|
Scopine
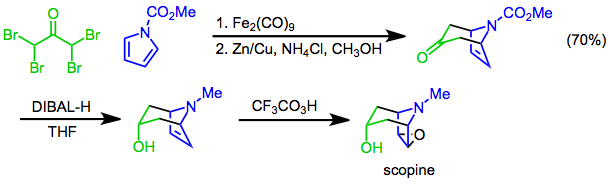
Scopine is a tropane alkaloid found in a variety of plants including '' Mandragora'' root, '' Senecio mikanioides'' (''Delairea odorata''), ''Scopolia carniolica'', and '' Scopolia lurida''. Scopine can be prepared by the hydrolysis of scopolamine. It can also be prepared in three steps from ''N''-methoxycarbonylpyrrole and 1,1,3,3-tetrabromoacetone; the reagents are combined in a +3cycloaddition, followed by a diastereoselective reduction with diisobutylaluminum hydride, and finally a Prilezhaev epoxidation with trifluoroperacetic acid affords scopine. See also * Aposcopolamine * Umbelliferone Umbelliferone, also known as 7-hydroxycoumarin, hydrangine, skimmetine, and ''beta''-umbelliferone, is a natural product of the coumarin family. It absorbs ultraviolet light strongly at several wavelengths. There are some indications that this ch ... References {{reflist Tropane alkaloids Tropane alkaloids found in Solanaceae Epoxides Heterocyclic compounds with 1 ring< ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tropane Alkaloid
Tropane alkaloids are a class of bicyclic [3.2.1] alkaloids and secondary metabolites that contain a tropane ring in their chemical structure. Tropane alkaloids occur naturally in many members of the plant family Solanaceae. Certain tropane alkaloids such as cocaine and scopolamine are notorious for their psychoactive effects, related usage and cultural associations. Particular tropane alkaloids such as these have pharmacological properties and can act as anticholinergics or stimulants. Classification Anticholinergics Anticholinergic drugs and deliriants: * Atropine, racemate, racemic hyoscyamine, from the deadly nightshade (''Atropa belladonna'') * Hyoscyamine, the ''levo''-isomer of atropine, from henbane (''Hyoscyamus niger''), mandrake (''Mandragora officinarum'') and the sorcerers' tree (''Latua pubiflora''). * Hyoscine hydrobromide, Scopolamine, from henbane and ''Datura'' species (Jimson weed) All three acetylcholine-inhibiting chemicals can also be found in the leave ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tropane Alkaloids
Tropane alkaloids are a class of bicyclic .2.1alkaloids and secondary metabolites that contain a tropane ring in their chemical structure. Tropane alkaloids occur naturally in many members of the plant family Solanaceae. Certain tropane alkaloids such as cocaine and scopolamine are notorious for their psychoactive effects, related usage and cultural associations. Particular tropane alkaloids such as these have pharmacological properties and can act as anticholinergics or stimulants. Classification Anticholinergics Anticholinergic drugs and deliriants: * Atropine, racemic hyoscyamine, from the deadly nightshade ('' Atropa belladonna'') * Hyoscyamine, the ''levo''- isomer of atropine, from henbane (''Hyoscyamus niger''), mandrake (''Mandragora officinarum'') and the sorcerers' tree (''Latua pubiflora''). * Scopolamine, from henbane and '' Datura'' species (Jimson weed) All three acetylcholine-inhibiting chemicals can also be found in the leaves, stems, and flowers in v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diisobutylaluminum Hydride
Diisobutylaluminium hydride (DIBALH, DIBAL, DIBAL-H or DIBAH) is a reducing agent with the formula (''i''-Bu2AlH)2, where ''i''-Bu represents isobutyl (-CH2CH(CH3)2). This organoaluminium compound is a reagent in organic synthesis. Properties Like most organoaluminum compounds, the compound's structure is most probably more than that suggested by its empirical formula. A variety of techniques, not including X-ray crystallography, suggest that the compound exists as a dimer and a trimer, consisting of tetrahedral aluminium centers sharing bridging hydride ligands. Hydrides are small and, for aluminium derivatives, are highly basic, thus they bridge in preference to the alkyl groups. DIBAL can be prepared by heating triisobutylaluminium (itself a dimer) to induce beta-hydride elimination: :(''i''-Bu3Al)2 → (''i''-Bu2AlH)2 + 2 (CH3)2C=CH2 Although DIBAL can be purchased commercially as a colorless liquid, it is more commonly purchased and dispensed as a solution in an organic sol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tropane Alkaloids Found In Solanaceae
Tropane is a nitrogenous bicyclic organic compound. It is mainly known for the other alkaloids derived from it, which include atropine and cocaine, among others. Tropane alkaloids occur in plants of the families Erythroxylaceae (including coca) and Solanaceae (including mandrake, henbane, deadly nightshade, datura, potato, tomato). Structurally, tropane is cycloheptane with a nitrogen bridge between carbons 1 and 5 and an additional methyl group attached to the nitrogen. While carbons 1 and 5 are asymmetric carbons, tropane itself is optically inactive due to mirror symmetry. 8-Azabicyclo .2.1ctane (tropane without the ''N''-methyl group) is known as nortropane or nor-tropane. See also * Phenyltropane * Tropane alkaloid * Tropine Tropine is a derivative of tropane containing a hydroxyl group at the third carbon. It is also called 3-tropanol. Tropine is a central building block of many chemicals active in the nervous system, including tropane alkaloids. Some of these comp . ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Umbelliferone
Umbelliferone, also known as 7-hydroxycoumarin, hydrangine, skimmetine, and ''beta''-umbelliferone, is a natural product of the coumarin family. It absorbs ultraviolet light strongly at several wavelengths. There are some indications that this chemical is antimutagenic, it is used in sunscreens. Umbelliferone has been reported to have antioxidant properties. It is a yellowish-white crystalline solid that has a slight solubility in hot water, but high solubility in ethanol. Natural occurrences and name Umbelliferone's name is from the umbelliferae family of plants, and the plant family in turn was named for their umbrella-shaped inflorescences, each called an umbel. Umbelliferone occurs in many familiar plants from the Apiaceae (Umbelliferae) family such as carrot, coriander and garden angelica, as well as in plants from other families, such as the mouse-ear hawkweed (''Hieracium pilosella'', Asteraceae) or the bigleaf hydrangea (''Hydrangea macrophylla'', Hydrangeaceae, under ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aposcopolamine
