonds allows it to be used to decompose complex mixtures of hydrocarbons, such as coal, in order to determine structural details.
Aromatic systems containing heteroatoms are resistant to this ring-opening as heteroatom oxidation occurs preferentially and deactivates the ring towards electrophilic attack by the peroxy acid. For example, purines, pyridines, and quinolines instead form ''N''-oxides, while sulfur systems like octafluoro dibenzothiophene are converted to sulfones.
Aromatic systems with ring-activating substituents can be oxidised to form phenols instead of undergoing a ring-opening reaction. Mesitylene, for example, reacts with trifluoroperacetic acid to form mesitol (2,4,6-trimethylphenol). Researchers attempting to form a lactone by Baeyer–Villiger oxidation of 7-oxodeacetamido colchicine were unable to prepare the desired product, but did achieve oxidation of the aromatic ring to produce a phenol-derivative in high yield:

peroxy acid
A peroxy acid (often spelled as one word, peroxyacid, and sometimes called peracid) is an acid which contains an acidic –OOH group. The two main classes are those derived from conventional mineral acids, especially sulfuric acid, and the peroxy ...
analog of trifluoroacetic acid, with the condensed structural formula . It is a strong oxidizing agent for organic oxidation reactions, such as in Baeyer–Villiger oxidations of ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s. It is the most reactive of the organic peroxy acids, allowing it to successfully oxidise relatively unreactive alkenes to epoxides where other peroxy acids are ineffective. It can also oxidise the chalcogens in some functional groups, such as by transforming selenoethers to selones. It is a potentially explosive material and is not commercially available, but it can be quickly prepared as needed. Its use as a laboratory reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
was pioneered and developed by William D. Emmons
William D. Emmons (November 18, 1924 – December 8, 2001) was an American chemist and published with William S. Wadsworth a modification to the Wittig-Horner reaction using phosphonate-stabilized carbanions, now called the Horner-Wadsworth-Emmons ...
.
Properties
Atstandard ambient temperature and pressure
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union o ...
, trifluoroperacetic acid is a colourless liquid with a boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
of 162 °C. It is soluble in acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not clas ...
, dichloromethane
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with ...
, diethyl ether, and sulfolane
Sulfolane (also ''tetramethylene sulfone'', systematic name: 1λ6-thiolane-1,1-dione) is an organosulfur compound, formally a cyclic sulfone, with the formula (CH2)4SO2. It is a colorless liquid commonly used in the chemical industry as a solvent ...
, and readily reacts with water. Like all peroxy acids, it is potentially explosive and requires careful handling. It is not commercially available, but can be made in the lab and stored for up to several weeks at −20 °C. Some preparative methods result in mixtures containing residual hydrogen peroxide and trifluoroacetic acid, and heating such a mixture is extremely hazardous; the hydrogen peroxide can be decomposed using manganese dioxide
Manganese dioxide is the inorganic compound with the formula . This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for is for dry-cell ...
for safety before heating.
Preparation
Trifluoroperacetic acid can be easily prepared by an '' Organic Syntheses'' process of treating trifluoroacetic anhydride with a concentrated (90%)aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be re ...
of hydrogen peroxide:
: + → +
As the anhydride will form trifluoroacetic acid in contact with water, an excess of the anhydride also serves to remove the solvent from the peroxide reactant:
: + → 2
A more dilute hydrogen peroxide solution (30%) can be used to form trifluoroperacetic acid for some reactions from trifluoroacetic acid.
: + → +
In order to avoid the danger of handling pure or highly concentrated solutions of hydrogen peroxide, hydrogen peroxide – urea can be used to give the peracid. This method involves no water, so it gives a completely anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achie ...
peracid, which is an advantage when the presence of water leads to side reactions during certain oxidation reactions.
: + → + +
In cases where a pH buffering agent is needed for a synthesis and where the presence of water is tolerated, another approach has been developed. Reacting trifluoroacetic anhydride with sodium percarbonate, , yields trifluoroperacetic acid and sodium carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions ...
, obviating the need for an additional buffer.
