|
Trapping Experiment
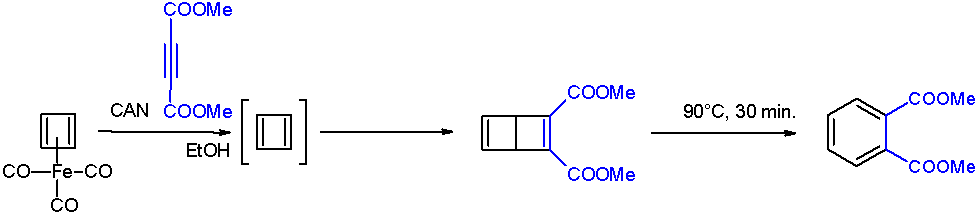
In chemistry Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ..., a chemical trap is a chemical compound that is used to detect unstable compounds. The method relies on efficiency of bimolecular reactions with reagents to produce a more easily characterize trapped product. In some cases, the trapping agent is used in large excess. Case studies Cyclobutadiene A famous example is the detection of cyclobutadiene released upon oxidation of cyclobutadieneiron tricarbonyl. When this degradation is conducted in the presence of an alkyne, the cyclobutadiene is trapped as a bicyclohexadiene. The requirement for this trapping experiment is that the oxidant (ceric ammonium nitrate) and the trapping agent be mutually compatible. : Diphosphorus Diphosphorus is an old target of chemists sinc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure. Structure and reactivity The compound is the prototypical antiaromatic hydrocarbon with 4 π-electrons. It is the smallest 'n''annulene ( annulene). Its rectangular structure is the result of the Jahn–Teller effect, which distorts the molecule and lowers its symmetry, converting the triplet to a singlet ground state. The electronic states of cyclobutadiene have been explored with a variety of computational methods. The rectangular structure is consistent with the existence of two different 1,2-dideutero-1,3-cyclobutadiene valence iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutadieneiron Tricarbonyl
Cyclobutadieneiron tricarbonyl is an organoiron compound with the formula Fe(C4H4)(CO)3. It is a yellow solid that is soluble in organic solvents. It has been used in organic chemistry as a precursor for cyclobutadiene, which is an elusive species in the free state. Preparation and structure It was first prepared in 1965 by Pettit from 3,4-dichlorocyclobutene and diiron nonacarbonyl: :C4H4Cl2 + 2 Fe2(CO)9 → (C4H4)Fe(CO)3 + 2 Fe(CO)5 + 5 CO + FeCl2 The compound is an example of a piano stool complex. The C-C distances are 1.426 Å. Properties Oxidative decomplexation of cyclobutadiene is achieved by treating the tricarbonyl complex with ceric ammonium nitrate. The released cyclobutadiene is trapped with a quinone, which functions as a dienophile. Cyclobutadieneiron tricarbonyl displays aromaticity as evidenced by some of its reactions, which can be classified as electrophilic aromatic substitution: : It undergoes Friedel-Crafts acylation with acetyl chloride and alu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rowland Pettit
Rowland Pettit (February 6, 1927 – December 10, 1981) was an Australian-born American chemist. He was awarded an overseas scholarship from the Royal Commission 1851 from 1952 - 1954. He came to London to Queen Mary College to conduct research into "the molecular orbital theory of organic chemistry and its application". ttp://www.utexas.edu/faculty/council/2000-2001/memorials/SCANNED/pettit.pdf University of Texas:In Memoriam:Rowland Petti/ref> Pettit was noted for preparation of Cyclobutadieneiron tricarbonyl and the related trimethylenemethane complex. Pettit was head of the Department of Chemistry and W. T. Doherty Professor in Chemistry at the University of Texas, Austin, a member of the National Academy of Sciences, a member of the American Chemical Society, a member of the Chemical Society of London, a recipient of the American Chemical Society's the Southwest Regional Award, a member of the American Academy of Arts and Sciences. The University of Texas said that Pett ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphosphorus
Diphosphorus is an inorganic chemical with the chemical formula . Unlike nitrogen, its lighter pnictogen neighbor which forms a stable N2 molecule with a nitrogen to nitrogen triple bond, phosphorus prefers a tetrahedral form P4 because P-P pi-bonds are high in energy. Diphosphorus is, therefore, very reactive with a bond-dissociation energy (117 kcal/ mol or 490 kJ/mol) half that of dinitrogen. The bond distance has been measured at 1.8934 Å. Synthesis Diphosphorus has been generated by heating white phosphorus at 1100 kelvins (827 °C). Nevertheless, some advancements have been obtained in generating the diatomic molecule in homogeneous solution under normal conditions with the use of some transition metal complexes (based on, for example, tungsten and niobium). Methods for dissociation of bonds in P4 molecules via photoexcitation were also proposed. The molecule attracted attention in 2006, when a new method for its synthesis at milder temperatures emerged. This method i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silylene
