|
Azine
Azines are a functional class of organic compounds with the connectivity RR'C=N-N=CRR'. These compounds are the product of the condensation of hydrazine with ketones and aldehydes, although in practice they are often made by alternative routes. Ketazines are azines derived from ketones. For example, acetone azine is the simplest ketazine. Aldazines are azines derived from aldehydes. Preparation The usual method of industrial production is the peroxide process, starting from the ketone, ammonia, and hydrogen peroxide. : In the laboratory, azines are typically prepared by condensation of hydrazine with two equivalents of a carbonyl. Azines are also produced when chalcone reacts with a hydrazone to produce 3,5-diphenyl-1''H''-pyrazole, in a conversion also carried out with hydrazine hydrate. : Reactions Azines characteristically undergo hydrolysis to hydrazines. The reaction proceeds by the intermediacy of a hydrazone: :R2C=N-N=CR2 + H2O → R2C=N-NH2 + R2C=O :R2C=N-NH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazine Hydrate
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine hydrate (). Hydrazine is mainly used as a foaming agent in preparing polymer foams, but applications also include its uses as a precursor to polymerization catalysts, pharmaceuticals, and agrochemicals, as well as a long-term storable propellant for in-space spacecraft propulsion. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. the world hydrazine hydrate market amounted to $350 million. About two million tons of hydrazine hydrate were used in foam blowing agents in 2015. Hydrazines re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azine
Azines are a functional class of organic compounds with the connectivity RR'C=N-N=CRR'. These compounds are the product of the condensation of hydrazine with ketones and aldehydes, although in practice they are often made by alternative routes. Ketazines are azines derived from ketones. For example, acetone azine is the simplest ketazine. Aldazines are azines derived from aldehydes. Preparation The usual method of industrial production is the peroxide process, starting from the ketone, ammonia, and hydrogen peroxide. : In the laboratory, azines are typically prepared by condensation of hydrazine with two equivalents of a carbonyl. Azines are also produced when chalcone reacts with a hydrazone to produce 3,5-diphenyl-1''H''-pyrazole, in a conversion also carried out with hydrazine hydrate. : Reactions Azines characteristically undergo hydrolysis to hydrazines. The reaction proceeds by the intermediacy of a hydrazone: :R2C=N-N=CR2 + H2O → R2C=N-NH2 + R2C=O :R2C=N-NH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketazine
Azines are a functional class of organic compounds with the connectivity RR'C=N-N=CRR'. These compounds are the product of the condensation of hydrazine with ketones and aldehydes, although in practice they are often made by alternative routes. Ketazines are azines derived from ketones. For example, acetone azine is the simplest ketazine. Aldazines are azines derived from aldehydes. Preparation The usual method of industrial production is the peroxide process, starting from the ketone, ammonia, and hydrogen peroxide. : In the laboratory, azines are typically prepared by condensation of hydrazine with two equivalents of a carbonyl. Azines are also produced when chalcone reacts with a hydrazone to produce 3,5-diphenyl-1''H''-pyrazole, in a conversion also carried out with hydrazine hydrate. : Reactions Azines characteristically undergo hydrolysis to hydrazines. The reaction proceeds by the intermediacy of a hydrazone: :R2C=N-N=CR2 + H2O → R2C=N-NH2 + R2C=O :R2C=N-NH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone Azine
Acetone azine is the simplest ketazine. It is an intermediate in some hydrazine manufacturing processes. Synthesis Acetone azine can be prepared from acetone and hydrazine: :2 (CH3)2CO + N2H4 → 2 H2O + CH3)2C=Nsub>2 It can also be produced from acetone (2 eq.), ammonia (2 eq.) and hydrogen peroxide (1 eq.). The first step is the formation of acetone imine, Me2C=NH; this is then oxidized by hydrogen peroxide through a complex mechanism to give 3,3-dimethyloxaziridine, which reacts with a further molecule of ammonia to produce acetone hydrazone. The hydrazone then condenses Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor to ... with a further molecule of acetone to produce the azine. The acetone azine product is distilled out of the reaction mixture as its azeotrope with water ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pechiney-Ugine-Kuhlmann Process