:3 + 4 → 6 + 4 + 3
Trifluoroperacetic acid can also be generated '' in situ'', allowing it to react promptly with the target substrate rather than pre-synthesizing a batch of the reagent for later use.
Uses
 Trifluoroperacetic acid is primarily used as an oxidising agent. In September 1953, the '' Journal of the American Chemical Society'' published work by
Trifluoroperacetic acid is primarily used as an oxidising agent. In September 1953, the '' Journal of the American Chemical Society'' published work by William D. Emmons
William D. Emmons (November 18, 1924 – December 8, 2001) was an American chemist and published with William S. Wadsworth a modification to the Wittig-Horner reaction using phosphonate-stabilized carbanions, now called the Horner-Wadsworth-Emmons ...
and Arthur F. Ferris reporting that this reagent, generated ''in situ'', was capable of oxidising aniline to nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor t ...
. Over the following two years, Emmons reported a preparative method for this reagent and published six further manuscripts in this journal on its applications;Emmons is remembered in part as the pioneer and developer of trifluoroperacetic acid as a laboratory reagent, which has since become useful as a
reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
for many different types of synthetic reactions.
One example is the formation of the hypervalent iodine compound , which is used to carry out the Hofmann rearrangement under acidic conditions. The hypervalent compound is accessible in two ways, and which is chosen usually depends on what materials are available: it can be prepared from its acetate analogue by an exchange reaction, or by reacting iodobenzene with a combination of trifluoroperacetic acid and trifluoroacetic acid:

Baeyer–Villiger oxidation
 Trifluoroperacetic acid is one of the strongest reagents used for Baeyer–Villiger oxidations, as a consequence of its high acidity relative to similar peracids and peroxides. This reaction converts
Trifluoroperacetic acid is one of the strongest reagents used for Baeyer–Villiger oxidations, as a consequence of its high acidity relative to similar peracids and peroxides. This reaction converts ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s to either straight-chain esters or lactones, and is named for Adolf von Baeyer and Victor Villiger
Victor Villiger (1 September 1868 – 10 June 1934) was a Swiss-born German chemist and the discoverer of the Baeyer-Villiger oxidation.
Life
He studied at University of Geneva and, following his graduation, began his doctoral studies with ...
, who first reported it 1899. The reaction is believed to proceed via a Criegee intermediate and demonstrates good regioselectivity and chemoselectivity for the position of oxygen atom insertion, along with retention of stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereois ...
at the adjacent position, as can be seen in the following example. The disodium phosphate
Disodium phosphate (DSP), or disodium hydrogen phosphate, or sodium phosphate dibasic, is the inorganic compound with the formula Na2HPO4. It is one of several sodium phosphates. The salt is known in anhydrous form as well as forms with 2, 7, 8, ...
() is added as a pH buffer to prevent the highly acidic trifluoroacetic acid byproduct from causing hydrolysis or transesterification of the ester product.

Epoxidation
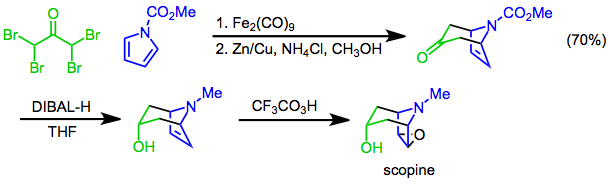
The Prilezhaev reaction involves the conversion of an alkene to an epoxide using a peracid as the oxidant and was first reported in 1909. The reaction has been used as the final step of the synthesis ofscopine
Scopine is a tropane alkaloid found in a variety of plants including '' Mandragora'' root, '' Senecio mikanioides'' (''Delairea odorata''), ''Scopolia carniolica'', and '' Scopolia lurida''.
Scopine can be prepared by the hydrolysis of scopolami ...
, a tropane alkaloid. In this approach, a +3 cycloaddition mediated by diiron nonacarbonyl is used to construct the bicyclic skeleton, the hydroxyl functional group is then introduced by diastereoselective reduction of the ketone with diisobutylaluminum hydride, and the preparation completed with a Prilezhaev trifluoroperacetic acid epoxidation.
