Cycloaddition on:
[Wikipedia]
[Google]
[Amazon]
In

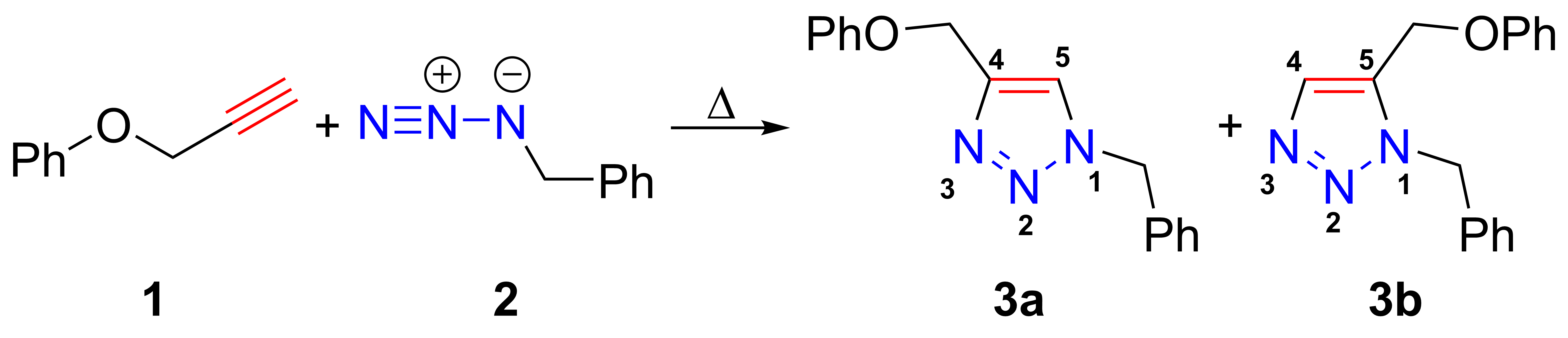


pyridine(diimine)">Diiminopyridine.html" ;"title="/nowiki>Diiminopyridine">pyridine(diimine)catalysts contain a redox active ligand in which the central iron atom can coordinate with two simple, unfunctionalized olefin double bonds. The catalyst can be written as a resonance between a structure containing unpaired electrons with the central iron atom in the II oxidation state, and one in which the iron is in the 0 oxidation state. This gives it the flexibility to engage in binding the double bonds as they undergo a cyclization reaction, generating a cyclobutane structure via C-C reductive elimination; alternatively a cyclobutene structure may be produced by beta-hydrogen elimination. Efficiency of the reaction varies substantially depending on the alkenes used, but rational ligand design may permit expansion of the range of reactions that can be catalyzed.
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, a cycloaddition is a chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct
In chemistry, an adduct (; alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is ...
in which there is a net reduction of the bond multiplicity". The resulting reaction is a cyclization
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where ...
reaction. Many but not all cycloadditions are concerted and thus pericyclic. Nonconcerted cycloadditions are not pericyclic. As a class of addition reaction
In organic chemistry, an addition reaction is an organic reaction in which two or more molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, ...
, cycloadditions permit carbon–carbon bond formation without the use of a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
or electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
.
Cycloadditions can be described using two systems of notation. An older but still common notation is based on the size of linear arrangements of atoms in the reactants. It uses parentheses
A bracket is either of two tall fore- or back-facing punctuation marks commonly used to isolate a segment of text or data from its surroundings. They come in four main pairs of shapes, as given in the box to the right, which also gives their n ...
: where the variables are the numbers of linear atoms in each reactant. The product is a cycle of size . In this system, the standard Diels-Alder reaction is a (4 + 2)-cycloaddition, the 1,3-dipolar cycloaddition is a (3 + 2)-cycloaddition and cyclopropanation
In organic chemistry, cyclopropanation refers to any chemical process which generates cyclopropane () Ring (chemistry), rings. It is an important process in modern chemistry as many useful compounds bear this motif; for example pyrethroid insectic ...
of a carbene with an alkene a (2 + 1)-cycloaddition.