The peroxide process is a method for the industrial production of hydrazine. In this process hydrogen peroxide is used as an oxidant instead of sodium hypochlorite, which is traditionally used to generate hydrazine. The main advantage of the peroxide process to hydrazine relative to the traditional Olin Raschig process is that it does not coproduce salt. In this respect, the peroxide process is an example of green chemistry. Since many millions of kilograms of hydrazine are produced annually, this method is of both commercial and environmental significance.Jean-Pierre Schirmann, Paul Bourdauducq "Hydrazine" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. . Production Ketazine formation In the usual implementation, hydrogen peroxide is used together with acetamide. This mixture does not react with ammonia directly but does so in the presence of methyl ethyl ketone to give the oxaziridine. : Balanced equations for the individual steps are as follows. Imi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxide Process
The peroxide process is a method for the industrial production of hydrazine. In this process hydrogen peroxide is used as an oxidant instead of sodium hypochlorite, which is traditionally used to generate hydrazine. The main advantage of the peroxide process to hydrazine relative to the traditional Olin Raschig process is that it does not coproduce salt. In this respect, the peroxide process is an example of green chemistry. Since many millions of kilograms of hydrazine are produced annually, this method is of both commercial and environmental significance.Jean-Pierre Schirmann, Paul Bourdauducq "Hydrazine" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. . Production Ketazine formation In the usual implementation, hydrogen peroxide is used together with acetamide. This mixture does not react with ammonia directly but does so in the presence of methyl ethyl ketone to give the oxaziridine. : Balanced equations for the individual steps are as follows. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Herbicide
Herbicides (, ), also commonly known as weedkillers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page for EPA reports on pesticide use ihere Selective herbicides control specific weed species, while leaving the desired crop relatively unharmed, while non-selective herbicides (sometimes called total weedkillers in commercial products) can be used to clear waste ground, industrial and construction sites, railways and railway embankments as they kill all plant material with which they come into contact. Apart from selective/non-selective, other important distinctions include ''persistence'' (also known as ''residual action'': how long the product stays in place and remains active), ''means of uptake'' (whether it is absorbed by above-ground foliage only, through the roots, or by other means), and ''mechanism of action'' (how it works). Historica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazone
Hydrazones are a class of organic compounds with the structure . They are related to ketones and aldehydes by the replacement of the oxygen =O with the = functional group. They are formed usually by the action of hydrazine on ketones or aldehydes. Synthesis Hydrazine, organohydrazines, and 1,1-diorganohydrazines react with aldehydes and ketones to give hydrazones. : Phenylhydrazine reacts with reducing sugars to form hydrazones known as osazones, which was developed by German chemist Emil Fischer as a test to differentiate monosaccharides. Uses Hydrazones are the basis for various analyses of ketones and aldehydes. For example, dinitrophenylhydrazine coated onto a silica sorbent is the basis of an adsorption cartridge. The hydrazones are then eluted and analyzed by HPLC using a UV detector. The compound carbonyl cyanide-''p''-trifluoromethoxyphenylhydrazone (abbreviated as FCCP) is used to uncouple ATP synthesis and reduction of oxygen in oxidative phosphorylation in molecu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or " high-test peroxide", decomposes explosively when heated and has been used as a propellant in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes slowly when exposed to light, and rapidly in the presence of organic or reactive compounds. It is typically stored with a stabilizer in a weakly acidic solution in a dark bottle to block light. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases. Properties The boiling poi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrazole
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ... atoms and two adjacent nitrogen atoms, which are in Arene substitution pattern, ortho-substitution. Pyrazole is a weak base, with p''K''b 11.5 (p''K''a of the conjugate acid 2.49 at 25 °C). Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol. Preparation and reactions Pyrazoles are synthesized by the reaction of α,β-unsaturated aldehydes with hydrazine and subsequent dehydrogenation: : Substituted pyrazoles are prepared by condensation of 1,3-diketones ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both caust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |






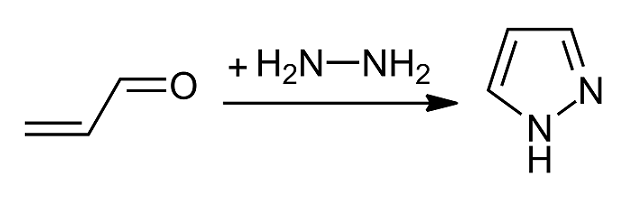
-3D-balls.png)
4-3D-balls.png)