 The high reactivity of trifluoroperacetic acid relative to other peroxy acids allows it to successfully oxidize relatively electron-poor alkenes such as 1-hexene and α,β-unsaturated esters such as
The high reactivity of trifluoroperacetic acid relative to other peroxy acids allows it to successfully oxidize relatively electron-poor alkenes such as 1-hexene and α,β-unsaturated esters such as methyl methacrylate
Methyl methacrylate (MMA) is an organic compound with the formula CH2=C(CH3)COOCH3. This colorless liquid, the methyl ester of methacrylic acid (MAA), is a monomer produced on a large scale for the production of poly(methyl methacrylate) (PMMA ...
, substrates that are generally resistant to peroxy-acid epoxidation. Including additional buffered trifluoroacetic acid in the mixture gives a vicinal hydroxy–trifluoroacetate structure instead of an epoxide, which can be converted to the diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
The most common industrial diol is e ...
by treatment with acidic methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
, such as in the following conversion of 1-dodecene to 1,2-dodecanediol.
 In the case of an allyl alcohol compound with a proximate carbonyl functional group, the epoxide can undergo a ring-expansion reaction to form a dioxolane. The process below was used as part of the total synthesis of neosporol, a
In the case of an allyl alcohol compound with a proximate carbonyl functional group, the epoxide can undergo a ring-expansion reaction to form a dioxolane. The process below was used as part of the total synthesis of neosporol, a natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical syn ...
:
 The preparation of the isomeric compound sporol involved a similar dioxolane formation. In this case, the use of trifluoroperacetic acid derived from hydrogen peroxide, which therefore presumably contained traces of water, gave mostly a hemiacetal rather than the closed-ring dioxolane. The use of the urea complex, which gave a water-free material, successfully gave the dioxolane as the major product. The dioxolane is expanded to the
The preparation of the isomeric compound sporol involved a similar dioxolane formation. In this case, the use of trifluoroperacetic acid derived from hydrogen peroxide, which therefore presumably contained traces of water, gave mostly a hemiacetal rather than the closed-ring dioxolane. The use of the urea complex, which gave a water-free material, successfully gave the dioxolane as the major product. The dioxolane is expanded to the 1,3-dioxane
1,3-Dioxane or ''m''-dioxane is a chemical compound with the molecular formula C4H8O2. It is a saturated six-membered heterocycle with two oxygen atoms in place of carbon atoms at the 1- and 3- positions. The corresponding five-membered rings are ...
system found in sporol at a later step in the synthesis.
Heteroatom oxidation
Functional groups containing heteroatoms in low oxidation states can be oxidised by trifluoroperacetic acid. Common cases include the oxidation of iodine (for example, the formation of the hypervalent iodine compound from iodobenzene mentioned earlier), nitrogen, sulfur, and selenium. In the case of nitrogen-containing compounds, known transformations include oximes and aromatic primary amines to nitro compounds (even with electron-withdrawing substituents, for example, pentafluoroaniline to pentafluoronitrobenzene),nitrosamine
In organic chemistry, nitrosamines (or more formally ''N''-Nitrosamines) are organic compounds with the chemical structure , where R is usually an alkyl group. They feature a nitroso group () bonded to a deprotonated amine. Most nitrosamines are ...
s to nitramines, formation of aromatic ''N''-oxides and aromatic azine ''N''-oxides, and conversion of nitroso compounds to nitro compounds or nitramines. For example, a mixture of hydrogen peroxide and trifluoroperacetic acid oxidises the nitroso-substituted pyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The other ...