A more recent, IUPAC-preferred notation, first introduced by Woodward and Hoffmann
Hoffmann is a German language, German surname.
People A
*Adolph Hoffmann (1858–1930), German politician
*Albert Hoffmann (horticulturist), Albert Hoffmann (1846–1924), German horticulturist
*Alexander Hoffmann (politician), Alexander Hoffma ...
, uses square brackets
A bracket is either of two tall fore- or back-facing punctuation marks commonly used to isolate a segment of text or data from its surroundings. They come in four main pairs of shapes, as given in the box to the right, which also gives their n ...
to indicate the number of ''electrons'', rather than carbon atoms, involved in the formation of the product. In the 'i'' + ''j'' + ...notation, the standard Diels-Alder reaction is a + 2cycloaddition, while the 1,3-dipolar cycloaddition is also a + 2cycloaddition.
Thermal cycloadditions and their stereochemistry
Thermal cycloadditions are those cycloadditions where the reactants are in the ground electronic state. They usually have (4''n'' + 2) π electrons participating in the starting material, for some integer ''n''. These reactions occur for reasons oforbital symmetry
In chemistry, molecular symmetry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explai ...
in a suprafacial-suprafacial (''syn''/''syn'' stereochemistry) in most cases. Very few examples of antarafacial-antarafacial (''anti''/''anti'' stereochemistry) reactions have also been reported. There are a few examples of thermal cycloadditions which have 4''n'' π electrons (for example the + 2cycloaddition). These proceed in a suprafacial-antarafacial sense (''syn''/''anti'' stereochemistry), such as the cycloaddition reactions of ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The na ...
and allene
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon atoms (, where R is hydrogen, H or some organyl group). Allenes are classified as diene#Classes, cumulated dienes ...
derivatives, in which the orthogonal
In mathematics, orthogonality (mathematics), orthogonality is the generalization of the geometric notion of ''perpendicularity''. Although many authors use the two terms ''perpendicular'' and ''orthogonal'' interchangeably, the term ''perpendic ...
set of p orbitals allows the reaction to proceed via a crossed transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
, although the analysis of these reactions as sub>π2s + π2ais controversial. Strained alkenes like ''trans''-cycloheptene derivatives have also been reported to react in an antarafacial manner in + 2cycloaddition reactions.
Doering (in a personal communication to Woodward) reported that heptafulvalene and tetracyanoethylene can react in a suprafacial-antarafacial 4 + 2
4 (four) is a number, numeral and digit. It is the natural number following 3 and preceding 5. It is a square number, the smallest semiprime and composite number, and is considered unlucky in many East Asian cultures.
Evolution of the H ...
cycloaddition. However, this reaction was later found to be stepwise, as it also produced the Woodward-Hoffmann forbidden suprafacial-suprafacial product under kinetic conditions.
Erden and Kaufmann had previously found that the cycloaddition of heptafulvalene and N-phenyltriazolinedione also gave both suprafacial-antarafacial and suprafacial-suprafacial products.

Photochemical cycloadditions and their stereochemistry
Cycloadditions in which 4n π electrons participate can also occur viaphotochemical
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible (400–750&nb ...
activation. Here, one component has an electron promoted from the HOMO
''Homo'' () is a genus of great ape (family Hominidae) that emerged from the genus ''Australopithecus'' and encompasses only a single extant species, ''Homo sapiens'' (modern humans), along with a number of extinct species (collectively called ...
(π bonding) to the LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
(π* antibonding
In theoretical chemistry, an antibonding orbital is a type of molecular orbital that weakens the chemical bond between two atoms and helps to raise the energy of the molecule relative to the separated atoms. Such an orbital has one or more node ...
). Orbital symmetry is then such that the reaction can proceed in a suprafacial-suprafacial manner. An example is the DeMayo reaction. Another example is shown below, the photochemical dimerization of cinnamic acid
Cinnamic acid is an organic compound with the formula phenyl, C6H5-CH=CH-Carboxylic acid, COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxy ...
. The two ''trans'' alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s react head-to-tail, and the isolated isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the exi ...
s are called '' truxillic acids''.