Aposcopolamine (apohyoscine) is a bio-active isolate of ''Datura ferox'' and several species of ''Physochlaina'',Gorinova, N.I., Atanassov, A.I. and Velcheva, M.P. ''In Vitro Culture and the Production of Physochlaine and Other Tropane Alkaloids'' - paper in ''Biotechnology in Agriculture and Forestry, Vol. 43 Medicinal and Aromatic Plants XI'' (ed. by Y.P.S. Bajaj) pub. Springer-Verlag Berlin Heidelberg 1999. - plants belonging to the Nightshade family, Solanaceae in which tropane alkaloids are of frequent occurrence, particularly in tribes Datureae and Hyoscyameae. See also *Hydroxyzine *Isovoacristine *Umbelliferone Umbelliferone, also known as 7-hydroxycoumarin, hydrangine, skimmetine, and ''beta''-umbelliferone, is a natural product of the coumarin family. It absorbs ultraviolet light strongly at several wavelengths. There are some indications that this ch ... References Tropane alkaloids Epoxides {{alkaloid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoroperacetic Acid
Trifluoroperacetic acid (trifluoroperoxyacetic acid, TFPAA) is an organofluorine compound, the peroxy acid analog of trifluoroacetic acid, with the condensed structural formula . It is a strong oxidizing agent for organic oxidation reactions, such as in Baeyer–Villiger oxidations of ketones. It is the most reactive of the organic peroxy acids, allowing it to successfully oxidise relatively unreactive alkenes to epoxides where other peroxy acids are ineffective. It can also oxidise the chalcogens in some functional groups, such as by transforming selenoethers to selones. It is a potentially explosive material and is not commercially available, but it can be quickly prepared as needed. Its use as a laboratory reagent was pioneered and developed by William D. Emmons. Properties At standard ambient temperature and pressure, trifluoroperacetic acid is a colourless liquid with a boiling point of 162 °C. It is soluble in acetonitrile, dichloromethane, diethyl ether, and sulf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prilezhaev Reaction
The Prilezhaev reaction, also known as the Prileschajew reaction or Prilezhaev epoxidation, is the chemical reaction of an alkene with a peroxy acid to form epoxides. It is named after Nikolai Prilezhaev, who first reported this reaction in 1909. A widely used peroxy acid for this reaction is ''meta''-chloroperoxybenzoic acid (''m''-CPBA), due to its stability and good solubility in most organic solvents. The reaction is performed in inert solvents (C6H14, C6H6, CH2Cl2, CHCl3, CCl4) between -10 and 60 °C with the yield of 60-80%. An illustrative example is the epoxidation of ''trans''-2-butene with ''m''-CPBA to give ''trans''-2,3-epoxybutane: The oxygen atom that adds across the double bond of the alkene is taken from the peroxy acid, generating a molecule of the corresponding carboxylic acid as a byproduct. The reaction is highly stereospecific in the sense that the double bond stereochemistry is generally transferred to the relative configuration of the epoxide with es ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diastereoselective
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter, they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two. Diastereomers differ from enantiomers in that the latter are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another. Enantiomers of a compound with more than one stereocenter are also diastereomers of the other stereoisomers of that compound that are not their mirror image (that is, excluding the opposing enantiomer). Diastereomers h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mandragora (genus)
''Mandragora'' is a plant genus belonging to the nightshade family (Solanaceae). Members of the genus are known as mandrakes. Between three and five species are placed in the genus. The one or two species found around the Mediterranean constitute the mandrake of ancient writers such as Dioscorides. Two or three further species are found eastwards into China. All are perennial herbaceous plants, with large tap roots and leaves in the form of a rosette. Individual flowers are bell-shaped, whitish through to violet, and followed by yellow or orange berries. Like many members of the Solanaceae, species of ''Mandragora'' contain highly biologically active alkaloids that make the plants poisonous. Their roots in particular have a long use in traditional medicine. Mandrakes are involved in many myths and superstitions. Description Species of ''Mandragora'' are perennial herbaceous plants. They have large vertical tap roots, sometimes forked. Their stems are short or virtually absent. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the Multiplicity (chemistry)#Molecules, bond multiplicity". The resulting reaction is a cyclization reaction. Many but not all cycloadditions are Concerted reaction, concerted and thus pericyclic. Nonconcerted cycloadditions are not pericyclic. As a class of addition reaction, cycloadditions permit carbon–carbon bond formation without the use of a nucleophile or electrophile. Cycloadditions can be described using two systems of notation. An older but still common notation is based on the size of linear arrangements of atoms in the reactants. It uses parentheses: where the variables are the numbers of linear atoms in each reactant. The product is a cycle of size . In this system, the standard Diels-Alder reaction is a (4 + 2)-cyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

iodo)benzene-3D-balls.png)