4,6-diamino-5-nitrosopyrimidine-2-thiol to its nitro analogue while also removing the thiol moiety by oxidative hydrolytic desulfurization:
 In the case of chalcogen elements, sulfide moieties (R–S–R) can be oxidised by trifluoroperacetic acid to
In the case of chalcogen elements, sulfide moieties (R–S–R) can be oxidised by trifluoroperacetic acid to sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
(R–S(O)–R) and/or sulfone (R–S(O)2–R) forms, depending on the conditions used. In the analogous selenium system, trifluoroperacetic acid oxidation of selenoethers (R–Se–R) produces selones (R–Se(O)2–R) without the formation of the related selenoxides (R–Se(O)–R) as an isolable product, a reaction which is particularly effective when the R is an aryl group. A general approach to the formation of sulfinyl chloride
Sulfinyl halide have the general formula R−S(O)−X, where X is a halogen. They are intermediate in oxidation level between sulfenyl halides, R−S−X, and sulfonyl halides, R−SO2−X. The best known examples are sulfinyl chlorides, thermol ...
s (RS(O)Cl) is the reaction of the corresponding thiol with sulfuryl chloride (). In cases where the sulfenyl chloride
In organosulfur chemistry, a sulfenyl chloride is a functional group with the connectivity , where R is alkyl or aryl. Sulfenyl chlorides are reactive compounds that behave as sources of . They are used in the formation of and bonds. According ...
(RSCl) results instead, a subsequent trifluoroperacetic acid oxidation affords the desired product, as in the case of 2,2,2-trifluoro-1,1-diphenyl ethanethiol:
 The trifluoroperacetic acid oxidation of thiophene illustrates competing pathways for reaction, with both ''S''-oxidation and epoxidation being possible. The major pathway initially forms the sulfoxide, but this chemical promptly undergoes a Diels-Alder-type dimerisation before any further oxidation occurs—neither thiophene-''S''-oxide or thiophene-''S'',''S''-dioxide are found among the products of the reaction. The dimer can then be oxidized further, converting one of the ''S''-oxide moieties to an ''S'',''S''-dioxide. In the minor reaction pathway, a Prilezhaev epoxidation results in the formation of thiophene-2,3-epoxide that rapidly rearranges to the
The trifluoroperacetic acid oxidation of thiophene illustrates competing pathways for reaction, with both ''S''-oxidation and epoxidation being possible. The major pathway initially forms the sulfoxide, but this chemical promptly undergoes a Diels-Alder-type dimerisation before any further oxidation occurs—neither thiophene-''S''-oxide or thiophene-''S'',''S''-dioxide are found among the products of the reaction. The dimer can then be oxidized further, converting one of the ''S''-oxide moieties to an ''S'',''S''-dioxide. In the minor reaction pathway, a Prilezhaev epoxidation results in the formation of thiophene-2,3-epoxide that rapidly rearranges to the isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
thiophene-2-one. Trapping experiments demonstrate that this epoxide pathway is not an alternative reaction of the ''S''-oxide intermediate, and isotopic labeling experiments demonstrate that a 1,2-hydride shift (an NIH shift) occurs and thus that a cationic intermediate is involved. The choice of trifluoroperacetic acid preparation method is important as water suppresses the minor reaction pathway, likely because it acts as a competing base.

Oxidation with acidic rearrangement
The use of trifluoroperacetic acid withboron trifluoride
Boron trifluoride is the inorganic compound with the formula BF3. This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
Structure and bondin ...
causes oxidation of alkenes and aromatic rings with concomitant rearrangement of the molecular skeleton.