:
Supramolecular effects can influence these cycloadditions. The cycloaddition of ''trans''-1,2-bis(4-pyridyl)ethene is directed by resorcinol
Resorcinol (or resorcin) is a phenolic compound. It is an organic compound with the formula C6H4(OH)2. It is one of three isomeric benzenediols, the 1,3-isomer (or ''meta- (chemistry), meta''-isomer). Resorcinol crystallizes from benzene as co ...
in the solid-state in 100% yield.
Some cycloadditions instead of π bonds operate through strained cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane ...
rings, as these have significant π character. For example, an analog for the Diels-Alder reaction is the quadricyclane- DMAD reaction:
In the (i+j+...) cycloaddition notation i and j refer to the number of atoms involved in the cycloaddition. In this notation, a Diels-Alder reaction is a (4+2)cycloaddition and a 1,3-dipolar addition such as the first step in ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the Saturated and unsaturated compounds, unsaturated bonds are Bond cleavage, cleaved with ozone (). Multiple carbon–carbon bond are replaced by carbonyl () groups, such as aldehydes ...
is a (3+2)cycloaddition. The IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
preferred notation however, with +j+...takes electrons into account and not atoms. In this notation, the DA reaction and the dipolar reaction both become a +2ycloaddition. The reaction between norbornadiene and an activated alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
is a +2+2ycloaddition.
Types of cycloaddition
Diels-Alder reactions
The Diels-Alder reaction is perhaps the most important and commonly taught cycloaddition reaction. Formally it is a +2cycloaddition reaction and exists in a huge range of forms, including the inverse electron-demand Diels–Alder reaction, hexadehydro Diels–Alder reaction and the related alkyne trimerisation. The reaction can also be run in reverse in theretro-Diels–Alder reaction
The retro-Diels–Alder reaction (rDA reaction) is the reverse of the Diels–Alder reaction, Diels–Alder (DA) reaction, a +2cycloelimination. It involves the formation of a diene and dienophile from a cyclohexene. It can be accomplished spon ...
.
:
Reactions involving heteroatoms are known, including the aza-Diels–Alder reaction and oxo-Diels–Alder reaction.
Huisgen cycloadditions
The Huisgen cycloaddition reaction is a (2+3)cycloaddition. :
Nitrone-olefin cycloaddition
The Nitrone-olefin cycloaddition is a (3+2)cycloaddition. :Cheletropic reactions
Cheletropic reactions are a subclass of cycloadditions. The key distinguishing feature of cheletropic reactions is that on one of the reagents, both new bonds are being made to the same atom. The classic example is the reaction ofsulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
with a diene
In organic chemistry, a diene ( ); also diolefin, ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nome ...
.
:Other
Other cycloaddition reactions exist: (4+3) cycloadditions, +4cycloadditions, + 2photocycloadditions, metal-centered cycloaddition and +4photocycloadditionsFormal cycloadditions
Cycloadditions often have metal-catalyzed and stepwiseradical
Radical (from Latin: ', root) may refer to:
Politics and ideology Politics
*Classical radicalism, the Radical Movement that began in late 18th century Britain and spread to continental Europe and Latin America in the 19th century
*Radical politics ...
analogs, however these are not strictly speaking pericyclic reactions. When in a cycloaddition charged or radical intermediates are involved or when the cycloaddition result is obtained in a series of reaction steps they are sometimes called formal cycloadditions to make the distinction with true pericyclic cycloadditions.
One example of a formal +3ycloaddition between a cyclic enone and an enamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.
The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and the r ...
catalyzed by ''n''-butyllithium is a Stork enamine / 1,2-addition cascade reaction
A cascade reaction, also known as a domino reaction or tandem reaction, is a chemical process that comprises at least two consecutive reactions such that each subsequent reaction occurs only in virtue of the chemical functionality formed in the p ...
:
Iron-catalyzed 2+2 olefin cycloaddition
IronReferences
{{Authority control Cycloadditions"> Reaction mechanisms Ring forming reactions">Reaction mechanisms">Cycloadditions"> Reaction mechanisms Ring forming reactions