Silylene is a chemical compound with the formula SiH2. It is the silicon analog of methylene, the simplest carbene. Silylene is a stable molecule as a gas but rapidly reacts in a bimolecular manner when condensed. Unlike carbenes, which can exist in the singlet or triplet state, silylene (and all of its derivatives) are singlets. Silylenes are formal derivatives of silylene with its hydrogens replaced by other substituents. Most examples feature amido (NR2) or alkyl/aryl groups. Silylenes have been proposed as reactive intermediates. They are carbene analogs. Synthesis and properties Silylenes are generally synthesized by thermolysis or photolysis of polysilanes, by silicon atom reactions ( insertion, addition or abstraction), by pyrolysis of silanes, or by reduction of 1,1-dihalosilane. It has long been assumed that the conversion of metallic Si to tetravalent silicon compounds proceeds via silylene intermediates: :Si + Cl2 → SiCl2 :SiCl2 + Cl2 → SiCl4 Similar considera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbene Analog
Carbene analogs in chemistry are carbenes with the carbon atom replaced by another chemical element. Just as regular carbenes they appear in chemical reactions as reactive intermediates and with special precautions they can be stabilized and isolated as chemical compounds. Carbenes have some practical utility in organic synthesis but carbene analogs are mostly laboratory curiosities only investigated in academia. Carbene analogs are known for elements of group 13, group 14, group 15 and group 16. Group 13 carbene analogs In group 13 elements the boron carbene analog is called a borylene or boranylidene. Group 14 carbene analogs The heavier group 14 carbenes are silylenes, R2Si:, germylenes R2Ge: (example diphosphagermylene), stannylenes R2Sn: and plumbylenes R2Pb:, collectively known as metallylenes and regarded as monomers for polymetallanes. The oxidation state for these compounds is +2 and stability increases with principal quantum number (moving down a row in the periodic tab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyldichlorosilane
Dimethyldichlorosilane is a tetrahedral, organosilicon compound with the formula Si(CH3)2Cl2. At room temperature it is a colorless liquid that readily reacts with water to form both linear and cyclic Si-O chains. Dimethyldichlorosilane is made on an industrial scale as the principal precursor to dimethylsilicone and polysilane compounds. History The first organosilicon compounds were reported in 1863 by Charles Friedel and James Crafts who synthesized tetraethylsilane from diethylzinc and silicon tetrachloride.Silicon: Organosilicon Chemistry. Encyclopedia of Inorganic Chemistry Online, 2nd ed.; Wiley: New Jersey, 2005. However, major progress in organosilicon chemistry did not occur until Frederick Kipping and his students began experimenting with diorganodichlorosilanes (R2SiCl2) that were prepared by reacting silicon tetrachloride with Grignard reagents. Unfortunately, this method suffered from many experimental problems. In the 1930s, the demand for silicones increased ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylsilane
Trimethylsilane is the organosilicon compound with the formula (CH3)3SiH. It is a trialkylsilane. The Si-H bond is reactive. It is less commonly used as a reagent than the related triethylsilane, which is a liquid at room temperature. Trimethylsilane is used in the semiconductor industry as precursor to deposit dielectrics and barrier layers via plasma-enhanced chemical vapor deposition (PE-CVD). It is also used a source gas to deposit TiSiCN hard coatings via plasma-enhanced magnetron sputtering (PEMS). It has also been used to deposit silicon carbide hard coatings via low-pressure chemical vapor deposition (LP-CVD) at relatively low temperatures under 1000 °C. It is an expensive gas but safer to use than silane (SiH4); and produces properties in the coatings that cannot be undertaken by multiple source gases containing silicon and carbon. See also * Dimethylsilane * Trimethylsilyl A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



_【_Pictures_taken_in_Japan_】_(cropped).jpg)