For alkenes, the reaction gives a ketone product, though the mechanistic process is not simply epoxidation followed by a BF3-catalyzed Wagner–Meerwein rearrangement:
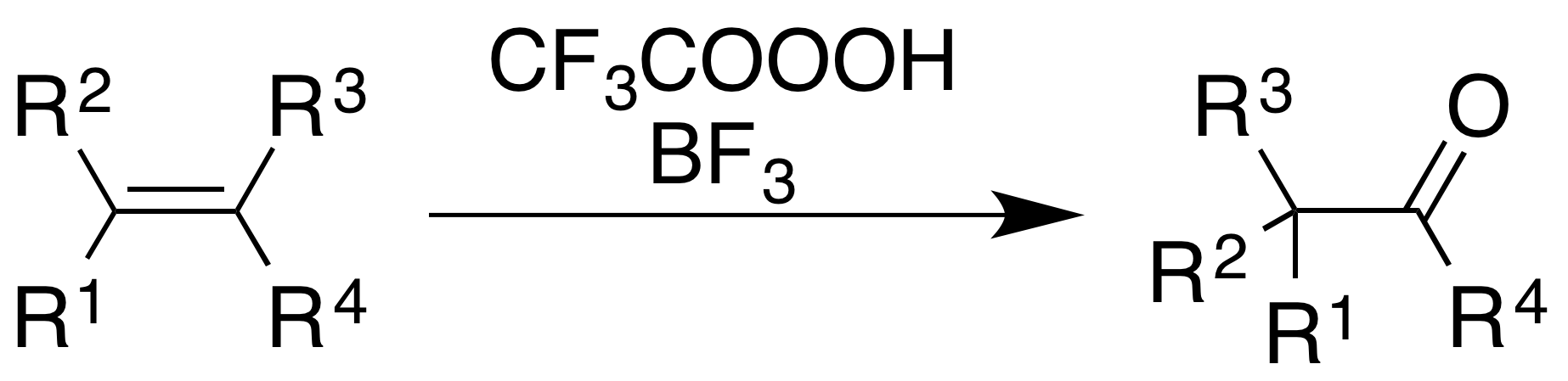 For aromatics, an example demonstrated in an ''Organic Syntheses'' report is the conversion of hexamethylbenzene to 2,3,4,5,6,6-hexamethyl-2,4-cyclohexadienone:
For aromatics, an example demonstrated in an ''Organic Syntheses'' report is the conversion of hexamethylbenzene to 2,3,4,5,6,6-hexamethyl-2,4-cyclohexadienone:

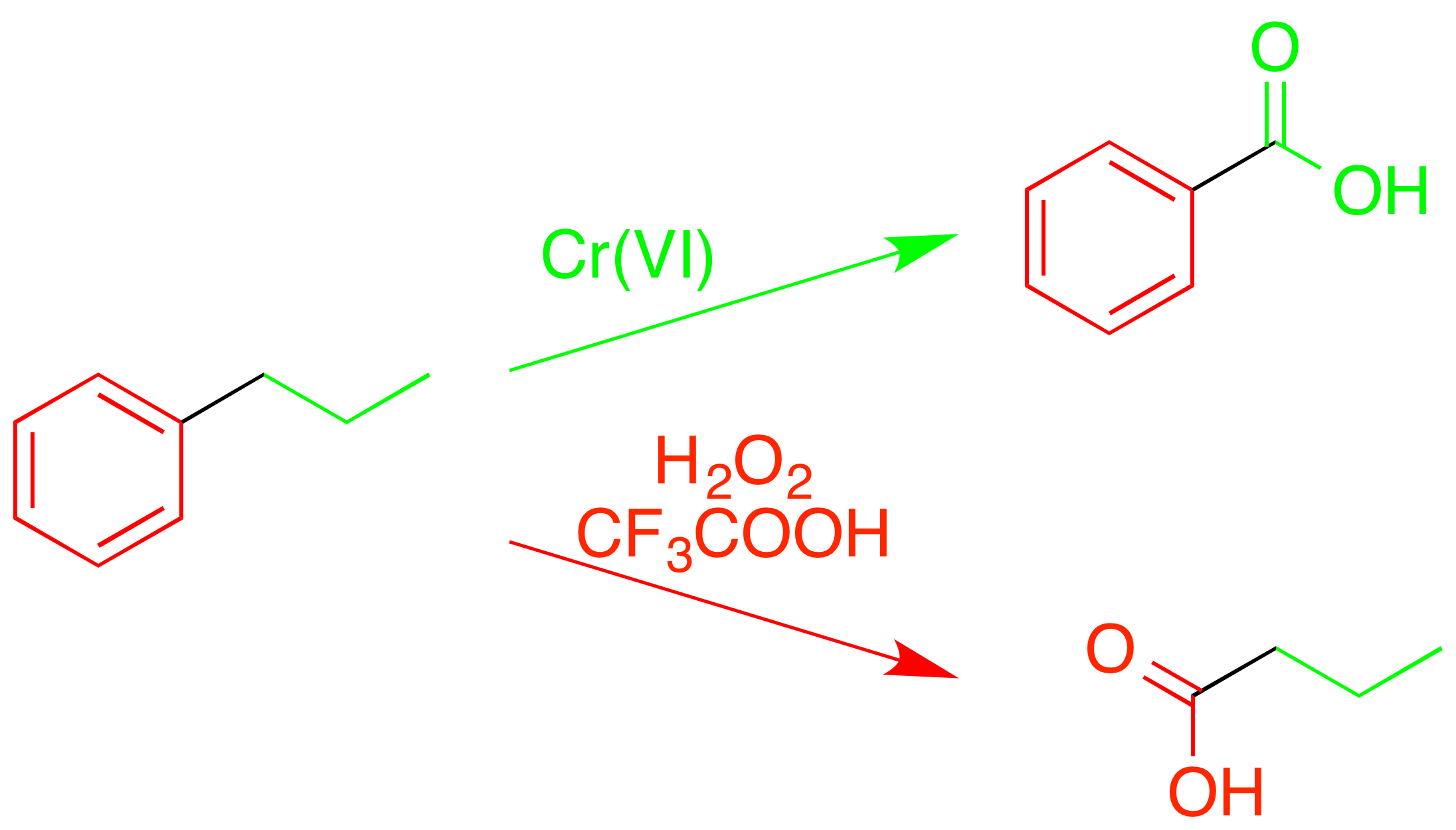
Oxidative cleavage of arenes
In addition to simple oxidation of aromatic rings to form carbonyl compounds (see ), trifluoroperacetic acid can fullycleave
Cleave may refer to:
*Cleave (surname)
*Cleave (fiber), a controlled break in optical fiber
*RAF Cleave, was an airfield in the north of Cornwall, England, May 1939 - Nov 1945
*The process of protein cleaving as a form of post-translational modifi ...
the carbon–carbon bonds within the ring. Unlike other oxidations of alkylaromatic structures, which yield benzoic acids and related compounds by cleavage of the alkyl chain at the reactive benzylic position, trifluoroperacetic acid causes an "inverse oxidation", cleaving the aromatic ring itself while leaving the alkyl group intact.
 This selectivity for certain types of bonds allows it to be used to decompose complex mixtures of hydrocarbons, such as coal, in order to determine structural details.
Aromatic systems containing heteroatoms are resistant to this ring-opening as heteroatom oxidation occurs preferentially and deactivates the ring towards electrophilic attack by the peroxy acid. For example, purines, pyridines, and quinolines instead form ''N''-oxides, while sulfur systems like octafluoro dibenzothiophene are converted to sulfones.
Aromatic systems with ring-activating substituents can be oxidised to form phenols instead of undergoing a ring-opening reaction. Mesitylene, for example, reacts with trifluoroperacetic acid to form mesitol (2,4,6-trimethylphenol). Researchers attempting to form a lactone by Baeyer–Villiger oxidation of 7-oxodeacetamido colchicine were unable to prepare the desired product, but did achieve oxidation of the aromatic ring to produce a phenol-derivative in high yield:
This selectivity for certain types of bonds allows it to be used to decompose complex mixtures of hydrocarbons, such as coal, in order to determine structural details.
Aromatic systems containing heteroatoms are resistant to this ring-opening as heteroatom oxidation occurs preferentially and deactivates the ring towards electrophilic attack by the peroxy acid. For example, purines, pyridines, and quinolines instead form ''N''-oxides, while sulfur systems like octafluoro dibenzothiophene are converted to sulfones.
Aromatic systems with ring-activating substituents can be oxidised to form phenols instead of undergoing a ring-opening reaction. Mesitylene, for example, reacts with trifluoroperacetic acid to form mesitol (2,4,6-trimethylphenol). Researchers attempting to form a lactone by Baeyer–Villiger oxidation of 7-oxodeacetamido colchicine were unable to prepare the desired product, but did achieve oxidation of the aromatic ring to produce a phenol-derivative in high yield:

Notes
References
{{Good article Trifluoromethyl compounds Organic peroxy acids Reagents for organic chemistry